Abstract
The morbidity and mortality rates of cardiovascular disease are markedly higher in patients with diabetes than in non-diabetic patients, including patients with ischemia–reperfusion injury (IRI). However, the cardiovascular protective effects of Empagliflozin (EMPA) on IRI in diabetes mellitus have rarely been studied. In this study, we established a cardiomyocyte hypoxia/reoxygenation (H/R) injury model to mimic myocardial I/R injuries that occur in vivo. H9C2 cells were subjected to high glucose (HG) treatment plus H/R injury to mimic myocardial I/R injuries that occur in diabetes mellitus. Next, different concentrations of EMPA were added to the H9C2 cells and its protective effect was detected. STAT3 knockdown with recombinant plasmids was used to determine its roles. Our results showed that H/R injury-induced cell apoptosis, necroptosis, oxidative stress, and endoplasmic reticulum stress were further promoted by HG conditions, and HG treatment plus an H/R injury inhibited the activation of JAK2/STAT3 signaling. EMPA was found to protect against H/R-induced cardiomyocyte injury under HG conditions and activate JAK2/STAT3 signaling, while down-regulation of STAT3 reversed the protective effect of EMPA. When taken together, these findings indicate that EMPA protects against I/R-induced cardiomyocyte injury by activating JAK2/STAT3 signaling under HG conditions. Our results clarified the mechanisms that underlie the cardiovascular protective effects of EMPA in diabetes mellitus and provide new therapeutic targets for IRI in diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
A hypoxia/reoxygenation (H/R) injury model was established to mimic myocardial I/R injuries in vivo.
-
Treatment of H9C2 cells with high glucose (HG) combined with an H/R injury was used to mimic the myocardial I/R in diabetes mellitus.
-
EMPA protects against I/R-induced cardiomyocyte injury under HG conditions.
-
STAT3 is involved in the effects of EMPA on cardiomyocyte H/R injuries under high glucose conditions.
Introduction
Diabetes is a complex and heterogeneous disease that affects people at different stages of life [1]. In the developing world, type 2 diabetes is growing at an alarming rate, as people gain access to Western-style diets [2]. Type 2 diabetes mellitus (T2DM) is characterized by a series of metabolic disorders, including hyperglycemia, insulin resistance, dyslipidemia, and nonalcoholic fatty liver disease (NAFLD), which accounts for 90–95% of diabetes mellitus cases affecting nearly 463 million individuals worldwide [1, 3].
Cardiovascular disease is an increasing complication of type 2 diabetes [4]. The morbidity and mortality rates of cardiovascular disease in patients with diabetes are markedly higher than those in non-diabetic patients [5]. The cardiovascular complications of T2DM can include myocardial infarction, coronary heart disease, and atherosclerosis [6,7,8]. Ischemia/reperfusion (I/R) is a pathological event that occurs in numerous disease states [9]. Ischemia, the first event, refers to the restriction of blood supply to an organ, usually as a result of a blockage within the arterial blood supply by an embolus. Ischemia is almost always associated with cellular metabolic imbalances and deleterious hypoxia. The second event is the reperfusion, or restoration of blood flow and reoxygenation of the affected ischemic area [9, 10]. Ischemia/reperfusion injury (IRI) refers to the fact that tissues and organs are sometimes unable to recover their normal function after the restoration of blood supply [11]. Due to impaired cardiovascular function in diabetic patients, IRI is more likely to occur in those patients. IRI causes tissue damage by enhancing oxidative stress [12] and endoplasmic reticulum stress [13], leading to cell apoptosis [14] or necroptosis [15, 16]. Glucose control is a central focus in the management of T2DM [17], and reducing hyperglycemia has been shown to decrease the cardiovascular complications of diabetes [4, 18]. Sodium-sugar cotransporter 2 (SGLT2) is a sodium-dependent glucose transport protein [19] which mediates the majority of glucose reabsorption (~ 90%) [20]. Inhibition of SGLT2-mediated glucose transport is a rational strategy for treating T2DM [21, 22].
