Abstract
European eel proteins hydrolysates (EPHs) were produced from A. anguilla muscle protein and protein isolate using Purafect®, goby crude proteases and B. invictae proteases. EPHs had high protein contents and displayed diverse molecular mass distributions. Muscle protein hydrolysates (MPHs) and protein isolate hydrolysates (PIHs) obtained both by Purafect® had the highest antibacterial activity. PIHs obtained by B. invictae proteases had the highest emulsifying stability. MPHs and PIHs obtained by goby proteases and PIHs obtained by B. invictae proteases had higher chelating effects. MPHs obtained by B. invictae proteases and PIHs obtained by Purafect® had the highest ability to prevent bleaching of β-carotene at 4 mg/ml (96.68% and 93.66% respectively). The maltodextrin-muscle protein hydrolysates dispersions were totally stable by the creaming index and the zeta potential values (−36.73 and −42.53 mV) compared to the maltodextrin-protein isolate hydrolysates dispersions and had the best electrosprayability approving the best ability to form microcapsules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Practical Applications
European eel protein hydrolysates (EPHs) were produced from muscle (MPH) and protein isolate (PIH) by means of the enzymatic hydrolysis. Various sources of crude proteases was used such as Purafect, bacterial proteases and goby fish viscera proteases. Result revealed that MPH-P and PIH-P exhibited higher techno-functional properties and biological activities such as antioxidant and antibacterial activities. In order to ensure the stability of eel protein hydrolysates, when incorporating in food or pharmaceutic products, the microencapsulation of protein hydrolysates via maltodextrin was investigated. The microstructure revealed the higher homogeneity and the small particle size of the MPHs microcapsules, explained by the higher stability and homogeneity of maltodextrin-MPHs dispersions. This study encourages the production of the microcapsules of eel protein hydrolysates as a safe and ecofriendly biomaterials and the evaluation of their biological potential in-vitro and in-vivo.
Introduction
Fishing industry by-products are discarded after industrial processing without attempting regaining for economical and/or environmental improvement (Lajmi et al. 2019). However, these fish materials could be a potential source of bioactive compounds that can be extracted and used to replace other synthetic ingredients (Taghvaei et al. 2014). Among these compounds, proteins are used in various food preparations due to their great importance. Generally, fish protein hydrolysates (FPH) are small fragments of peptides that contain 2–20 amino acids showing various biological bioactivities (Martínez-Alvarez et al. 2016) including antioxidant (Sripokar et al. 2019), anti-hypertensive (Lassoued et al. 2015), anti-inflammative (Ahn et al. 2015) and anti-ACE (Lajmi et al. 2019) activities. Nevertheless, bioactive peptides could present some disadvantages such as chemical instability, bitter taste and a high hygroscopicity under the influence of temperature, light and oxygen. The bioavailability and stability of peptides are rarely studied as major concerns despite strong evidence that in-vitro bioactivity is not always available for animal models and human subjects (Mohan et al. 2015). To protect and improve the stability of these compounds, organoleptic properties, shelf life, and especially biological activities of peptides, different encapsulation strategies have been developed, such as the film hydration technique, spray drying or coacervation (Mohan et al. 2015) and electrohydrodynamic processes (Wen et al. 2017). This latter technique has been recently used as a promising method to protect bioactive substances. The main advantage of this method is the absence of heating step, which allows the protection of bioactive compounds against thermal degradation and improving their encapsulation efficiency (Wen et al. 2017; Lim et al. 2019). In electrohydrodynamic processing, jets from the polymeric/biopolymeric solutions are electrically charged by electrostatic forces, causing the whipping of these jets and, subsequent solvent evaporation during the flight between the ejector and collector, generating dry ultrathin structures. The process is called electrospinning when ultrathin continuous fibers are produced, while when non-continue structures (or capsules) are obtained, the process is called ‘electrospraying’ (Pérez-Masiá et al. 2014). This process remains attractive for industrial implementation and it can be of interest in a broad variety of applications such as textile, tissue engineering, drug delivery and encapsulation (Lim et al. 2019). The obtained microfibers and microparticles are versatile for the encapsulation of bioactive compounds (e.g., micronutrients, nutraceuticals, probiotics). This novel method has been used in the food sector and constitutes a good alternative for protecting bioactive compounds against environmental stress factors such as oxygen, light, temperatures, etc. (Desai and Park 2005). Natural macromolecules, such as proteins, polysaccharides, and lipids, are used as carrier systems for food peptide encapsulation (Mohan et al. 2015). One of the most important carrier agents using as coating material to protect various heat-sensitive compounds is maltodextrin (Jafari et al. 2008). Complex formation between maltodextrin and proteins is influenced by several parameters such as protein/maltodextrin ratio, pH, ionic strength, total polymer concentration, molecular weight. Some other parameters such as agitation, pressure or temperature have also been shown to influence the complex formation. These parameters have been reported in detail for various protein-polysaccharide pairs (Schmitt and Turgeon 2011).
