Abstract
Studies in animal models of myocardial ischemia-reperfusion revealed that the administration of insulin-like growth factor (IGF-1) can provide substantial cardioprotective effect. However, the mechanisms by which IGF-1 prevents myocardial ischemia-reperfusion injury are not fully understood. This study addresses whether mitochondrial bioenergetic pathways are involved in the cardioprotective effects of IGF-1. Single cardiomyocytes from adult rats were incubated in the absence or presence of IGF-1 for 60 min and subjected to 60 min hypoxia followed by 30 min reoxygenation at 37°C. Mitochondrial function was evaluated by assessment of enzyme activities of oxidative phosphorylation and Krebs cycle pathways. Hypoxia/reoxygenation (HR) caused significant inhibition of mitochondrial respiratory complex IV and V activities and of the Krebs cycle enzyme citrate synthase, whereas pretreatment with IGF-1 maintained enzyme activities in myocytes at or near control levels. Mitochondrial membrane potential, evaluated with JC-1 staining, was significantly higher in IGF-1 + HR- treated myocytes than in HR alone, with levels similar to those found in normal control cardiomyocytes. In addition, IGF-1 reduced both HR-induced lactate dehydrogenase (LDH) release and malondialdehyde production (an indicator of lipid peroxidation) in cardiomyocytes. These results suggest that IGF-1 protects cardiomyocytes from HR injury via stabilizing mitochondria and reducing reactive oxidative species (ROS) damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondria constitute over one-third of cardiomyocyte volume and exclusively support the energy requirements of excitation–contraction coupling, thin–thick filament interaction, and ion (including calcium) homeostasis required during the cardiac work cycle. High-energy phosphates (including ATP and phosphocreatine) are mainly produced by oxidation of carbon fuels with oxygen in mitochondria through the electron transport chain (ETC). Furthermore, mitochondrial Ca++ signaling modulates the Krebs cycle and mitochondrial matrix dehydrogenases that maintain the nicotinamide adenine dinucleotide (NADH) redox potential and ATP synthesis.
It is well-established that both ATP synthesis and Ca++ homeostasis are driven by proton motive force (the H+ electrochemical gradient) resulting in the generation of a membrane potential across the inner mitochondrial membrane. The maintenance of the electrochemical gradient and mitochondrial membrane potential relies on membrane integrity, sufficient aerobic metabolism, and normal oxidative phosphorylation (OXPHOS) of carbon fuels.
Myocardial ischemia/reperfusion is a common event in coronary artery disease, heart surgery (including cardiac transplantation) and thrombolytic therapy. The key factor leading to tissue injury and subsequent cell death is the anoxia and reoxygenation that happens to somatic cells. In particular, re-introduction of oxygen to ischemic tissues or cells lead to the burst of reactive oxidative species (ROS), mainly derived from mitochondria due to the impairment of respiratory ETC. Furthermore, mitochondria play a critical role in the early progression of apoptosis with the release to the cytosol of apoptotic factors, which activate caspases and enhance genomic DNA fragmentation. Therefore, mitochondrial dysfunction might be important as a major causative agent in tissue injury during ischemia-reperfusion (I/R).
Several lines of evidence indicate that insulin-like growth factor 1 (IGF-1), a 70 amino acid polypeptide, has a wide protective effect on I/R injury in a variety of tissues including the heart. IGF-1 has been shown to ameliorate I/R-induced acute renal failure [1], to recover neurons from severe cerebral hypoxic-ischemic injury [2], to improve cardiac function and reduce structural damage during I/R [3–9], and to prevent apoptosis and promote survival of cardiomyocytes [7–11]. Davani et al. observed that the histological and functional cardiac improvements generated by IGF-1 treatment in an ex vivo model of myocardial I/R injury were accompanied by the maintenance of the ratio of mitochondrial to nuclear DNA in the mouse heart after I/R [4]. Using cultured neonatal rat cardiomyocytes, Lai et al. found that IGF-1 can prevent the loss of the mitochondrial electrochemical gradient and membrane depolarization resulting from doxorubicin-induction and demonstrated that IGF-1 signaling to the organelle involved the PI3 kinase-Akt pathway [12].
These findings, suggesting that IGF-1 has salutary effects on heart mitochondria, are the primary rational for the present study. We tested the hypothesis that IGF-1 exerts protective effects in adult rat cardiomyocytes stressed by hypoxia/reoxygenation by preventing damage to mitochondrial bioenergetic function and membrane potential. Our findings indicate that IGF-I is able to stabilize mitochondrial function and enable cardiomyocytes to become more resistant to hypoxia and reoxygenation injury.