Empagliflozin (EMPA), an SGLT2 inhibitor, is an effective and generally well-tolerated antihyperglycemic agent approved for the treatment of adults with T2DM [23]. Beyond lowering glucose, EMPA exerts a favorable effect on a series of non-glycemic effects, including causing modest reductions in body weight and blood pressure [24], and providing some cardiovascular and renal protection [25]. In non-diabetic rats, low-dose EMPA treatment improves systolic heart function after a myocardial infarction [26]. Clinical studies have shown that EMPA reduces cardiovascular morbidity and mortality in patients with type 2 diabetes mellitus [27, 28], and attenuates ischemia–reperfusion injuries [29,30,31]. Previous studies revealed that EMPA attenuates transient cerebral ischemia/reperfusion injuries in hyperglycemic rats [32]. However, the mechanisms by which EMPA exerts its cardiovascular protective effects in patients with diabetes mellitus remain unclear.
In this study, we cultured rat H9C2 cardiomyocyte cells and used them to establish a cellular model of a hypoxia/reoxygenation (H/R) injury that occurs in vivo. High glucose (HG) treatment combined with induction of an H/R injury in H9C2 cells was used to mimic the myocardial I/R that occurs in diabetes mellitus. Next, the effects of EMPA on cardiomyocyte H/R injuries that occur under high glucose conditions and the underlying molecular mechanisms for the effects were investigated.
Materials and methods
Cell lineage and cell culture
Rat H9C2 cardiomyocytes were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 5.5 mM glucose (Control group) or 25 mM glucose (High glucose group), plus 100 μg/mL of penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere for 18 h. A hypoxic condition was achieved by culturing the H9C2 cells in serum- and glucose-deficient DMEM in an incubator chamber (MIC-101, Billups-Rothenberg, San Diego, CA, USA) filled with 5% CO2, 95% N2, and 1% O2.
Western blot analysis
Total proteins were extracted from each group of cells, and separated by 10% SDS-PAGE; after which, the protein bands were transferred onto PVDF membranes (Millipore, Burlington, MA, USA) that were subsequently blocked with 5% non-fat milk. The membranes were then incubated with the following primary antibodies: Caspase 3 (ab184787, Abcam, Cambridge, UK), Bcl-2 (ab194583, Abcam), Bax (ab182734, Abcam), p-RIP1(AF7088, Affinity Biosciences, Cincinnati, OH, USA), p-RIP3 (AF7443, Affinity Biosciences), p-MLKL (AF7420, Affinity Biosciences), GRP78 (ab108615, Abcam), eIF-2α (ab5369, Abcam), p-eIF-2α (ab214434, Abcam), PERK (ab229912, Abcam), p-PERK ( DF7576, Affinity Biosciences), CHOP (SAB4500632, Sigma-Aldrich, St. Louis, MO, USA), p-JAK2 (ab32101, Abcam), p-STAT3 (ab32143, Abcam), and β-actin (ab8227, Abcam). Nest, the membranes were incubated with an HRP-conjugated secondary antibody and developed with an ECL kit (Perkin-Elmer Inc., Waltham, MA, USA). β-actin served as an internal control.
Cell transfection
Plasmids constructed with short hairpin RNA targeting STAT3 (si-STAT3) were used to achieve the knockdown of STAT3 (GenePharma, Shanghai, China). Cells were transfected with si-STAT3 plasmids or mock-vectors for 48 h and then collected for use in subsequent experiments. Lipofectamine®3000 reagent (Invitrogen) was used to perform all transfections.
Cell counting kit-8 (CCK-8) assay
A CCK-8 assay kit (Beyotime, Nanjing, China) was used for determinations of cell viability. Cells were cultured for 24 h in a 96-well flat-bottomed plate (5000 cells/well), and then transfected, treated, and cultured in a normal medium. Next, CCK-8 solution (20 μL) was added to each well at times ranging from 0 to 48 h, and the cells were incubated or an additional 4 h. Cell viability was evaluated by detecting the absorbance of each well at 450 nm.
