Abstract
Objectives: To determine the association between the combustion of wood, animal dung, coal and paraffin (polluting fuels) for cooking and heating and 1–59 month old mortality in South Africa, whilst adjusting for a number of confounders. Methods: Data from 3,556 children (142 deaths) living in 2,828 households were extracted from the 1998 South African Demographic and Health Survey (SADHS) database. The SADHS was the first national health survey conducted across the entire country and provided the opportunity to examine the prevalence and determinants of various morbidity and mortality outcomes in a representative national population. Results: The results suggest that exposure to cooking and heating smoke from polluting fuels is significantly associated with 1–59 month mortality, after controlling for mother's age at birth, water source, asset index and household crowdedness (RR=1.95; 95% CI=1.04, 3.68). Conclusions: Although there is potential for residual confounding despite adjustment, the better documented evidence on outdoor air pollution and mortality suggest this association may be real. As nearly half of households in South Africa still rely on polluting fuels and women of childbearing age perform most cooking tasks, the attributable risk arising from this association, if confirmed, could be substantial. It is trusted that more detailed analytical intervention studies will scrutinise these results in order to develop integrated intervention programmes to reduce children's exposure to air pollution emanating from cooking and heating fuels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Notwithstanding the technical advances in developing countries that have improved the survival rate of children (children between 12 and 59 months of age, infants <12 months) mortality rates are still at least 10 times higher in developing countries than in developed countries [1]. Infant deaths mainly happen in the perinatal (<8 days) and neonatal periods (<28 days), mainly as a result of maternal characteristics, delivery factors, prematurity, intrauterine growth retardation, and congenital causes. Later deaths are more likely to be the result of infection and environmental factors [2].
Over 40% of the global burden of disease attributed to environmental factors falls on children (<5 years), who account for about 10% of the world's population [3]. Furthermore, air pollution is the largest single environment-related cause of ill health among children in most countries [4]. In other countries, it is the second, after the scarcity of safe water. Globally, 2.6% of all ill health is attributable to indoor smoke from polluting fuels that are mostly used in poor regions [5].
According to a recent report published by the World Health Organisation (WHO) substantial evidence currently exists concerning the adverse effects of air pollution–especially outdoor PM10—on different pregnancy outcomes and infant health [6]. The evidence is sufficient to infer a causal relationship between particulate air pollution and respiratory deaths in the postneonatal period (28 days to 12 months).
As exposure-response relationships and risk estimates for postneonates have been derived for outdoor air pollution in developed country urban settings, it raises a number of issues about their suitability for application to indoor air pollution exposure due to the combustion of wood, animal dung, coal, crop residues or paraffin (hereafter “polluting fuels”) for cooking and heating in developing countries. The applicability of exposure-response relationships and risk estimates to children in the age bracket 12 to 59 months is also uncertain. Although outdoor sources often dominate air pollution emissions, indoor sources frequently dominate air pollution exposures [7]. Exposure is a function of both the pollutant concentration in an environment and the person-time spent in that environment. Since most people spend the majority of their time in homes, schools and workplaces, human exposure to air pollution is largely a function of pollutant levels in indoor settings (which can arise from outdoor sources, and vice versa).
Of 11 million South African households, 41% and 48% still used polluting fuels for cooking and heating, respectively during 2001, even when access to electricity was available [8]. The majority (70%) of the households used electricity for lighting. Polluting fuels are at the low end of the energy ladder in terms of combustion efficiency and cleanliness [9] and burning these fuels produces a large number of health-damaging air pollutants, including: PM10 (particles with aerodynamic diameters below 10 μm), PM2.5 (particles with aerodynamic diameters below 2.5 μm), sulphur dioxide (SO2) (mainly from coal), carbon monoxide (CO), nitrogen oxides (NO2 and NO), formaldehyde, benzene, polycyclic aromatic hydrocarbons (such as the carcinogen benzo[a]pyrene) and many other toxic organic compounds. The fuels are typically burned in simple, inefficient and mostly unvented household cooking stoves in small, confined, and poorly ventilated indoor areas. Cooking stoves are typically used for several hours each day at times when people are present indoors, resulting in much higher exposure to air pollutants than that from outdoor sources [10].