Materials and methods
Materials
IGF-1 and all the reagents used for mitochondrial enzyme assays as well as cell culture media were purchased from Sigma Chemicals (St. Louis, MO). JC-1 was from Biotium (CA). Collagenase II was from Worthington (NJ) and hyauronidase from Sigma.
Cell dissociation and culture
Single cardiomyocytes from hearts of 20 adult male Sprague-Dawley rats were isolated with collagenase (type II, Worthington) using a previously described method [13], and cultured in Petri dishes (0.2–1 × 104/cm2) with Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with (in mM): 10 HEPES, 5 taurine, 5 creatine, 5 glucose, 4 pyruvate, 5 NaHCO3, and 1% penicillin-streptomycin at 37°C in a 5% CO2 incubator.
Hypoxia/reoxygenation
One hour after preculture of cardiomyocytes in the presence or absence of IGF-I (100 nM), the media was replaced with 95% N2/5% CO2-bubbled DMEM containing 5 mM glucose with or without IGF-I. Hypoxia was achieved in a customized chamber by continuous positive N2-flow for 60 min at 37°C. Subsequently, reoxygenation was induced by switching to 95% O2/CO2 for 30 min after the culture media was changed to DMEM containing 10 mM glucose.
Assays of mitochondrial enzyme activities
Quantitative assays of specific mitochondrial enzyme activities were performed in cardiomyocyte homogenates prepared in STE buffer (in mM, 320 sucrose, 10 Tris–HCl, 1 EDTA, pH 7.4). Specific activity levels of respiratory complexes I, II, IV and V, as well as the Krebs cycle enzyme, citrate synthase (CS) were measured following previously described methods [14]. Complex I activity was measured by the oxidation of NADH by ubiquinone-1 at 340 nm, complex II at 600 nm with the reduction of ubiquinone-2 and dichlorophenolindophenol by succinate, complex IV (cytochrome c oxidase) activity was assessed by the oxidation of dithionite-reduced cytochrome c at 550 nm and complex V (oligomycin-sensitive ATP synthase) assayed by NADH oxidation using a coupled enzyme assay with pyruvate kinase and lactate dehydrogenase (LDH) at 340 nm by spectrophotometry at room temperature. Complexes I and V were assayed in the presence and absence of rotenone and oligomycin, respectively. CS was measured at 412 nm by the reaction of sodium oxaloacetate, acetyl-coenzyme A and 5-dithiobis-(2 nitrobenzoic) acid.
Detection of LDH release
Culture media was collected after Hypoxia/reoxygenation (HR) and the activity of LDH, leaked out from cardiomyocytes, was assayed in 1.2 ml of phosphate buffer (0.1 mol/l, pH 7.4) with 50 μl of NADH (2.5 mg/ml phosphate buffer). The rate of NADH oxidation was determined following decrease in absorbance at 340 nm at room temperature with the use of a spectrophotometer (U-2000, Hitachi).
Measurement of MDA production
The product of lipid peroxidation, malondialdehyde (MDA), was determined in cardiomyocyte homogenates using thiobarbituric acid assay [15]. A homogenate extract (containing 200 μg protein) was incubated with 2.0 ml of TCA-TBA-HCl reagent in boiling water for 15 min. After centrifugation, the absorbance of the sample (supernatant) was measured at 535 nm at room temperature with the use of a spectrophotometer.
Assessment of mitochondrial membrane potential (ΔΨm)
After HR, cardiomyocytes were loaded with 0.5 mM of JC-1 (a lipophilic and cationic dye that exhibits potential-dependent accumulation in negatively charged mitochondria) in DMEM at 37°C for 15 min and washed twice with DMEM. The cells were visualized under a fluorescent microscope equipped with red and green filters. Fluorescent images were taken with a CCD camera (Q Imaging Micropublisher), saved in a Mac computer, and analyzed offline with Image-J software (NIH). At low concentration (low ΔΨm,) JC-1 exists mainly in a monomeric form, which emits green fluorescence. JC-1 at high concentration (high ΔΨm) forms aggregates called “J” complexes, which emit red fluorescence. Thus, a reduction in the ratio of red to green fluorescence indicated a fall in ΔΨm.