Therefore, the aims of this study were to produce novel European eel protein hydrolysates from muscle and protein isolate, to evaluate their biological activities (antioxidant and antibacterial activities) and techno-functional properties (emulsifying, foaming, water and oil binding capacities). Additionally, protein hydrolysates with bioactive properties have been electrosprayed using maltodextrin as coating material.
Materials and Methods
Materials
European eel (A. anguilla) and goby (Zosterissessor ophiocephalus) fishes were purchased from the fish market of Sfax city, Tunisia. Muscle of A. anguilla was separated, rinsed three times with distilled water to remove salts and other contaminants and stored at −20 °C, until it used for protein hydrolysates production; while viscera of goby fish were used for alkaline proteases extraction.
Endogenous viscera proteases from goby were prepared as described by Nasri et al. (2013). The production of proteases from the B. invictae AH1 was carried according the protocol described by Hammami et al. (2016). In order to measure the protease activity, the method of Kembhavi et al. (1993) using casein as a substrate, was used.
The maltodextrin (MD) is used for the preparation of MD-protein hydrolysate dispersion. Xanthan gum and Span-20, used as an emulsifying agents, were supplied by Sigma-Aldrich.
Preparation of A. anguilla Protein Isolate and European Eel Protein Hydrolysates
The protein isolate was extracted from European eel muscle by pH-shift processing according to the method of Taktak et al. (2018). The fish muscle was minced (1:9; w/v) with cold water (4 °C), followed by pH adjustment to 11.5 using 2 N NaOH solution. The homogenate was centrifuged at 9500 g for 20 min at 4 °C. Three layers were obtained: i: the upper layer was neutral lipid; ii: the middle layer was soluble proteins; iii: the bottom layer was insoluble materials. The soluble proteins were then precipitated at their nominal isoelectric point (pH 5.5) using 2 N HCl and centrifuged (9500 g for 20 min). Finally, the obtained pellet was resuspended in distilled water, followed by pH adjustment to 7.0 using 2 N NaOH solution. European eel protein isolate (EPI) was freeze-dried using freeze-dryer lab (Modulyo D Freeze dryer-230, Thermo Fisher Scientific, USA) at a temperature of −50 °C and a pressure of about 121 mbar.
To prepare European eel protein isolate hydrolysates (EPHs), freeze dried European eel protein isolate (EPI), at a concentration of 4% (w/v), was resuspended in distilled water and homogenized at 20,000 g for 1 min using Ultra-turrax T18 model homogenizer (IKA, Germany), followed by pH adjustment to the optimum values for each enzyme. For the preparation of hydrolysates from A. anguilla fresh muscle (MPHs), 100 g of fillet were mixed with 200 ml distilled water by KENWOOD blender BL440. After the preparation of protein mixtures, the pH and the temperature of solutions were adjusted to the optimum values for each enzyme: Purafect® (pH 10.0, 50 °C), crude proteases extracted from goby visceral (pH 9.0, 45 °C) and proteases prepared from B. invictae AH1 (pH 10.0, 50 °C). The enzymes were added at the same enzyme/substrate-ratio of 6:1 (unit of enzyme/mg of protein) to compare hydrolytic efficiencies. During the reaction, the pH of the mixture was maintained constant at the desired value by continuous addition of 4 N NaOH solution. After incubation for 500 min, the reactions were stopped by heating the solutions for 20 min at 90 °C to inactivate enzymes. Then, EPHs were recovered from the supernatant after centrifugation at 9500 g for 20 min and freeze-dried. Protein hydrolysates obtained from fresh muscle of European eel using Purafect®, goby crude proteases and B. invictae proteases were noted as MPH-P, MPH-G MPH-A, respectively; while hydrolysates produced from European eel protein isolate with the same proteases were named protein isolate hydrolysates, PIH-P, PIH-G and PIH-A, respectively. Undigested muscle and protein isolate were prepared at the same conditions, without proteases addition, and were noted as UN-MP and UN-PI, respectively.