Flow cytometry for cell apoptosis
An Annexin V-FITC Apoptosis Detection Kit (Keygen, China) was used to quantify the numbers of apoptotic cells. In brief, live cells were collected with 0.25% pancreatin, washed twice with ice-cold PBS, and then resuspended in 500 μL of binding buffer. Next, the cells were incubated with 5 μL of antibody against Annexin V-FITC and 5 μL of propidium iodide (PI) for 15–20 min in the dark. After the incubation, cell apoptosis was detected with a BD Accuri C6 flow cytometer (BD, Franklin Lakes, NJ, USA). The excitation wavelength (Ex) used was 488 nm, and the emission wavelength (Em) was 530 nm.
TdT-mediated dUTP nick end labeling (TUNEL) assay
The DNA fragments of apoptotic cells were detected using the TUNEL assay. Specifically, the treated cells in each group were collected (2 × 104 cells per well, from an 8-well plate), fixed with 4% formaldehyde, and embedded onto glass slides. DNA fragment 3ʹ-OH terminal nucleotides were labeled with biotin-dUTP at 37 °C for 1 h in the dark. The slides were then mounted using a DAPI containing solution to stain the nuclei. Nuclear fluorescence of the DNA fragments was detected under a fluorescence microscope (BX41, Olympus, Japan).
Measurements of MDA levels and SOD activity
MDA levels and SOD activity were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions.
Immunofluorescence (IF) staining
Cells were fixed with cold methanol (100%, stored at − 20 °C) for 2 min, washed 3 times with 800 μL of PBS, and then blocked with bovine serum albumin (BSA, 2%) for 1 h. Next, the cells were incubated with primary antibodies against p-STAT3 (ab32143, Abcam) at 4 °C overnight. After incubation, the cells were washed with PBS and treated with an Alexa Fluor 568‐labeled secondary antibody (1:1000 diluted in PBS buffer with 2% BSA) for 2 h in the dark. The cell nuclei were stained with DAPI. Images of the stained cells were acquired using a fluorescence microscope (Olympus, Japan).
Statistical analysis
All statistical data were analyzed using IBM SPSS Statistics for Window, Version 20 software (IBM Corp., Armonk, NY, USA). Results are expressed as a mean value ± standard deviation (SD). All data were analyzed for normality using the Shapiro–Wilk test. The student's t-test was used to compare the significance of differences between two groups and one-way ANOVA followed by Dunnett’s T3 test were used to compare the significance of differences among more than two groups. The Whitney U test was for analysis of non-parametric data. A P-value < 0.05 was considered to be statistically significant.
Results
Establishment of a cardiomyocytes hypoxia/reoxygenation (H/R) injury model
To confirm the effects of hypoxia/reoxygenation on cardiomyocytes, rat cardiomyocytes (H9C2) were exposed to hypoxic conditions for 2 h, followed by reoxygenation for 0–12 h. The CCK8 assay, flow cytometry, and TUNEL assay were used to detect cell viability and cell apoptosis, respectively. Figure 1A shows that the viability of H9C2 cells was significantly decreased after exposure to hypoxic conditions. Exposure to hypoxic conditions for 2 h increased the numbers of apoptotic cells, and reoxygenation for 4 h, 8 h, and 12 h still promoted the apoptosis of H9C2 cells (Fig. 1B). Similarly, TUNEL assays showed that hypoxia and hypoxia/reoxygenation increased the numbers of apoptotic cells with typical DNA fragmentation (Fig. 1C). In addition, the total proteins of cells from each group were extracted, and the protein levels of cells undergoing apoptosis and necrosis were examined. Figure 1D shows that hypoxia/reoxygenation markedly increased the expression of pro-apoptosis and necrosis-related proteins (Caspase 3, Bax, p-RIP3, p-RIP1, and p-MLKL), and decreased the expression of Bcl-2 (an anti-apoptosis protein). Finally, the MDA content and SOD activity of each group were measured with commercial kits. The results showed that hypoxia treatment significantly increased the MDA content of H9C2 cells, and the MDA content was further increased by reoxygenation; while hypoxia significantly decreased the SOD activity of H9C2 cells, and the SOD activity was further decreased by reoxygenation (Fig. 1E–F). These results suggested that the cardiomyocyte hypoxia/reoxygenation injury model had been successfully established. Next, a 2 h period of hypoxia and an 8 h period of reoxygenation were selected for use in subsequent experiments.