Statistical analysis
All quantitative data are presented as mean ± SEM. Multiple comparisons among groups were analyzed by t-test as well as ANOVA analysis. A level of P < 0.05 was accepted as statistically significant.
Results
LDH release
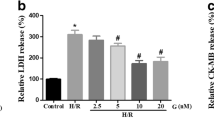
In energy production, LDH is a key enzyme that catalyzes the conversion of lactate to pyruvate. Cardiomyocytes are rich in LDH, particularly LDH-5 (M4) and LDH-1 (H4). LDH-1 normally resides in mitochondria, whereas LDH-5 is present not only in mitochondria but also in myofibrils, cytosol, and matrix surrounding mitochondria [16]. When cells are damaged or dead, LDH is leaked out into culture media. Figure 1 shows that substantially elevated LDH levels in culture media occurred as a result of HR. The accumulation of LDH in the media was significantly prevented by IGF-1 pretreatment, indicating that IGF-1 significantly reduced cell damage following HR stress. However, no significant difference was noted between the control and IGF-1 treated groups with respect to overall cardiomyocyte survival (data not shown).
Mitochondrial membrane potential (ΔΨm)
Negatively charged mitochondrial membrane (ΔΨm) is essential to allow ATP synthase to produce ATP by adequate electrochemical gradient across the mitochondrial membrane. An increase in membrane permeability to ions or small molecules might be mediated in part by an increase in the opening of mitochondrial membrane permeability transition pore (PTP), leading to the loss of electrochemical gradient and collapse of ΔΨm. We used JC-1 as an indicator of mitochondrial membrane potential to test whether IGF-1 could preserve ΔΨm in cardiomyocytes subjected to HR stress. As shown in Fig. 2a and b, red fluorescence intensity, in particular, the ratio of red to green fluorescence was significantly decreased in HR-treated cardiomyocytes compared to control (0.8 ± 0.08 in HR vs. 1.98 ± 0.17 in control), suggesting that HR caused the loss of mitochondrial electrochemical gradient in cultured adult cardiomyocytes. On the other hand, in cardiomyocytes pretreated with IGF-1 the ratio of red to green fluorescence after HR was significantly higher compared to HR alone (1.89 ± 0.07 in IGF-1 + HR vs. 0.8 ± 0.08 in HR, P < 0.0001), indicating that IGF-1 prevented the collapse of the mitochondrial electrochemical gradient. These data suggest that rapid IGF-1 signaling is able to effectively maintain or to promote full recovery of ΔΨm in cardiomyocytes undergoing HR stress.
(A) Representative fluorescence microscopic images of JC-1 staining in myocytes. IGF-1 (100 nM) was added to the culture medium 1 h prior and during HR. A to F showed the changes in mitochondrial fluorescence intensity in myocytes with or without HR stress. A, B, and C show cells with red filter; D, E, and F with green filter. (B) IGF-I rescued Δψm in myocytes subjected to HR. Mitochondrial membrane depolarization was characterized by the reduction of red/green ratio. Data derived from four independent experiments are shown. n=number of cells determined; *P < 0.0001 versus control; # P < 0.0001 versus HR
Respiratory chain and oxidative phosphorylation function
Since the preservation of ΔΨm suggested a salutary effect of IGF-1 treatment on cardiomyocyte mitochondria, we further determined if IGF-1 protected mitochondrial respiratory enzyme activities in cardiomyocytes subjected to HR. As indicated in Fig. 3a, HR by itself inhibited complex IV activity by 25%, which was prevented by IGF-1 pretreatment of myocytes. In addition, activity of complex V, the enzyme primarily responsible for mitochondrial ATP synthesis, was inhibited by over 50% when myocytes underwent HR. However, in cardiomyocytes treated with IGF-1, HR caused only a slight decrease (<15%) in complex V activity. These data indicate that IGF-1 preserves mitochondrial ETC function in cardiomyocytes during HR. Analysis of cardiomyocyte respiratory complex I and II activities also shows a similar trend of IGF-1 mediated protection against HR inhibition (Fig. 3B).