Hydrolysis Degree (HD)
The hydrolysis degree (HD) defined, in the substrate studied, as the percent ratio of peptide bonds cleaved to the total number of peptide bonds, was determined from the amount of base (NaOH) added in order to keep the pH constant through the hydrolysis as described by the method of Adler-Nissen (1986).
Physicochemical Characterization
Chemical Analysis
The moisture and ash contents were governed according to the AOAC standard methods 930.15 and 942.05, respectively. Total nitrogen content of samples was determined using the Kjeldahl method following the AOAC method number 984.13(AOAC 2000). Crude protein was estimated by multiplying total nitrogen content by the factor of 6.25. Fat content was conducted gravimetrically after Soxhlet extraction of freeze-dried samples with hexane for 2 h using Nahita Model 655, Navarra. All measurements were performed in triplicate.
Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) Analysis
The hydrophobicity of peptides from EPHs was determined using RP-HPLC; LC-10, Shimadzu, Kyoto, Japan. Each hydrolysate (20 mg/ml) was filtered at 0.45 μm and was injected into C18 Eurosphère-100 column (250 mm × 8 mm). The column was equilibrated with solvent A (1 ml/l trifluoroacetic acid in ultrapure water) and peptides were eluted with a linear increase in solvent B (1 ml/l trifluoroacetic acid in acetonitrile) from 0% at 0 min to 40% at 60 min. The flow rate was 1 ml/min. The elution was monitored at 215 nm using a UV–Visible spectrophotometer (Cecil CE 2021, Lab Equip Instruments Ltd., Ontario, Canada).
Characterization of Hydrolysates by Size Exclusion Chromatography
The molecular weight (MW) distribution of EPHs and the undigested proteins was carried out by size-exclusion HPLC on Biobasic SEC-120 column (Thermo Fisher, LC1260, USA) with UV detection at 215 nm eluted at 0.35 ml/min with 0.5 M phosphate buffer (pH 7.0). All samples were loaded to the column at a concentration of 40 mg/ml, which was previously calibrated with bovine serum albumin (66 kDa), egg albumin (44 kDa), cytochrome C (12 kDa) and insulin (5.8 kDa).
Determination of Functional Properties
Solubility of undigested proteins and their hydrolysates was investigated over a wide range of pH values from pH 2.0 to pH 10.0 as described by Tsumura et al. (2005).
The emulsifying activity index (EAI) and emulsifying stability index (ESI) of the intact proteins, MPHs and PIHs are carried out according to the method of Pearce and Kinsella (1978). The microscopic observation of each emulsion (20 μl) was carried out after 24 h using a CX31-12C04 microscope (Motic 2048 × 1536 pixels, Olympus Co., Tokyo, Japan). The emulsion images were captured by a charge-coupled device camera (Olympus Co., Tokyo, Japan) connected to the microscope.
Foam expansion (FE) and foam stability (FS) of undigested proteins and their hydrolysates were conducted referring to the method of Shahidi et al. (1995).
Water binding capacity (WBC) was measured referring to Mac-Connel et al. (1974) and the Oil binding capacity (OBC) was carried out according to Lin et al. (1974).
Determination of Antioxidant Activities
The DPPH free radical-scavenging activity of UN-MP, UN-PI and their hydrolysates was determined as described by Bersuder et al. (1998). A volume of 500 ul of each sample at different concentrations (1–6 mg/ml) was added to 375 ul of 99.5% ethanol and 125 ul of 0.02% DPPH in 99.5% ethanol. The mixtures were then kept at room temperature in the dark for 60 min, and the reduction of DPPH radical was measured at 517 nm using a UV–Visible spectrophotometer (T70, UV/VIS spectrometer, PG Instruments Ltd., China). The control was conducted in the same manner, except that distilled water was used instead of sample. The DPPH radical scavenging activity was calculated as follows:
where Ac is the absorbance of the control reaction and As is the absorbance of the samples. DPPH has an absorption at 517 nm which disappear upon reduction by an antiradical compound. Lower absorbance of the reaction mixture indicated higher DPPH radical-scavenging activity. BHA was used as positive control. The test was carried out in triplicate.