In such settings, daily average and peak exposures to air pollutants often far exceed air quality safety levels [11]. Bailie et al. [12] conducted an indoor exposure assessment study during winter in a poor urban environment in South Africa, where a range of fuel types was used, including paraffin. The mean maximum hourly average was 28 μg·m−3 (range 0–451 μg·m−3) for NO2, 1414 μg·m−3 (range 0–17,723 μg·m−3) for SO2, and 34 mg·m−3 (range 0–388 mg·m−3) for CO. The number of households for which NO2, CO and SO2 standards were exceeded by the maximum hourly averages was six (9%), 20 (30%) and 28 (42%) respectively. The hourly WHO standards are 200 μg·m−3 and 30 mg·m−3, respectively, for NO2 and CO [13]. The hourly California standard is 655 μg·m−3 for SO2 [14]. Hourly WHO or U.S. EPA standards do not exist for SO2 [13, 14]. Concentrations of total suspended particulates (TSP) ranged from 7–433 μg·m−3. The investigators did not compare TSP levels with international standards because TSP measurements now focus on PM10 and PM2.5 particles. To date, hourly standards or guidelines do not exist for PM10 or PM2.5.
Indoor air pollution exposure is generally much higher among women, who tend to do most of the cooking, and among young children who stay indoors and who are often carried on their mother's back or lap while she is cooking. A local study by Thomas et al. [15] reported that, on average, 14% of households had children (<6 years) who were usually or always present when their mothers were cooking with polluting fuels. This percentage increased to 18% of households in the lowest wealth quintile. In lower-wealth quintile homes, paraffin stoves burned on average four hours per day, with the evening meal taking over half of this time to prepare. Muller et al. [16] established in another local study that individuals spend on average two hours cooking indoors during both winter and summer.
Studies indicate that exposure to indoor air pollution more than doubles the risk of pneumonia [17, 18]. Globally, pneumonia and other acute lower respiratory infections are responsible for about 33% of all deaths among children under five years of age in developing countries [17, 19]. Children under three years of age, and especially children in their first year, are at greatest risk of serious respiratory illnesses [20].
The pathogenic mechanisms by which air pollution can increase the risk of adverse health outcomes—which may ultimately lead to death—are not fully understood. However, it is known that PM10, PM2.5, polycyclic aromatic hydrocarbons, volatile organic compounds, NO2 and SO2 cause inflammation of the airways and lungs and impair the immune response [13, 17, 21]. Exposure to CO may also result in systemic effects by reducing the oxygen-carrying capacity of the blood [13, 17, 21].
It has been suggested that infants and young children are more susceptible to environmental toxicants. The inhaled pollutant dose per unit body weight is likely to be greater, because their body weight is smaller and their respiratory rate higher than adults [22, 23]. Infants’ developing lungs and immune system put them at greater risk of respiratory infections and may make them particularly vulnerable to the immune effects of inhaled pollutants, especially in the first year of life while lung maturation is still ongoing [20, 24, 25].
Over the past 20 years, the hazards of indoor air pollution have been documented by a growing body of literature [17], but very few studies have focused on their impact on infant and child mortality. The few studies conducted in developing countries have tended to investigate the association between infant and child mortality and outdoor air pollution [26–29].
This study will therefore investigate whether the use of cooking and heating fuel significantly increases the risk of dying during the first 1–59 months of life, and will also consider a number of possible confounders or effect modifiers. The findings from the study will contribute to the current body of knowledge on this topic. The 1998 South African Demographic and Health Survey (SADHS) was the first national health survey conducted across the entire country [30]. Data from this survey provided the opportunity to examine the prevalence and determinants of various morbidity and mortality outcomes in a representative national population rather than a selected high risk population, as has been the case in most previous studies in developed countries.
Methods
Study population and setting
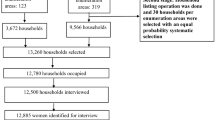
The 1998 SADHS had a cross-sectional design and was a national household survey of the population living in private households in the country. Detailed information on the survey design is outlined elsewhere [30]. The sampling frame for the SADHS was the list of approximately 86,000 enumeration areas (EAs) created by Central Statistics (now Statistics South Africa, SSA) for the Census conducted in October 1996. The EAs ranged from about 100–250 households and were stratified by nine provinces, urban and non-urban residence, and EA type.
The first stage (proportional stratified sampling) of the two-stage sampling led to a total of 972 EAs being selected for the SADHS (690 in urban areas and 282 in non-urban areas). The second stage involved a systematic random sample of 10 and 20 houses in selected urban and rural EAs, respectively.