(A) IGF-1 prevented HR-induced inhibition of mitochondrial complex IV and V activity in myocytes. Data shown are derived from 7 sets of separate experiments. *P < 0.005 versus control;# P < 0.03 versus HR. (B) Effects of IGF-1 on HR-induced mitochondrial complex I and II activities. The activity of complex I and II was assayed in myocyte homogenates after HR (see methods). Data derived from 7 independent experiments are shown. *P < 0.04 versus control; # P < 0.03 versus HR
Krebs cycle activity
Furthermore, we evaluated cardiomyocyte mitochondrial bioenergetic function by determining the activity levels of a key rate-limiting enzyme in the Krebs cycle pathway, citrate synthase (CS), an enzyme encoded by nuclear DNA, which is located in the mitochondrial matrix. HR reduced CS activity by over 21% (Fig. 4). However, in IGF-1 pretreated myocytes, CS activity remained unchanged after HR, indicating that IGF-1 signaling is able to prevent Krebs cycle and oxidative metabolism dysfunction in cardiomyocytes stressed by HR.
Lipid peroxidation
Reoxygenation in aerobic cells, especially in cardiomyocytes, results in a burst of ROS production, which in turn causes extensive lipid peroxidation and cellular damage. ROS production was indirectly gauged by determining levels of cardiomyocyte lipid peroxidation as assessed by the measurement of MDA. As shown in Fig. 5, HR increased MDA level near or above 75% compared to control, while IGF-1 pretreatment of cardiomyocytes reduced HR-induced MDA production by 67% (P < 0.04 vs HR alone). These findings demonstrated that exposure of cardiomyocytes to IGF-1 reduces the oxidative injury promoted by HR.
IGF-1 reduced lipid peroxidation in myocytes following HR stress. The extent of HR-induced lipid peroxidation was assessed by the measurement of MDA in myocyte homogenates from 7 sets of independent experiments. *P < 0.0004 versus control; # P < 0.006 versus HR and P < 0.05 versus control, respectively
Discussion
Mitochondria play a critical role in reperfusion or reoxygenation injury of cardiac muscle. The mechanisms involved in this process include impaired ETC function that leads to generation of ROS and augments cellular oxidative stress, increased mitochondrial Ca++ accumulation that promotes PTP opening, produces a profound mitochondrial depolarization, and causes the release of cytochrome c and other apoptosis-inducing proteins [17–20]. Recently, it has been suggested that modulation of mitochondrial function can stem the effects (and reduce levels) of oxidative stress and ultimately protect cardiac cells from injury. In agreement with this hypothesis, our study, for the first time showed evidence that IGF-1 preserves mitochondrial Krebs cycle, ETC function, and ΔΨm in cardiomyocytes subjected to HR stress, and these effects may in part account for the protective action of IGF-1 against myocardial I/R injury.
Normal mitochondrial electrochemical gradient or membrane potential, the driving force for OXPHOS, is essential for the survival of cardiomyocytes during and after HR. Sustained or irreversible depolarization leads to irreversible cell injury or death. Previously it has been shown that cardiomyocyte ΔΨm dropped rapidly when exposed to short periods (15–25 min) of hypoxia and recovered partially upon reoxygenation with a subset of hypoxic cardiomyocytes developing sustained depolarization after reoxygenation [19]. Similarly, Honda et al. reported that rabbit myocytes subjected to anoxia and reoxygenation exhibited irreversible mitochondrial depolarization and ultimately developed hypercontracture leading to cell death [20]. Although we were unable to dynamically monitor the changes in ΔΨm using our experimental conditions, significant mitochondrial depolarization was found in the majority of cardiomyocytes after HR, indicating that those cells were unable to recover from the damage. We also found that sustained depolarization occurred in conjunction with HR, as did irreversible mitochondrial enzyme dysfunction. Whether this membrane depolarization develops as a consequence, acts as a stimulus or is a parallel event with mitochondrial enzymatic dysfunction remains presently undetermined.
Ischemia results in early and progressive cardiac mitochondrial structural and functional damage [21, 22]. A variety of animal models of cardiac ischemia (e.g., pig, rat and dog) have shown that I/R causes inhibition of cardiomyocyte mitochondrial function manifested by reduced levels of respiratory complex activities [23, 24], with complex IV [25, 26], and complex V [27] activities most severely inhibited. There is also evidence that subpopulations of myocardial mitochondria can be detected whose enzymes are more susceptible to ischemic damage [25]. Some studies have suggested that damage of the ETC components is exacerbated upon reperfusion [28]. Consistent with these observations, our findings documented a significant reduction in ETC (e.g., complex IV and V) and Krebs cycle CS activities in cultured cardiomyocytes exposed to HR.