Establishment of a cardiomyocyte hypoxia/reoxygenation (H/R) injury model. A After hypoxia/reoxygenation treatment, the viability of H9C2 cardiomyocytes was detected by the CCK-8 assay. B Cell apoptosis was examined by flow cytometry. C Apoptotic DNA fragments were detected by the TUNEL assay. D Western blot assays were performed to detect the levels of proteins associated with cell apoptosis and necroptosis. E–F MDA levels and SOD activity were measured using commercial kits. ns no significant difference; *P < 0.05, **P < 0.01, ***P < 0.001
High glucose combined with hypoxia/reoxygenation treatment induced cardiomyocyte injury and inhibited JAK2/STAT3 signaling
After establishing the cardiomyocyte H/R model, we explored the effects of high glucose (HG) on H9C2 cells prior to induction of an H/R injury. H9C2 cells were cultured in high glucose medium for 18 h and then subjected to H/R treatment. The CCK8 assay, flow cytometry, and TUNEL assay were used to detect cell viability and cell apoptosis, respectively. Figure 2A shows that the viability of H9C2 cells was significantly decreased after either high glucose or H/R treatment, and HG further reduced cell viability under conditions of H/R treatment. Results of flow cytometry and TUNEL assays showed that either HG or H/R treatment could increase the numbers of apoptotic H9C2 cells and HG further promoted cell apoptosis under conditions of H/R treatment (Fig. 2B–C). Western blot analyses demonstrated that HG or H/R treatment markedly increased the levels of Caspase 3, Bax, p-RIP3, p-RIP1, and p-MLKL protein expression, and decreased Bcl-2 expression. Furthermore, HG markedly promoted the alteration of proteins under conditions of H/R treatment (Fig. 2D). In addition, assays for MDA content and SOD activity showed that either HG or H/R treatment significantly increased the MDA levels in H9C2 cells, and those levels were further increased by HG when HG was combined with an H/R injury. Either HG or H/R treatment significantly decreased SOD activity in the H9C2 cells, and SOD activity was further decreased when HG was combined with an H/R injury (Fig. 2E–F).
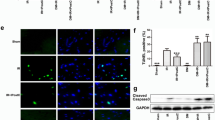
High glucose combined with hypoxia/reoxygenation treatment induced cardiomyocyte injury and inhibited JAK2/STAT3 signaling. A–C Cell viability in each group was detected by the CCK-8 assay, cell apoptosis was detected by flow cytometry, and apoptotic DNA fragments were detected by the TUNEL assay. D Western blotting was used to detect the levels of proteins associated with cell apoptosis and necrosis. E–F MDA levels and SOD activity were measured with commercial kits. G Western blotting was performed to detect the levels of endoplasmic reticulum stress-related proteins and STAT3 pathway-related proteins. H IF staining was performed to detect the intensity of p-STAT3 protein expression in each group of cells. *P < 0.05, **P < 0.01, ***P < 0.001, vs. Control group; #P < 0.05, ##P < 0.01, vs. I/R group
To explore the mechanism underlying changes in cell phenotype, the levels of endoplasmic reticulum stress (ERS)-related proteins (GRP78, p-PERK, p-eIF-2, and CHOP) and STAT3 pathway-related proteins (p-JAK2 and p-STAT3) were detected. Figure 2G shows that either HG or H/R treatment markedly increased the levels of endoplasmic reticulum stress in H9C2 cells, and markedly decreased activation of the STAT3 pathway; while HG promoted ERS and further inhibited STAT3 pathway activation under conditions of H/R treatment. Finally, IF staining of p-STAT3 in H9C2 cells further confirmed that activation of the STAT3 pathway was inhibited by HG or H/R treatment, and HG combined with H/R treatment further inhibited that activation (Fig. 2H).