The ability of intact proteins and EPHs to reduce iron (III) was determined resorting to the method of Yildirim et al. (2001). An aliquot of 1 ml sample of each hydrolysate at different concentrations (1–5 mg/ml) was mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide solution. The reaction mixtures were incubated for 30 min at 50 °C. After incubation, 2.5 ml of 10% (w/v) TCA was added and the reaction mixtures were then centrifuged at 10,000 × g for 10 min. Finally, 2.5 ml of the supernatant solution from each sample mixture was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% (w/v) ferric chloride. After a 10 min reaction time, the absorbance of the resulting solutions was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. The control was conducted in the same manner, except that distilled water was used instead of sample. Values presented are the mean of triplicate analyses.
The ability of EPHs and undigested proteins to prevent bleaching of β-carotene was assessed as described by Koleva et al. (2002). A stock solution of β-carotene/linoleic acid was prepared by dissolving 0.5 mg of β-carotene, 25 μl of linoleic acid and 200 μl of Tween 40 in 1 ml of chloroform. The chloroform was completely evaporated under vacuum in a rotatory evaporator at 40 °C; then 100 ml of distilled water were added and the resulting mixture was vigorously stirred. The emulsion obtained was freshly prepared before each experiment. Aliquots (2.5 ml) of the β-carotene/linoleic acid emulsion were transferred to test tubes containing 0.5 ml of samples at different concentrations. Following incubation for 2 h at 50 °C, the absorbance of each sample was measured at 470 nm. BHA was used as a positive standard. The control tube contained no sample. Tests were carried out in triplicate. The antioxidant activity was evaluated in terms of bleaching of β-carotene using the following formula:
where A0 and A′0 are the absorbances of the test sample and the control, respectively, measured at time zero; and At and A′t are the absorbances of the sample and the control, respectively, measured after incubation for 120 min. Values presented are the mean of triplicate analyses.
The chelating activity of samples towards ferrous ion (Fe2+) was made referring to the method of Decker and Welch (1990). Briefly, 100 µl of each sample, prepared at different concentrations, were added to 50 µl of 2 mM FeCl2 and 450 µl of water. The mixtures were incubated at room temperature for 3 min and the reaction was initiated by the addition of 200 µl of 5 mM of ferrozine solution. The mixtures were then vigorously shaken and left to stand at room temperature for 10 min. Control tubes were prepared by the same manner, substituting the sample with water. The absorbance of solutions was measured at 562 nm. EDTA was used as reference. Chelating activity (%) was then calculated as the following equation. Where ODC, ODB and ODS represent the absorbances of the control, the blank and the sample reaction tubes, respectively.
Determination of Antibacterial Activity
Microbial Strains
Antibacterial activities of EPHs were tested against four Gram-positive bacteria: Staphylococcus aureus (ATCC 6538), Micrococcus luteus (LB 14110), Micrococcus luteus (BRM7) (Ben Salem et al. 2011) and Listeria monocytogenes (ATCC 19117) and four Gram-negative bacteria: Escherichia coli (ATCC 8739), Salmonella enterica (ATCC 14028), Pseudomonas aeruginosa (ATCC 49189) and Enterobacter sp.
Agar Diffusion Method
The antibacterial activity assay was achieved according to the method illustrated by Berghe and Vlieinck (1991). The protein hydrolysates were dissolved in distilled water at a concentration of 100 mg/ml. Culture suspension (200 μl) of the tested microorganisms (106 colony-forming units (cfu)/ml of bacteria cells estimated by absorbance at 600 nm) were spread on Muller–Hinton agar. Then, eight bores (3 mm depth, 4 mm diameter) were made using a sterile borer and were loaded with 100 μl of the sample at 100 mg/ml. The Petri dishes were kept, first for 1 h at 4 °C, and then incubated for 24 h at 37 °C. Antimicrobial activity was evaluated by measuring the diameter of the growth inhibition zones in millimeters (including well diameter of 4 mm). Antimicrobial activity was estimated by determining the zone of growth inhibition (diameter expressed in millimeters) around the wells.