An important effect of mitochondrial ETC dysfunction is ROS generation. Inhibition of complex I and III activities favors the leakage of electrons from the ETC resulting in superoxide anions (O −2 ) formation [29]. The O −2 could be further converted to hydrogen peroxide (H2O2) by superoxide dismutase (MnSOD) present in the mitochondrial matrix and can also be converted to highly-reactive hydroxyl radicals (OH−) via H2O2 by Fenton reaction. Furthermore, electron leakage at complex I and II results in the generation of lipid peroxide. These ROS may affect cellular components including proteins, lipids, and DNA and can further damage mitochondria leading to the formation of a vicious cycle. Our findings of extensive lipid peroxidation in the HR treated cardiomyocytes not only indicate increased ROS production, but also may have an impact on the activity and stability of mitochondrial enzymes by modifying the lipid environment similar to that reported with oxidized cardiolipin affecting complex IV activity [26]. However, it is important to note that while our findings of both mitochondrial enzymatic dysfunction and a parallel increase in lipid peroxidation in hypoxic cardiomyocytes, and their reversal by IGF-1 treatment, are suggestive of a contributory role of mitochondria in the modulation of hypoxia and IGF-1 cardioprotective responses, they do not directly show a definitive cause and effect relationship. Further studies employing a time-course approach may be helpful in establishing the precise sequence of events and in defining cause-effect relationships.
Another consequence of ischemia/HR on mitochondria is the irreversible change in membrane permeability mediated by opening of the nonspecific PTP megachannel composed of the adenine nucleotide translocase (ANT) in the inner membrane, cyclophilin D (CyP-D) in the matrix, and the voltage-dependent anion channel (VDAC) in the outer membrane [17, 20]. Using the mitochondrial DOG-entrapment technique, it has been shown that PTP opening occurs upon reperfusion and not during ischemia [18]. Opening of PTP may result from sustained mitochondrial membrane depolarization, ROS burst and/or mitochondrial Ca++ overload, which leads to uncoupling, swelling, and loss of nucleotides and other small molecules from the mitochondrial matrix. Furthermore, PTP opening and swelling are thought to contribute to mitochondrial outer membrane rupture resulting in the release of cytochrome c and other apoptosis-inducing factors from the mitochondrial intermembrane space (e.g., smac/Diablo, endoG, and AIF) into the cytosol, critical early events of the intrinsic apoptotic pathway [30].
Our data suggest that mitochondria constitute a crucial target in the treatment of HR injury. Improving or stabilizing mitochondrial respiratory function may help to interrupt, dampen or prevent the ROS burst-mediated cellular damage cycle, to eliminate the release of cytochrome c and AIF, and to promote cell recovery from HR injury. Several lines of evidence support this approach.
Cyclosporine (CsA) and NIM811, which inhibit PTP opening were found to protect the heart against I/R injury [31] as did deletion of CyP-D in mice [32, 33]. Zhao et al. using another mitochondrial-based cardioprotective approach employed cell-permeable peptide antioxidants targeting the inner mitochondrial membrane which were able to inhibit mitochondrial ROS production, PTP and swelling, and to prevent 3-nitropropionic acid-induced mitochondrial depolarization [34]. These peptides significantly induced recovery of cardiac function following I/R injury. A mitochondrial-targeted antioxidant (MitoQ) has been shown to be particularly potent in preventing lipid peroxidation and mitochondrial damage [35]. Feeding MitoQ to rats significantly prevented heart dysfunction, cell death, and ETC defects caused by I/R. These findings suggest that stabilization of mitochondria may be a practical way to prevent myocardial I/R injury [36].
Several observations have demonstrated that IGF-1 protects the heart against post-ischemic reperfusion injury [3–8]. In vivo studies have shown that IGF-1 pretreatment reduces myocyte apoptosis in rat heart subjected to I/R [3]. Moreover, ex vivo studies demonstrated that IGF-1 treatment improved recovery of cardiac performance and reduced infarct size as well as apoptotic cell death following I/R [3–7]. In addition, IGF-1 overexpression may offer protection of cardiomyocytes from apoptosis after either ischemia or post-ischemic reperfusion [9,10]. The anti-apoptotic effect of IGF-1 has also been confirmed in cultured cardiomyocytes [11]. Furthermore, positive regulatory actions of IGF-1 on myocyte Ca++ handling and contractility may contribute to cardioprotection [37–40] and activating normal mitochondrial function, upon which IGF-1’s effect is not known.