In addition to the main survey of households a women's health questionnaire was administered individually to women (15–49 years) in all the households selected for the main survey. The SADHS questionnaires were translated into nine of the eleven official languages of South Africa and checked by back translation [30]. The questionnaires were pretested in November/December 1996 as part of a pilot study.
Interviewers were trained over several weeks. Interviews were conducted after working hours and interviewers were instructed to return twice if a suitable respondent was not found at home. Fieldwork commenced in late January 1998 and was completed in September 1998. The response rate at the household level was 97% of 12,860 households in 966 EAs. For the women's health survey, the overall response rate was 92%.
Ethical approval was granted by the Ethics Committee of the South African Medical Research Council to conduct the 1998 SADHS. The survey obtained informed consent from each respondent (in this case, mothers of the children included in the women's health questionnaire) before questions were asked. The analysis presented in this paper is based on secondary analysis of the existing survey data with all identifying information removed.
Outcome and potential risk factor definitions
The outcome variable was ascertained by asking a woman whether she had given birth to any children and whether any of them were still alive and, if not, at what age the child or children had passed away. The main independent variable—exposure to cooking and space heating smoke—was ascertained indirectly by type of fuels used. The question was: ‘What does your household use for cooking and heating?’ Respondents indicated all the different fuel types that were used. The households were grouped into two categories representing the extent of exposure to cooking and heating smoke: polluting fuels (if either wood, dung, coal or paraffin was used in the fuel combination without using liquid petroleum gas (LPG)/natural gas or electricity) and clean fuels (if LPG/natural gas or electricity was used exclusively as the fuel type).
Confounding variables related to the household included: access to flush toilet facilities (coded 0 if own/shared; coded 1 if bucket/pit larine or no facility/bush/field); access to clean water (coded 0 if using piped water in dwelling/site/yard, tap water/water carrier/tanker, borehole/well or bottled water; coded 1 if water from dam/river/stream/spring, rain water tank was used); number of people per room (coded 0 if ≤2; coded 1 if >2); nutritional status (coded 0 if household was going hungry never/seldom; coded 1 if household was going hungry often/sometimes) and asset index (AI) as an indicator of socio-economic status. The AI score was calculated by using a modified approach of Mishra [31]. The following scores were summed: 3 for a car or tractor; 2 each for a scooter/motorcycle, TV, telephone, refrigerator, electricity, wood/vinyl/asphalt/ceramic/cement/carpet of main floor material; 1 each for a bicycle or radio. The AI score was then divided into two categories (<35 and ≥35). Confounding variables pertaining to a child included: age (coded 0 if 1–11 months old; coded 1 if 12–59 months old); sex (coded 0 if male; coded 1 if female); birth order (coded 0 if 1; coded 1 if >1); preceding birth interval (coded 0 if <24 months; coded 1 if ≥24 months); and whether the child was breastfed (coded 0 if yes; coded 1 if no). The only confounding variable related to the biological mother of a child was age at birth (coded 0 if <19 years; coded 1 if 19–24 years; coded 2 if 25–34 years; coded 3 if ≥35 years). As maternal education status was only ascertained at the time of the survey and not at the birth of the child, it was not included in the analysis. The child's year of birth was included to capture a time trend in child mortality (continuous variable).
Data analysis
All subsequent statistical analyses of results were done with the use of STATA version 8. The household (n=12,209) and women's questionnaire (n=5,066) data were linked with a unique identification variable, containing the cluster number and household number. The merge data set had 5,060 observations. This analysis included only children whose mothers had indicated their ethnic identity as African/Black. This ethnic group comprised 82% (n=4,114) of the data with 237 deaths, compared to 25 deaths from the other three ethnic groups. Under apartheid, South Africans were categorised into one of four socially defined groups: White (mainly European ancestry), Asian (Indian sub-continent ancestry), African or Black (descent primarily from one of a number of Bantu language groups in Southern Africa) and Coloured (general grouping, including a mixture of Black, Malay, European and indigenous Khoisan ancestry). It is anticipated that race will be linked to socio-economic status and educational status for many years.
The following exclusions were made from the 4,114 observations: residual category of other fuels due to unknown nature of fuels in that category (n=6; 0.1%), all children from multiple births (n=113; 2.8%), all children who did not live with their mothers (n=331; 8.5%), women who only visited the household during the survey (n=77; 1.9%) and deaths before 1 month (n=27; 0.7%). The cause of death for newborns is difficult to determine and may be due to quite different risk factors. Eventually 3,556 children, of whom 142 were deaths, were included in the analysis.