Empagliflozin (EMPA) exerted a protective effect in the cardiomyocyte H/R model under high glucose conditions and activated JAK2/STAT3 signaling
Due to the wide use of EMPA in treatment of diabetes mellitus, we explored the effects of EMPA on cardiomyocyte H/R injuries under high glucose conditions. H9C2 cardiomyocytes were cultured in high glucose medium and exposed to different concentrations of EMPA (0.1 μM, 0.5 μM, and 1 μM) followed by an H/R injury. CCK8 assays, flow cytometry, and TUNEL assays were used to determine cell viability and cell apoptosis, respectively. Results showed that EMPA significantly increased cell viability, and decreased the numbers of apoptotic cells in the H/R-induced cell injury model (Fig. 3A–C). Western blot analyses showed that EMPA markedly decreased the expression of Caspase 3, Bax, p-RIP3, p-RIP1, and p-MLKL proteins, and increased Bcl-2 expression (Fig. 3D). In addition, EMPA significantly decreased the cellular MDA levels and increased the SOD activity resulting from H/R-induced oxidative changes (Fig. 3E–F). Figure 3 G shows that EMPA markedly decreased the levels of endoplasmic reticulum stress in H9C2 cells, and markedly increased activation of the STAT3 pathway involved in H/R-induced protein changes. Finally, IF staining of p-STAT3 in H9C2 cells confirmed that EMPA markedly increased activation of the STAT3 pathway under conditions of H/R injury (Fig. 3H).
Empagliflozin (EMPA) exerted a protective effect in the cardiomyocyte H/R model under high glucose conditions and activated JAK2/STAT3 signaling. H9C2 cardiomyocytes were cultured in high glucose medium and exposed to different concentrations of EMPA (0.1 μM, 0.5 μM, and 1 μM) followed by hypoxia/reoxygenation treatment. A–C Cell viability in each group was detected by the CCK-8 assay, cell apoptosis was detected by flow cytometry, and apoptotic DNA fragments were detected by the TUNEL assay. D Western blotting was performed to detect the levels of proteins associated with cell apoptosis and necrosis. E–F MDA levels and SOD activity were measured with commercial kits. G Western blotting was performed to detect the levels of endoplasmic reticulum stress-related proteins and STAT3 pathway-related proteins. H IF staining was performed to detect the intensity of p-STAT3 protein expression in each group of cells. ns, no significant difference; *P < 0.05, **P < 0.01, ***P < 0.001
Knockdown of STAT3 reversed the effects of EMPA on cardiomyocyte H/R injury under high glucose conditions
To determine whether STAT3 is involved in the cellular protective effect of EMPA on cardiomyocytes after H/R injury, H9C2 cardiomyocytes were transfected with an si-STAT3/control plasmid and/or treated with EMPA. CCK8 assays and flow cytometry, and TUNEL assays were used to detect cell viability and cell apoptosis, respectively. Figure 4A shows that the viability of H9C2 cells was significantly increased by EMPA, and restored by STAT3 interference. Flow cytometry and TUNEL assays demonstrated that EMPA decreased the numbers of apoptotic H9C2 cells, and STAT3 interference reversed EMPA-induced cell apoptosis inhibition (Fig. 4B–C). Western blot assays showed that STAT3 interference reversed EMPA-inhibited cell apoptosis and necrosis (Fig. 4D). In addition, STAT3 interference significantly reversed the decrease in MDA levels and increase in SOD activity induced by EMPA (Fig. 4E–F). As seen in Fig. 4G, EMPA inhibited endoplasmic reticulum stress, and increased the level of STAT3 pathway activation, while STAT3 interference reversed those changes. Finally, IF staining of p-STAT3 further confirmed that STAT3 pathway activation was increased by EMPA treatment and restored by STAT3 interference (Fig. 4H).