IGF-1 signaling can trigger multiple signal cascade pathways in cardiomyocytes [7–12, 41]. Activation of the PI3K-Akt pathway and/or ERK 1/2 kinase cascade by IGF-1 can initiate efficient anti-cell death mechanisms, and show evidence of mitochondrial involvement. For instance, systemic IGF-1 treatment in rat has been reported to up-regulate Bcl-Xl expression but down-regulates pro-apoptotic Bax protein in heart mitochondria, as well as significantly reducing PTP opening and cytochrome c release in response to I/R injury [7]. In addition, overexpression of Bcl-Xl affects mitochondrial membrane potential, matrix swelling, and prevents cell death induced by OXPHOS inhibitors [42]. These findings suggest that IGF-1 may regulate mitochondrial function and in turn protect myocytes against a lethal stimulus like HR.
Interestingly, our findings showed that IGF-1 can rapidly target mitochondria, maintaining respiratory enzyme activity after HR, likely acting at a non-genomic level. A rapid protective action of IGF-1 on mitochondria was also reported by Lai et al. [12]. Doxorubicin, a drug widely used in chemotherapy, was found to cause rapid loss of the mitochondrial electrochemical gradient and depolarization in cultured neonatal myocytes, which was prevented by IGF-1 pretreatment. Also, high glucose has been found to cause mitochondrial depolarization in neurons, which was prevented by IGF-1 as well [43].
Improved mitochondrial ETC activity may reduce the leakage of electrons and reduce ROS production. In fact, several observations have shown that IGF-1 can reduce ROS generation induced by angiotensin II [44] or hyperglycemia [45]. Similarly, our data provides indirect evidence that IGF-1 reduce ROS generation and lipid peroxidation in cardiomyocytes during HR.
IGF-1 has been reported to decrease Ca++-stimulated mitochondrial cytochrome c release and inhibit Ca++-sensitive mitochondrial swelling [7], suggesting that IGF-1 signaling can modulate PTP in heart. Juhaszova et al. reported that glycogen synthase kinase-3 (GSK-3), a potential downstream target of the IGF-signaling pathway can regulate PTP opening in cardiomyocytes [46]. Also, IGF-1 can prevent mitochondrial DNA damage following I/ R injury [4], and promote increased tonic mitochondrial ATP synthesis in rat heart myocytes [7]. These salutary actions of IGF-1 would enable mitochondria to be more resistant to insult stress and protect cardiac muscle against oxidative stress.
In conclusion, our data showed that the activation of IGF-1 signaling pathways provide full protection to rat cardiomyocytes against HR injury, which might be associated with stabilization of mitochondrial respiratory chain function and reduction of oxidative stress.
References
Noguchi S, Kashihara Y, Ikegami Y, Morimoto K, Miyamoto M, Nakao K (1993) Insulin-like growth factor-I ameliorates transient ischemia-induced acute renal failure in rats. J Pharmacol Exp Ther 267:919–926
Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K (1992) A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun 182:593–599
Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM (1995) Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci USA 92:8031–8035
Davani EY, Brumme Z, Singhera GK, Cote HC, Harrigan PR, Dorscheid DR (2003) Insulin-like growth factor-1 protects ischemic murine myocardium from ischemia/reperfusion associated injury. Crit Care 7:R176-R183
Otani H, Yamamura T, Nakao Y, Hattori R, Kawaguchi H, Osako M, Imamura H (2000) Insulin-like growth factor-I improves recovery of cardiac performance during reperfusion in isolated rat heart by a wortmannin-sensitive mechanism. J Cardiovasc Pharmacol 35:275–281
Friehs I, Stamm C, Cao-Danh H, McGowan FX, del Nido PJ (2001) Insulin-like growth factor-1 improves postischemic recovery in hypertrophied hearts. Ann Thorac Surg 72:1650–1656
Yamamura T, Otani H, Nakao Y, Hattori R, Osako M, Imamura H (2001) IGF-I differentially regulates Bcl-xL and Bax and confers myocardial protection in the rat heart. Am J Physiol Heart Circ Physiol 280:H1191–H1200
Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, et al (1997) Overexpression of insulin- like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest 100:1991–1999
Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, et al (2001) Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res 88:609–614
Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101:660–667
Ren J, Samson WK, Sowers JR (1999) Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol 31:2049–2061
Lai HC, Liu TJ, Ting CT, Sharma PM, Wang PH (2003) Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol Cell Endocrinol 205:99–106
Pi Y, Walker JW (2000) Diacylglycerol and fatty acids synergistically increase cardiomyocyte contraction via activation of PKC. Am J Physiol 279:H26–H34