Simple descriptive statistics were used to describe the characteristics of the sample and to calculate the mortality incidence rate for each characteristic. The 1998 SADHS report pointed out that the risk factors might be associated with each other [30]. Association among risk factors was investigated with χ 2 tests. It was observed that most of the risk factors were significantly associated at the 95% confidence level, although very poorly with associations varying from 0.01 to 0.40.
To estimate the effects of the independent variables on 1–59 month old mortality, Cox proportional hazards analysis was performed, stratified by urban or rural. The time variable was either set as the child's current age (months since birth until interview date) or age of death (in months), respectively, for those who were still alive and those who were deceased at the interview date. Time was fitted to estimate the hazard rate ratio (hereafter denoted “relative risk”) of mortality. The results were presented as the relative risk of mortality with 95% confidence intervals. The proportional hazards assumption was confirmed for each independent variable. First univariate analyses were run and subsequently a multivariate analysis with all the significant independent variables identified from the former procedure. No interactions between the potential risk factors under investigation were explored in the data analyses.
Results
The data presented here represent a more detailed analysis of the first national survey of under-five mortality in South Africa. Table 1 lists the characteristics of the 3,556 children from 2,828 households. The overall mortality incidence rate in the five years preceding the study was 1.473/1000 person-months. Two-thirds of children lived in rural households and 75% of deaths occurred in rural areas.
Children aged 12–59 months were somewhat less represented than the other age group. Children were relatively equally distributed by sex. A minority of the children were first borns (32%). Most of the children were born to mothers aged 19–24 and 25–34 years and spaced within 24 months of their siblings. The majority of the children were breastfed (89%) at some stage (Table 1).
Most of the children lived in rural areas (65%). The majority of the children lived in households that used polluting fuels for cooking and heating, either exclusively or in combination with clean fuels (79%) or in households that did not have access to flush toilets (75%) or clean water (70%). More than half of the children lived in households with a low asset index (55%). Most of the children lived in crowded households (>2 people/room) (71%) with poor nutritional status (hungry often/sometimes) (62%). Children's births were equally distributed from 1993–1997, with the lowest number during 1998 (Table 1).
In the univariate analysis only mother's age at birth, fuel use, access to clean water, asset index and people/room were significantly associated with an increased risk in 1–59 month old mortality (Table 1). In the adjusted model, all of these potential risk factors, except asset index, were still significantly associated with an increased risk in mortality (Table 2). The impact of mother's age at birth on under-five mortality was not affected much in the adjusted model. Children born to mothers aged 19–24 and 25–34 years were significantly less at risk from dying than those born to mothers younger than 19 years, whilst older mothers (≥35 years) had no significant health benefit. Compared to households using clean fuels, children in households using polluting fuels had a significant higher risk of dying: from 2.22 (95% CI=1.22, 4.04; p=0.009) to 1.95 (95% CI=1.04, 3.68; p=0.039) in the univariate and adjusted analyses. Indeed, the negative effect of polluting fuels in the univariate model exceeded that of lack of clean water supplies. However, their effects were similar in the adjusted model. Living in households without clean water supplies increased the risk of dying almost twofold compared to the reference group (adjusted model: RR=1.96; 95% CI=1.32, 2.90; p=0.001). Surprisingly, children living in crowded households (>2 persons/room) had a significantly lower risk of dying (37%) than those who were living in less crowded households. The influence of a crowded household decreased somewhat (11%) from the univariate to the adjusted analyses.
Discussion
Results from this study suggest that exposure to cooking and heating smoke from polluting fuels is significantly associated with 1–59 month mortality, after controlling for mother's age at birth, water source, asset index and household crowdedness. It is intriguing that household crowdedness decreases the risk of mortality in the multivariate model. Household crowdedness has been associated with higher acute respiratory infection and TB rates, which may lead to more deaths [32, 33]. A possible explanation might be that less fuel is burnt for space heating when more people are sharing a dwelling.