Knockdown of STAT3 reversed the effects of EMPA on cardiomyocyte H/R injuries under high glucose conditions. H9C2 cardiomyocytes were transfected with an si-STAT3/control plasmid and/or treated with EMPA. A–C Cell viability in each group was detected by the CCK-8 assay, cell apoptosis was detected by flow cytometry, and apoptotic DNA fragments were detected by the TUNEL assay. D Western blotting was performed to detect the levels of proteins associated with cell apoptosis and necrosis. E–F MDA levels and SOD activity were measured using commercial kits. G Western blotting was performed to detect the levels of endoplasmic reticulum stress-related proteins and STAT3 pathway-related proteins. H IF staining was performed to detect the intensity of p-STAT3 protein expression in each group of cells. *P < 0.05, **P < 0.01, ***P < 0.001, vs. Control group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. EMPA group
Discussion
The prevalence of diabetes is increasing at a rapid rate worldwide. It is estimated that by 2045, there will be approximately 700 million diabetic patients between the ages of 20 and 79 [33]. Cardiovascular disease is a leading cause of morbidity and mortality in T2DM patients [5]. High glucose induces cardiomyocyte injuries by promoting apoptosis, ROS production, and pro-inflammatory responses in cardiomyocytes [34]. In diabetic rats, ischemia–reperfusion induced myocardial injuries can be alleviated by suppressing cardiac cell oxidative stress and apoptosis [35]. Maternal diabetes enhances myocardial I/R injuries in adult offspring by increasing ROS production [36]. A previous study showed that EMPA ameliorates high glucose induced-cardiac dysfunction in human cardiomyocytes [37]. EMPA is widely used as a new drug for type 2 diabetes. In clinical trials, EMPA has displayed robust cardiovascular protective outcomes in type 2 diabetes mellitus patients [7]. In vivo, it has been reported that EMPA protects the heart against ischemia/reperfusion-induced sudden cardiac death or heart failure [28, 38]. Chronic administration of EMPA was shown to reduce myocardial infarct size by activating STAT3 in microvascular endothelial cells [39]. In myocardial I/R injury mice with diabetes mellitus, EMPA was found to preserve cardiac systolic function independent of blood glucose levels [40]. In this study, we established an H/R injury model to mimic myocardial I/R injury in vivo. High glucose (HG) treatment combined with H/R injury in H9C2 cells was used to mimic myocardial I/R that occurs in diabetes mellitus. Our experimental results showed that HG promoted H/R injury-induced cell apoptosis, oxidative stress, and endoplasmic reticulum stress, while EMPA had obvious protective effects.
Although robust evidence suggests that EMPA has cardiovascular protective effects in diabetes mellitus, it remains important to elucidate the mechanism underlying those cardiovascular protective effects. As for the specific mechanism by which EMPA protects cardiomyocytes from H/R injury under high glucose conditions, previous studies reported that in diabetic rats, hyperglycemia-induced myocardial oxidative stress was produced by activating the PI3K/AKT and JAK2/STAT3 signaling pathways [41]. In STZ-induced type I diabetes models, phosphorylation and activation of STAT3 at Tyr705 was found to be decreased [42, 43]. In H9C2 cells, high glucose conditions (25 mM) remarkably decreased the non-ischemic baseline levels of STAT3 phosphorylation (Tyr705 and/or Ser727) and activation [44, 45]. Our current study showed that HG inhibited activation of the JAK2/STAT3 signaling pathway, which is consistent with the aforementioned reports. Furthermore, EMPA markedly decreased cell apoptosis, oxidative stress, and endoplasmic reticulum stress under conditions of H/R injury by increasing the activation of STAT3. Knockdown of STAT3 reversed the cardioprotective effects of EMPA. It has been reported that mitochondrial function and cell survival are partially STAT3-dependent [46, 47]. Thus, STAT3 may affect mitochondrial energy metabolism by enhancing mitochondrial function and promoting cell survival after H/R injury.
Conclusion
In conclusion, our study revealed that high glucose and H/R injury inhibited the activation of STAT3, and EMPA protected against H/R-induced cardiomyocyte injury by activating JAK2/STAT3 signaling under high glucose conditions (Fig. 5). This study clarifies the mechanisms underlying the cardiovascular protective effects of EMPA in diabetes mellitus and provides new targets for treating ischemia–reperfusion injuries in diabetes mellitus.
Data availability
All data generated or analyzed in this study are available in the published article.