Investigation of Protein Hydrolysates Encapsulation Through Electrospraying Process
The maltodextrin dispersion was prepared by dissolving 10, 20 and 40% (w/v) of the polymer in water to evaluate its electrosprayability. Then, 1% (w/w) of Xanthan gum (XG) and/or 5% (w/w) of surfactant (Span 20) was added to maltodextrin dispersion when needed. The best condition has been used to encapsulate European eel protein hydrolysates (EPH). In fact, the EPH (30% w/w of maltodextrin respecting the total solid mass) was dispersed in maltodextrin MD solution (40% w/v) previously dissolved in water. Subsequently, different homogenization conditions were used, homogenization with Ultraturrax (2 min and 5 min) and/or homogenization with ultrasonication 2 min. The microcapsules production of protein hydrolysates encapsulated using maltodextrin were processed using a homemade electrospraying apparatus, equipped with a variable high-voltage power supply. The solutions were introduced in a 10 mL plastic syringe and were pumped through a stainless-steel needle (14.43 mm of inner diameter). The needle was connected through a PTFE wire to the syringe, which was placed on a digitally controlled syringe pump. Processed samples were collected on a stainless-steel plate connected to the cathode of the power supply and placed facing the syringe in a horizontal configuration, fixed at an optimal distance of 10 cm. To determine the best conditions for production uniforms microcapsules, the EPH-MD solutions (30% of MD, w/w) were tested at different voltages (10, 12, 15 and 18 kV) and flow rates (0.04, 0.1, 0.15, 0.5 mL/h). SEM analysis using a Hitachi microscope (Hitachi S 4800) was used to assess the microcapsules morphological appearances regarding different conditions after coating with the gold-palladium mixture.
Statistical Analysis
Statistical analysis was performed with SPSS ver.17.0, professional edition. Comparison of variance was carried out using one-way analysis of variance (ANOVA) followed up by Duncan’s Multiple Range tests for the determination of statistically different groups. Differences were considered significant at p < 0.05. All tests were carried out in triplicate.
Results and Discussion
Characterizations of European Eel Protein Hydrolysates
Chemical Composition and HD of EPI
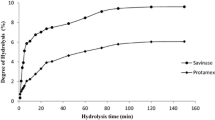
Actually, dietary fish proteins offer an interest source of bioactive peptides, which are commonly produced by enzymatic process. The nature of the enzyme and protein matrix significantly affect the biological activity of protein hydrolysates (Van der Ven et al. 2002). Therefore, six protein hydrolysates were prepared from European eel muscle (MPH) and protein isolate (EPI) by various proteases: Purafect®, crude enzyme preparations from B. invectea, and goby viscera proteases. The level of European eel protein hydrolysis by proteolytic proteases was assessed by determining the HD (Table 1). Regarding protein hydrolysates prepared from Anguilla muscle (MPH), the highest HD was obtained with goby proteases (18.15%), followed by Purafect (16.4%) and B. invectea (15.37%). For the proteolysis from EPI, the HDs were 22.79% (PIH-P), 20.33% (PIH-A) and 19.09% (PIH-G). Thus, all the hydrolysates prepared from protein isolate had the highest HD compared to that from muscle. Additionally, goby visceral proteases are most efficient for muscle protein hydrolysates and Purafect proteases are most efficient for protein isolate hydrolysates preparation.
Furthermore, the chemical composition analysis, illustrated in Table 1, revealed that the undigested protein isolate (UN-PI) presented higher protein content (94.33%) compared to the undigested muscle protein (UN-MP) (78.29%) (Table 1). A similar trend was reported by Pires et al. (2015) who found that protein solubilization of hake by-products at pH 11.0 before hydrolysis brought about a high reduction (90%) of lipid content. The lipid level of the UN-PI (3.41%) was significantly lower than that of the UN-MP (15%) (p < 0.05). Moreover MPH had the highest level of lipid compared to EPH which is attributed to the high amount of lipid in the initial raw materials.
After enzymatic hydrolysis, the obtained dried EPHs had a high protein contents ranging from 75.84 to 84.67%. This is in accordance with the previous study of Geirsdottir et al. (2010) who found that hydrolysates obtained from blue whiting protein isolate which is previously extracted by an alkaline solubilization method, exhibited a protein content in the range of 73.7–85.3%. Furthermore, the higher level of ash as in the protein hydrolysates compared to the undigested protein is probably due to the NaOH addition during hydrolysis in order to keep the pH constant (Nasri et al. 2013).