There are some important limitations to this study, which should be taken into account when the results are interpreted. The SADHS had a cross-sectional design. Cross-sectional studies are weak at proving causation as they are subject to difficulties in interpreting the temporal sequence of events since mortality and determinants are measured simultaneously. However, the better documented evidence on outdoor air pollution and mortality suggest this association may be real. The proportional hazards assumption was confirmed for each independent variable. However, this test cannot indicate slight changes in exposure variables over the five-year period. A large proportion (43%) of women lived in their current house when their child was born, so we assumed that the toilet facility and water source variables remained constant over time. Maternal educational status was not considered in the analysis as it was reported at the time of the survey and not at the time of death. It was assumed that maternal educational status will not be constant during a child's life.
Some biases within the study may influence the generalisability of the results. There is probable recall bias among respondents answering questions involving events occurring in the past, such as those about the household going hungry and breastfeeding patterns immediately after birth. Reporting bias may be present, since the possibility exists for respondents to answer in such a fashion that may be more socially desirable or in ways that they perceive would elicit a more approving response from the interviewers. There is also the potential for information bias.
Other factors that might be detrimental to children's health, such as mother's smoking status, mother's size and pre-pregnancy weight, child's birth weight, mother's exposure to other pollution sources and risk factors during pregnancy, outdoor air pollution, insecticide and fertiliser use, along with meteorological variables, were not recorded. Excluding these risk factors from the analysis might introduce substantial bias (differential or nondifferential). For these reasons the direction of bias on the calculated association measures is not easy to predict.
Excluding maternal smoking status from the analysis might introduce substantial bias as 24% of women in South Africa smoke [30]. However, the percentage of pregnant women who smoke is not known. Information on adult household members who were smokers was recorded in the adult health questionnaire from 50% of the 12,860 households surveyed during the 1998 SADHS [30]. Linking the adult health dataset—using a unique household identification code—to the dataset under investigation reduced the number of deaths to nine and the number of children to 321. Therefore, it was not possible to include the influence of environmental tobacco smoke (ETS) on 1–59 month mortality in the analyses. The degree of exposure to ETS depends on the number of smokers and the amount of tobacco smoked, as well as size and ventilation characteristics of the indoor space and the duration of exposure. ETS is a complex mixture of thousands of compounds of which some are similar to those emitted during polluting fuel combustion [34]. Although ETS and unvented gas cooking are the main indoor air pollution sources in developed countries, their emissions may be minute compared to the emissions from a mixture of 2–4 kg of polluting fuel used for cooking and heating on a daily basis in developing countries [35]. For this reason, indoor air pollution measurements in several developing countries have shown fuel type to be the best single indirect indicator of household air pollution levels [31, 36]. ETS may thus add to the air pollution mix from combustion of polluting fuels. Excluding ETS may underestimate the impact of this air pollution mixture on 1–59 month mortality.
Although most of the children were born in clinics or hospitals, their birth weight could not be included in the statistical analyses because of the large number of missing birth weight observations. Prior to excluding certain observations as stipulated in the data analysis section, the original dataset for Black children under-five had 1,520 missing birth weights values (38%). About 12% of those Black children who had birth weight recordings were born underweight (<2,500 g). The influence of premature births could not be included in the statistical analyses as this was not ascertained by the women's health questionnaire.
The current HIV/AIDS epidemic along with escalating number of TB infections could also influence the association between 1–59 month mortality and exposure to smoke from polluting fuels. The 1998 SADHS survey did not collect indicators of potential HIV/AIDS status. The TB prevalence rate was low (2.4% among 13,826 adults) [30]. Adult household members diagnosed with TB and who lived in households with smokers (34% among 13,826 adults), were not linked with the dataset as this considerably reduced the number of deaths to be analysed [30]. Furthermore, although only 28% of children were vaccinated, no deaths were reported for unvaccinated children. Most women had antenatal care check ups (97%).
Although breastfeeding was included in the multivariate regression analyses, the length of breastfeeding was not. Including length of breastfeeding may have introduced recall bias. It is known that breastfeeding protects against respiratory infections, particularly in the first year of life [20, 37]. Breastfeeding may also modify detrimental impacts of ETS exposure on respiratory illnesses [38–40].
The definition of a confounder is important to remember: it must be associated with both the exposure variable of interest and the health effect. As the association between the excluded aforementioned potential risk factors and polluting fuel use is not available from the literature, it is impossible to predict the direction of the potential bias on the association measure.