References
The L (2017) Diabetes: a dynamic disease. Lancet 389(10085):2163
Brody H (2012) Diabetes. Nature 485(7398):S1
Thomas R, Halim S, Gurudas S et al (2019) IDF Diabetes Atlas: a review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res Clin Pract 157:107840
Napoli R, Formoso G, Piro S et al (2020) Management of type 2 diabetes for prevention of cardiovascular disease. An expert opinion of the Italian Diabetes Society. Nutr Metab Cardiovasc Dis 30(11):1926–1936
Booth G, Kapral M, Fung K et al (2006) Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet (Lond, Engl) 368(9529):29–36
Newman J, Schwartzbard A, Weintraub H et al (2017) Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol 70(7):883–893
Wanner C, Lachin J, Inzucchi S et al (2018) Empagliflozin and clinical outcomes in patients with type 2 Diabetes Mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 137(2):119–129
Vaidya V, Gangan NandSheehan J (2015) Impact of cardiovascular complications among patients with Type 2 diabetes mellitus: a systematic review. Expert Rev Pharmacoecon Outcomes Res 15(3):487–497
Eltzschig HandEckle T (2011) Ischemia and reperfusion–from mechanism to translation. Nat Med 17(11):1391–1401
Yellon DandHausenloy D (2007) Myocardial reperfusion injury. N Engl J Med 357(11):1121–1135
Anaya-Prado R, Toledo-Pereyra L, Lentsch A et al (2002) Ischemia/reperfusion injury. J Surg Res 105(2):248–258
Zhao D, Yang JandYang L (2017) Insights for oxidative stress and mTOR signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev 2017:6437467
Li W, Li W, Leng Y et al (2020) Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol 39(2):210–225
Wang C, Zhu L, Yuan W et al (2020) Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J Cell Mol Med 24(12):6670–6679
Yang Z, Li C, Wang Y et al (2018) Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J Mol Cell Cardiol 125:185–194
Dong X, Liu H, Zhang M et al (2019) Postconditioning with inhaled hydrogen attenuates skin ischemia/reperfusion injury through the RIP-MLKL-PGAM5/Drp1 necrotic pathway. Am J Transl Res 11(1):499–508
Inzucchi S, Bergenstal R, Buse J et al (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38(1):140–149
La-Sala L, Pontiroli AE (2020) Prevention of diabetes and cardiovascular disease in obesity. Int J Mol Sci 21(21):8178
Wright E, Loo DandHirayama B (2011) Biology of human sodium glucose transporters. Physiol Rev 91(2):733–794
Gerich J (2010) Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabetic Med 27(2):136–142
Abdul-Ghani MandDeFronzo R (2008) Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 14(6):782–790
Wilding J (2014) The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 63(10):1228–1237
Frampton J (2018) Empagliflozin: a review in type 2 diabetes. Drugs 78(10):1037–1048
Tikkanen I, Narko K, Zeller C et al (2015) Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 38(3):420–428
Perrone-Filardi P, Avogaro A, Bonora E et al (2017) Mechanisms linking empagliflozin to cardiovascular and renal protection. Int J Cardiol 241:450–456
Goerg J, Sommerfeld M, Greiner B et al (2021) Low-dose empagliflozin improves systolic heart function after myocardial infarction in rats: regulation of MMP9, NHE1, and SERCA2a. Int J Mol Sci 22(11):5437
Lu Q, Liu J, Li X et al (2020) Empagliflozin attenuates ischemia and reperfusion injury through LKB1/AMPK signaling pathway. Mol Cell Endocrinol 501:110642
Hu Z, Ju F, Du L et al (2021) Empagliflozin protects the heart against ischemia/reperfusion-induced sudden cardiac death. Cardiovasc Diabetol 20(1):199
Kappel B, Lehrke M, Schütt K et al (2017) Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation 136(10):969–972
Levine M (2017) Empagliflozin for type 2 diabetes mellitus: an overview of phase 3 clinical trials. Curr Diabetes Rev 13(4):405–423
Woo V (2020) Cardiovascular effects of sodium-glucose cotransporter-2 inhibitors in adults with type 2 diabetes. Can J Diabetes 44(1):61–67