RP-HPLC Profiles
RP-HPLC separates protein hydrolysates into several peptide peaks based on their hydrophobic/hydrophilic character. The RP-HPLC elution profiles of EPHs are shown in Fig. 1. The order of appearance of hydrophobic and hydrophilic residues influences the peptide elution profiles (Mahmoud et al. 1992). Several peaks were detected, demonstrating the heterogeneous composition of European protein hydrolysates after proteolysis, and the chromatograms are divided into three zones. Zone 1 consisted of hydrophilic peptides. Zone 2 contained low hydrophobic peptides and zone 3 comprised high hydrophobic peptides. RP-HPLC revealed that protein hydrolysates from muscle MPH-P followed by MPH-G showed the highest content of hydrophilic peptides compared to the hydrolysates produced from protein isolate generating the most hydrophobic peptides (PIH-P and PIH-G). Furthermore, muscle protein hydrolysates produced with goby proteases (MPH-G) had a higher content of low hydrophobic peptides followed by the protein isolate hydrolysates (PIH-G) produced by the same proteases. Besides, protein isolate and muscle protein hydrolysates using B. invictae AH1 proteases (PIH-A and MPH-A) had the lowest content of hydrophilic peptides and the highest hydrophobicity. These findings could be related to the highest fatty acid composition of MPH-A (12.35%) and PIH-A (9%) prepared. Besides, the lower hydrophilic content of MPH-A using B. invictae AH1 could be correlated to the lower HD (15.37%) (Lassoued et al. 2015). The hydrophobic/hydrophilic ratio of peptides could be related to the protease’s specificity.
RP-HPLC profiles of European eel protein hydrolysates (EPHs). Results were showed as absorbance at 215 nm as function of the elution time (min). UN-MP and UN-PI represent undigested muscle and protein isolate prepared at the same conditions without proteases addition respectively. EPHs: European eel protein hydrolysates. MPH-P MPH-G and MPH-A: protein hydrolysates obtained from A. anguilla muscle by Purafect®, goby proteases and B. invictae proteases respectively. PIH-P, PIH-G and PIH-A: protein hydrolysates produced from protein isolate with the same proteases respectively
Molecular Weight Distribution
The various proteases applied to A. anguilla muscle proteins and its protein isolate displayed different spectra of substrate specificity leading to produce several type of protein hydrolysates containing peptides with different functional and antioxidant properties and molecular weights (MW) distributions. The peptide size distribution of the EPHs was carried out using the HPLC-SEC analysis. The SEC profiles and the relative percent of the MW peptides divided into 6 fractions are shown in Table 1.
The gel permeation HPLC spectra revealed highly heterogeneity of peptide length and intensities of several hydrolysates depending on the enzyme and initial protein matrices used. In addition, the MW profiles of undigested European eel proteins revealed a major fraction of high molecular weight (HMW) peptides eluted from 7 to 10 min, while UN-MP spectra showed clearly four peaks eluted tardily from 15 to 20 min (Data not shown).
The MW distribution of peptides (Table 1) shows that the proteolysis of A. anguilla muscle proteins reduced (p < 0.05) the intensity of peptides with HMW (>10 kDa) from 70.69% (undigested muscle) to 17.18%, 16.93% and 12.93% for proteins digested using Purafect, Goby and B. invectea AH1 proteases respectively. Furthermore, among hydrolysates obtained from fish mince, the highest levels of peptides below 500 kDa were obtained with MPH-G (30.79%) followed by MPH-A (21.55%) and MPH-P (12.69%).
On the other side, results show that the hydrolysis of European eel protein isolate by Purafect considerably reduced (p < 0.05) the percentage of HMW peptides (>10 kDa) to 5.75%, followed by B. invectea AH1 proteases (9.02%), while the decrease of HMW percentage was less accentuated with fish goby proteases (33.32%). For the lower molecular mass ranges (<0.5 kDa), PIH-P had higher percentage (34.47%) of LMW peptides fraction, followed by PIH-A (31.76%) and PIH-G (21.21%).