During the analysis it was assumed that confounding is additive and not multiplicative. If confounding is additive, then the confounding variable would produce the same additional risk of a health outcome in the exposed and unexposed; but if the health outcome is rare in the unexposed, it would follow that the confounder might account for a much larger proportion of health outcomes in that group. Conversely, if two exposures act multiplicatively, the proportional increase in health outcome rates due to confounding would be the same in exposed and unexposed; but if the health outcome is more prevalent in the exposed group, the absolute increase would be larger in the exposed. This issue thus has important implications for risk assessment and public health policy.
Evidence had shown that parents perceive the inherent healthiness of a child through knowledge of their own genetic endowments and the health characteristics of the household and neighbourhood [41–43]. If parents perceive that their child's inherent healthiness is low, they may alter their fertility decisions, leading to selection bias in the sample of potential births. However, we could not only consider first births in the analysis as this reduced the sample size considerably. Also, the number of children per age group decreased with increasing age and this is likely to be to the result of two contributing factors. First, close to 7% of the children in South Africa die by the time they reach five years of age and this high mortality rate may result in fewer older children in the sample [44]. Children from poor households are more likely to be sent away to live with relatives in urban areas as they get older. It was found that 331 children did not live with their mothers (8.1%; n=4,114).
The most common indicators used for measurement of socio-economic status are income level, occupation and educational level [45]. We cannot rule out that the results would have been different with the use of a measure other than an asset index. More than a third (35%) of the women never married, so we could not assume that the current partner's job or education remained the same over the five-year period.
It was not possible to indicate the categories of death recorded on the death certificates for the 142 children who died. Questionnaires from Demographic and Household Surveys typically do not include identifying variables, such as names.
It is hoped that future analytic studies will validate and improve the understanding of how pollution emanating from polluting fuel combustion detrimentally impact on 1–59 month mortality rates. In South Africa, detailed analytic epidemiology studies will have to compete with the demands on the public and research purse for work on common diseases of pressing current importance (e.g. HIV/AIDS). Furthermore, only 5% of the research budget is spent on health-related research in South Africa, compared to 30% of the research budget of developed countries [46]. It is important that analytical studies not merely redocument the impact of known risk factors. Instead, such studies—as did the very first ever published randomised control intervention study by Smith-Sivertsen et al. [7]—should provide a basis for designing technical or socio-behavioural interventions to minimise exposure to air pollution from cooking and heating fuels. Smith-Sivertsen et al. [7]—conducted a randomised study in a poor rural community in Guatemala. Traditional open fire stoves were replaced with new chimney stoves for the intervention group, who burned the same wood fuels as the exposed group. Participating households (n=534) all started the project with a child less than four months old or a pregnant woman. These households were followed until the child was 18 months old. At the end of follow-up, the exposed group received new chimney stoves to replace traditional open fire stoves. Health outcomes investigated were the incidence of acute lower respiratory infection in infants, along with respiratory and cardiovascular health in women. Preliminary results indicated that there was a 30% reduction in CO breath in the intervention group. The difference in CO breath between intervention homes and exposed homes was highly significant throughout the study period [47]. Symptoms rates were smaller for young women in the intervention group, most notably for phlegm and wheeze.
References
Katz J, West KP, Jr., Khatry SK, Christian P, LeClerq SC, Pradhan EK, Shrestha SR. Risk factors for early infant mortality in Sarlahi district, Nepal. Bull World Health Organ. 2003;81(10):717–25.
Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(Suppl 2):519–617.
Report of the International Conference on Environmental Threats to the Health of Children: Hazards and Vulnerability, Bangkok, Thailand, 3-7 March 2002. Geneva: World Health Organisation. Available at: http://whqlibdoc.who.int/hq/2002/ WHO_SDE_PHE_02.02.pdf. Accessed 20 March 2006.
Tamburlini G, von Ehrenstein OS, Bertollini R (eds). Children's environmental health: review of the evidence. European Environment Agency and WHO, Regional Office for Europe, Copenhagen, 2002. Available at: http://www.who.int/ceh/publications/evidence/en/. Accessed 20 March 2006.
Desai MA, Mehta S, Smith KR. Indoor smoke from solid fuels: Assessing the environmental burden of disease. Environmental burden of disease series No. 4. World Health Organisation. 2004. Available at: http://www.who.int/quantifying_ehimpacts/publications /en/Indoorsmoke.pdf. Accessed 20 March 2006.
The effects of air pollution on children's health and development: a review of the evidence. WHO Regional Office for Europe. WHO Monograph. 2005 Available at: http://www.euro.who. int/document/E83080.pdf. Accessed 20 March 2006.