Amin E, Rifaai RandAbdel-Latif R (2020) Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundam Clin Pharmacol 34(5):548–558
Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9 edition. Diabetes Res Clin Pract 157:107843
Cheng GandLi L (2020) High-glucose-induced apoptosis, ROS production and pro-inflammatory response in cardiomyocytes is attenuated by metformin treatment via PP2A activation. J Biosci 45:1–11
Kosuru R, Cai Y, Kandula V et al (2018) AMPK contributes to cardioprotective effects of pterostilbene against myocardial ischemia- reperfusion injury in diabetic rats by suppressing cardiac oxidative stress and apoptosis. Cell Physiol Biochem 46(4):1381–1397
Zhang L, Wang X, Wu Y et al (2018) Maternal diabetes up-regulates NOX2 and enhances myocardial ischaemia/reperfusion injury in adult offspring. J Cell Mol Med 22(4):2200–2209
Ng K, Lau Y, Dhandhania V et al (2018) Empagliflozin ammeliorates high glucose induced-cardiac dysfuntion in human iPSC-derived cardiomyocytes. Sci Rep 8(1):14872
Seefeldt J, Lassen T, Hjortbak M et al (2021) Cardioprotective effects of empagliflozin after ischemia and reperfusion in rats. Sci Rep 11(1):9544
Nikolaou P, Efentakis P, Abu Qourah F et al (2021) Chronic empagliflozin treatment reduces myocardial infarct size in nondiabetic mice through STAT-3-mediated protection on microvascular endothelial cells and reduction of oxidative stress. Antioxid Redox Signal 34(7):551–571
Ideishi A, Suematsu Y, Tashiro K et al (2021) Combination of Linagliptin and Empagliflozin preserves cardiac systolic function in an ischemia-reperfusion injury mice with diabetes mellitus. Cardiol Res 12(2):91–97
Lei S, Su W, Xia Z et al (2019) Hyperglycemia-induced oxidative stress abrogates remifentanil preconditioning-mediated cardioprotection in diabetic rats by impairing caveolin-3-modulated PI3K/Akt and JAK2/STAT3 signaling. Oxid Med Cell Longev 2019:9836302
Wang C, Li H, Wang S et al (2018) Repeated non-invasive limb ischemic preconditioning confers cardioprotection through PKC-ε/STAT3 signaling in diabetic rats. Cell Physiol Biochem 45(5):2107–2121
Xu J, Lei S, Liu Y et al (2013) Antioxidant N-acetylcysteine attenuates the reduction of Brg1 protein expression in the myocardium of type 1 diabetic rats. J Diabetes Res 2013:716219
Wang Y, Li H, Huang H et al (2016) Cardioprotection from emulsified isoflurane postconditioning is lost in rats with streptozotocin-induced diabetes due to the impairment of Brg1/Nrf2/STAT3 signalling. Clin Sci (Lond, Engl: 1979) 130(10):801–812
Deng F, Wang S, Zhang L et al (2017) Propofol through upregulating caveolin-3 attenuates post-hypoxic mitochondrial damage and cell death in H9C2 cardiomyocytes during hyperglycemia. Cell Physiol Biochem 44(1):279–292
Visavadiya N, Keasey M, Razskazovskiy V et al (2016) Integrin-FAK signaling rapidly and potently promotes mitochondrial function through STAT3. Cell Commun Signal 14(1):32
Zhang W, Jin Y, Wang D et al (2020) Neuroprotective effects of leptin on cerebral ischemia through JAK2/STAT3/PGC-1-mediated mitochondrial function modulation. Brain Res Bull 156:118–130
Acknowledgements
This research is supported by Scientific Research Fund of the Third Affiliated Hospital of Southern University of Science and Technology (No. 2020-D1).
Author information
Authors and Affiliations
Contributions
FZ and XC designed the research. FZ and XC collected the data. CZ and LC analyzed the data. FZ and XC wrote or revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, F., Cao, X., Zhao, C. et al. Empagliflozin activates JAK2/STAT3 signaling and protects cardiomyocytes from hypoxia/reoxygenation injury under high glucose conditions. J Thromb Thrombolysis 55, 116–125 (2023). https://doi.org/10.1007/s11239-022-02719-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-022-02719-0