Thus, for muscle protein hydrolysates, the highest level of peptides with low molecular weight (<0.5 kDa) was obtained by MPH-G (30.79%). However, for protein isolate hydrolysates, this highest level (<0.5 kDa) was attained for PIH-P (34.47%) followed by PIH-A (31.76%).
Determination of Functional Properties
Solubility
The investigation of solubility at different pH values is required to understand functional properties of proteins (Kristinsson and Rasco 2000). The solubility of UN-PI, UN-MP and their hydrolysates, at different pH values, showed that enzymatic hydrolysis improved the solubility of proteins (Fig. 2a). The increase of solubility of protein hydrolysates compared to that of the intact proteins could be related to the decrease of molecular weight and the increase of hydrophilic/hydrophobic ratio (Adler-Nissen 1986). In addition, the solubility of all hydrolysates decreased at pH 5.0, reaching 53.6–60.4% for PIH-A and PIH-P, respectively, and increased gradually below and above this value. Furthermore, EPHs had better solubility in acidic and alkaline pH values (100% for MPH-P, MPH-A, PIH-P and PIH-A at pH 10.0) than the undigested proteins (p < 0.05), except for UN-PI which had a similar solubility to its hydrolysates at pHs range from 8.0 to 10.0. Besides, MPH-P and PIH-P had the highest solubility reaching 100% at pH 9.0 and pH 10.0. It’s important to note that the solubility of PIH was higher than MPH at alkaline and acid conditions which could be explained by the difference in the physicochemical composition and the RP-HPLC profiles.
a Solubility profiles of UN-PI, UN-AM and their hydrolysates as a function of pH values (2–10), at a concentration of 2%. b Stability of emulsion prepared with 0.5% of undigested UN-AM, UN-PI and EPHs sample after incubation for 24 h by the optical microscopy. c Oil binding capacities (%) of UN-PI, UN-AM and their EPHs. d Water binding capacities (%) of UN-PI, UN-AM and their EPHs. UN-MP and UN-PI represent undigested muscle and protein isolate prepared at the same conditions without proteases addition respectively. EPHs: European eel protein hydrolysates. MPH-P MPH-G and MPH-A: protein hydrolysates obtained from A. anguilla muscle by Purafect®, goby proteases and B. invictae proteases respectively. PIH-P, PIH-G and PIH-A: protein hydrolysates produced from protein isolate with the same proteases respectively
Emulsifying Properties
The emulsifying properties are proportional to the solubility of the proteins and peptides. Samples with high solubility can diffuse and bind to the interface (Klompong et al. (2007). In this context, the emulsifying activity index (EAI) and emulsifying stability index (ESI) of all samples, prepared at different concentrations (0.5%, 1% and 2%; w/v), were determined at pH 10.0. Results, illustrated in Table 2, show that UN-MP exhibited the highest EAI (79 m2 g−1) followed by UN-PI (48.55 m2 g−1), at all concentrations. Besides, the EAIt0 of UN-MP and UN-PI decreased with increasing concentration from 0.5 to 2%. The decrease of protein–protein interactions at the interface of droplet oil could be explained by the aggregation or the precipitation of proteins at high concentrations. In addition, the EAIt10 and EAIt30 of undigested proteins and their hydrolysates decreased with concentrations increased, except for PIH-A (p < 0.05).
Among protein isolate hydrolysates, PIH-P had the highest emulsifying capacity of 48.33 m2g−1 at 0.5% which could be related to its highest levels of LMW peptides fraction (<0.5 kDa) reaching 34.47%. This is in line with previous works of Taheri et al. (2013) who found that molecular size stands for one of the factors which contribute to the emulsifying activity of protein hydrolysates.
Concerning the evaluation of emulsion stability, results show that, with the increase of sample concentration, the ESIt10 min increased significantly for undigested protein and their hydrolysates. In fact, the emulsifying stability of UN-PI (96.84%) was higher than UN-MP (78.46%) at a 2% concentration. This finding agreed with previous studies of Galla et al. (2012), Tanuja et al. (2012) and Betty et al. (2014) who found that increased amounts of larger molecular weight peptides contribute to the emulsion stability. Protein hydrolysates had a higher emulsifying stability by increasing the concentration from 0.5 to 2% reaching the maximum ESIt10min (98.77%) for PIH-A followed by MPH-A (95.55%), MPH-G (94%) and PIH-G (92%). This interesting ESI could be related to the high of hydrophobic peptide content able to stabilize protein emulsion. This finding is in agreement with previous studies, which reported that hydrophobic peptides conducted more stable emulsion (Chi et al. 2014; Li et al. 2013; Taheri et al. 2013).