Long X, Goldenthal MJ, Wu GM, Marin-Garcia J (2004) Mitochondrial Ca2+ flux and respiratory enzyme activity decline are early events in cardiomyocyte response to H2O2. J Mol Cell Cardiol 37:63–70
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Meth Enzymol 52:302–310
Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE (1999) Role of mitochondrial lactate dehydrogenase, lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA 96:1129–1134
Di Lisa F, Bernardi P (2006) Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res 70:191–199
Halestrap AP (2006) Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans 34:232–237
Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG (1995) Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol 486:1–13
Honda HM, Korge P, Weiss JN (2005) Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci 1047:248–258
Murry CE, Richard VJ, Reimer KA, Jennings RB (1990) Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 66:913–931
Lesnefsky EJ, Hoppel CL (2003) Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys 420:287–297
Rouslin W (1983) Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol 244:H743-H748
Borutaite V, Mildaziene V, Brown GC, Brand MD (1995) Control and kinetic analysis of ischemia-damaged heart mitochondria: which parts of the oxidative phosphorylation system are affected by ischemia? Biochim Biophys Acta 1272:154–158
Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL (1997) Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol 273:H1544-H1554
Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Serena D, Ruggiero FM (1999) Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic Biol Med 27:42–50
Ylitalo K, Ala-Rami A,Vuorinen K, Peuhkurinen K, Lepojarvi M, Kaukoranta P, Kiviluoma K, Hassinen I (2001) Reversible ischemic inhibition of F(1)F(0)-ATPase in rat and human myocardium. Biochim Biophys Acta 1504:329–339
Solaini G, Harris DA (2005) Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J 390:377–394
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M (2005) Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 38:367–374
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662
Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH (2004) Cell- permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279:34682–34690
Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA (2005) Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19:1088–1095
Lesnefsky EJ, He D, Moghaddas S, Hoppel CL (2006) Reversal of mitochondrial defects before ischemia protects the aged heart. FASEB J 20:1543–1545
Kinugawa S, Tsutsui H, Ide T, Nakamura R, Arimura K, Egashira K, Takeshita A (1999) Positive inotropic effect of insulin-like growth factor-1 on normal and failing cardiac myocytes. Cardiovasc Res 43:157–164
Cittadini A, Ishiguro Y, Stromer H, Spindler M, Moses AC, Clark R, Douglas PS, Ingwall JS, Morgan JP (1998) Insulin-like growth factor-1 but not growth hormone augments mammalian myocardial contractility by sensitizing the myofilament to Ca2+ through a wortmannin-sensitive pathway: studies in rat and ferret isolated muscles. Circ Res 83:50–59
Freestone NS, Ribaric S, Mason WT (1996) The effect of insulin-like growth factor-on adult rat cardiac contractility. Mol Cell Biochem 163–164:223–229
von Lewinski D, Voss K, Hulsmann S, Kogler H, Pieske B (2003) Insulin-like growth factor-1 exerts Ca2+-dependent positive inotropic effects in failing human myocardium. Circ Res 92:169–176
Foncea R, Andersson M, Ketterman A, Blakesley V, Sapag-Hagar M, Sugden PH, LeRoith D, Lavandero S (1997) Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J Biol Chem 272:19115–19124
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB (1997) Bcl-xL regulates the membrane potential, volume homeostasis of mitochondria. Cell 91:627–637
Leinninger GM, Russell JW, van Golen CM, Berent A, Feldman EL (2004) Insulin-like growth factor-I regulates glucose-induced mitochondrial depolarization and apoptosis in human neuroblastoma. Cell Death Differ 11:885–896
Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P (2001) IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50:1414–1424
Gustafsson H, Soderdahl T, Jonsson G, Bratteng JO, Forsby A (2004) Insulin-like growth factor type 1 prevents hyperglycemia-induced uncoupling protein 3 down-regulation and oxidative stress. J Neurosci Res 77:285–291
Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ (2004) Glycogen synthase kinase-3 beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113:1535–1549
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pi, Y., Goldenthal, M.J. & Marín-García, J. Mitochondrial involvement in IGF-1 induced protection of cardiomyocytes against hypoxia/reoxygenation injury. Mol Cell Biochem 301, 181–189 (2007). https://doi.org/10.1007/s11010-007-9410-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9410-0