Smith-Sivertsen T, Díaz E, Bruce N, Díaz A, Khalakdina A, Schei MA, McCracken J, Arana B, Klein R, Thompson L, Smith KR. Reducing indoor air pollution with a randomised intervention design-A presentation of the Stove Intervention Study in the Guatemalan Highlands. Norsk Epidemiologi. 2004;14(2):137–3.
Statistics South Africa. Census 2001. Census in Brief. Pretoria: Statistics South Africa. 2003 Available at: http://www.statssa. gov.za. Accessed 20 March 2006.
Smith KR, Liu Y. Indoor air pollution in developing countries. In: Samet JM, editor. Epidemiology of Lung Cancer, Lung Biology in Health and Disease Series 1994;74:151–84.
Smith KR. Indoor air pollution in developing countries: recommendations for research. Indoor Air. 2002;12:1–7.
World Health Organisation. Health and Environment for Sustainable Development: Five Years After the Earth Summit. Geneva: WHO, 1997.
Bailie RS, Pilotto LS, Ehrlich RI, Mbuli S, Truter R, Terblanche P. Poor urban environments: use of paraffin and other fuels as sources of indoor air pollution. J Epidemiol Community Health. 1999;53:585–6.
World Health Organisation. Air Quality Guidelines for Europe. 2nd Edition. WHO Regional Publications, European Series, No. 91. Regional Office for Europe, Copenhagen, Denmark: WHO, 2000. Available at: http://www.euro.who.int/air/activities/20050223_3. Accessed 20 March 2006.
United States Environmental Protection Agency, Criteria Air Pollutants. 2004. Available at: http://www.epa.gov/air/criteria.html. Accessed 20 March 2006.
Thomas EP, Thomas JR, Viljoen E, Potgieter F, Rossouw A, Tokota B, et al. Household Environment and Health in Port Elizabeth, South Africa. Stockholm Environment Institute and SA Medical Research Council ISBN: 91 88714 65 9 1999. Available at: http://www.mrc.ac.za/healthdevelop/householdpart1.pdf and http://www.mrc.ac.za/healthdevelop/householdpart2.pdf. Accessed 20 March 2006.
Muller E, Diab RD, Binedell M, Hounsome R. Health risk assessment of kerosene usage in an informal settlement in Durban, South Africa. Atmos Environ. 2003;37:2015–22.
Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ. 2000;78:1078–92.
Von Schirnding YE. Addressing the Links between Indoor Air Pollution, Household Energy and Human Health. WHO/HDE/HID/02.10, Washington, DC, USA, 2000. Available at: http://www.who.int/mediacentre/events/HSD_Plaq_10.pdf. Accessed 20 March 2006.
The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: World Health Organisation, 2002. Available at: http://www.who.int/whr/2002/en/index.html. Accessed 20 March 2006.
Phelan PD, Olinsky A, Robertson CF. Respiratory Illness in Children. Oxford: Blackwell Scientific Publications, 1994.
Patterson J, Hakkinen PJ, Wullenweber AE. Human health risk assessment: selected Internet and world wide web resources. Toxicology. 2002;173:123–43.
Cerna M, Jelinek R, Janoutova J, Kotesovec F, Benes I, Leixner M. Risk assessment of the common air pollutants in Teplice, Czech Republic. Toxicol Lett. 1998;96–97:203–8.
Gilliland FD, McConnell R, Peters J, Gong H Jr. A theoretical basis for investigating ambient air pollution and children's respiratory health. Environ Health Perspect 1999;107(Suppl 3):403–7.
Koenig JQ. Health Effects of Ambient Air Pollution: How Safe is the Air We Breathe? Boston: Kluwer Academic Publishers; 2000.
Braga AL, Saldiva PH, Pereira LA, Menezes JJ, Conceicao GM, Lin CA, et al. Health effects of air pollution exposure on children and adolescents in Sao Paulo, Brazil. Pediatr Pulmonol. 2001;31(2):106–13.
Conceicao GM, et al. Air pollution and child mortality: a time-series study in Sao Paulo, Brazil. Environ Health Perspect. 2001;109(Suppl 3):347–50.
Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH. Air pollution and infant mortality in Mexico City. Epidemiology. 1999;10(2):118–23.
Penna ML, Duchiade MP. Air pollution and infant mortality from pneumonia in the Rio de Janeiro metropolitan area. Bull Pan Am Health Organ 1991;25(1):47–54.
Saldiva PH, Lichtenfels AJ, Paiva PS, Barone IA, Martins MA, Massad E, et al. Association between air pollution and mortality due to respiratory diseases in children in Sao Paulo, Brazil: a preliminary report. Environ Res. 1994;65(2):218–25.
South Africa Demographic and Health Survey (SADHS). Sample Design. 1998, Available at: http://www.doh.gov.za/facts /1998/sadhs98/. Accessed 20 March 2006.
Mishra V. Indoor air pollution from biomass combustion and acute respiratory illness in preschool age children in Zimbabwe. Int J Epidemiol. 2003;32:847–53.
Weber MW, Milligan P, Hilton S, Lahai G, Whittle H, Mulholland EK, Greenwood BM. Risk factors for severe respiratory syncytial virus infection leading to hospital admission in children in the Western Region of The Gambia. Int J Epidemiol. 1999;28(1):157–62.
Drucker E, Alcabes P, Bosworth W, Sckell B. Childhood tuberculosis in the Bronx, New York. Lancet. 1994;343(8911):1482–85.
Morawska L, Zhang J. Combustion sources of particles. 1. Health relevance and source signatures. Chemosphere.;49(2002):1045–1058.
Brouwer R, Falcão MP. Wood fuel consumption in Maputo, Mozambique. Biomass and Bioenergy. 2004;27:233–45.
Mehta S, Smith KR, Balakrishnan K, et al. Using household characteristics to predict respirable particulate levels in rural households in Andhra Pradesh, India. Paper presented at the 9th International Conference on Indoor Air Quality and Climate, California: Monterey; 30 June–5 July 2002.
Heinig MJ. Host defense benefits of breastfeeding for the infant. Effect of breastfeeding duration and exclusivity. Pediatr Clin North Am. 2001;48(1):105–23.
Chulada PC, Arbes SJ, Jr., Dunson D, Zeldin DC. Breast-feeding and the prevalence of asthma and wheeze in children: analyses from the Third National Health and Nutrition Examination Survey, 1988–1994. J Allergy Clin Immunol. 2003;111(2):328–36.
Nafstad P, Jaakkola JJ. Breast-feeding, passive smoking, and asthma and wheeze in children. J Allergy Clin Immunol. 2003;112(4):807–808; author reply 808–809.
Woodward A, Douglas RM, Graham NM, Miles H. Acute respiratory illness in Adelaide children: breast feeding modifies the effect of passive smoking. J Epidemiolb Community Health. 1990;44(3):224–30.
Panis CWA, Lillard LA. Health inputs and child mortality: Malaysia. J Health Econ. 1994;13:455–89.
Olsen R, Wolpin KI. The Impact of Exogenous Child Mortality on Fertility: A Waiting Time Regression with Dynamic Regressors. Econometrica. 1983;51(3):731–49.
Rosenzweig MR, Schultz TP. Estimating a Household Production Function: Heterogeneity, the Demand for Health Inputs, and Their Effects on Birthweight. J Pol Econ. 1983;91(5):723–46.
Childhood under threat: The State of the World's Children 2005. UNICEF Report, Available at: http://www.unicef.org/ infobycountry/southafrica_statistics.html. Accessed 20 March 2006.
Liberatos P, Link BG, Kelsey JL. The measurement of social class in epidemiology. Epidemiol Rev. 1988;10:87–121.
Essential National Health Research in South Africa. The Council on Health Research for Development May 2001. Available at: http://www.doh.gov.za/docs/reports/2001/enhr/. Accessed 20 March 2006.
Smith-Sivertsen T, Bruce N, Pope D, Lie RT, Diaz A, McCracken J, Arana B, Jenny A, Khalakdina A, Diaz E, Klein RK, Smith KR. Indoor air pollution from biomass fuel use and lung health in young Guatemalan women: preliminary analysis of a randomised controlled trial. Epidemiology. 2005;16(5):S104-05.
Acknowledgments
The authors would like to thank the South African National Department of Health for supplying the 1998 SADHS data freely. The corresponding author received PhD scholarships from the South African National Research Foundation (NRF) (2001–2003) and the University of Pretoria (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wichmann, J., Voyi, K.V.V. Influence of Cooking and Heating Fuel Use on 1–59 Month Old Mortality in South Africa. Matern Child Health J 10, 553–561 (2006). https://doi.org/10.1007/s10995-006-0121-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-006-0121-z