The droplet shape analysis was carried out 24 h after emulsification by the optical microscopy observation of emulsion prepared at 0.5% of sample concentration. Results, shown in Fig. 2b, revealed that the smallest droplet size was obtained in the presence of protein hydrolysates.
Foaming Properties
Foam capacity (FC) and stability (FS) of UN-MP, UN-PI and EPHs, at various concentrations (0.5%, 1% and 2%; w/v) and at pH 10.0, are presented in Table 3. The FC of EPH was dose-dependent. In fact, Sanchez and Patino (2005) revealed that an increase in protein concentration resulted in a higher rate of diffusion of foam properties. Among undigested proteins, UN-PI exhibited a high foaming capacity reaching 105% at 0.5% concentration while UN-MP showed the maximum of FC (127%) at a concentration of 2%. The evaluation of undigested proteins and their hydrolysates revealed that UN-PI had the highest FS after 30 min at a concentration of 2% (56.25%). This result is related to their high content on protein and HMW peptide (67.88% of peptides with HMW >10 KDa). Several researchers reported that the high foaming stability are directly related to the high molecular size, cohesiveness and elasticity (Li et al. 2013; Betty et al. 2014; Chi et al. 2014). Likewise, Nalinanon et al. (2011) reported that low molecular weight peptides (~1 kDa) could not maintain well-ordered orientation of the molecule at the interface.
Among protein hydrolysates, those obtained by Purafect had the highest FC (157.5% and 151.5% for MPH-P and PIH-P respectively), followed by PIH-G (70.75%). Furthermore, the same hydrolysates are stable after 10 min (52%). This finding could be related to its higher content on hydrophilic peptides, compared to the other hydrolysates, as the foaming property is governed by the interface water/air adsorption of protein, and thereby hydrophilicity is crucial to assure the penetration and rearrangement of molecules at the interface between air-water (Elavarasan et al. 2014).
Water and Oil Binding Capacities
Oil binding capacity (OBC) analysis, illustrated in Fig. 2c, highlighted that the OBC of PIHs was higher (30.35–57.81%) than that obtained with MPHs (22 to 29%). Among all hydrolysates, PIH-A exhibited the highest OBC which could be attributed to its high content on hydrophobic peptides, compared to the hydrolysate obtained with goby and B. invectea proteases.
The hydrolysates with high oil binding capacity are a potential additive for the meat and confectionery industries (Gbogouri et al. 2004).
In addition, the highest WHC was obtained with undigested proteins, in a dependent-dose manner (Fig. 2d). In fact, UN-PI and UN-MP could retain 98.86 and 67.08% of water at 2% concentration, respectively, while the WHC of EPHs is ranged from 8.57 to 15.46%, at the same concentration. This high WHC of undigested protein is due to their high content with higher (>10 kDa) molecular weight (67.9 and 70.7 kDa for UN-PI and UN-MP respectively). The WBC and OBC recorded values could be influenced by the nature, charge and molecular weight of proteins and peptides.
Antioxidant Activities
DPPH Radical Scavenging Activity Assay
Antioxidants can terminate or retard the oxidation process by interacting with free radicals and forming species. The results of the DPPH free radical-scavenging activity, presented in Fig. 3a, indicated that intact proteins and their hydrolysates exhibited antioxidant activity against DPPH. A positive correlation between the peptide concentrations and their radical-scavenging potential was observed. These findings are in line with previous study of Nasri et al. (2014) which reported that the DPPH scavenging activity increased with increasing concentrations of goby fish protein hydrolysates. Furthermore, results showed that UN-MP, UN-PI and EPHs, prepared at a concentration of 5 mg/ml, manifested moderated antioxidant activity ranging from 23 to 42%, which was lower than that observed with BHA at the same concentration. For the hydrolysate obtained with gobie and B. invectea proteases, PIH had higher scavenging activity compared to MPH. Furthermore, among all hydrolysates PIH-G had the highest scavenging activity (42,58%) followed by PIH-A (37.93%) at the concentration 5 mg/ml.
a DPPH radical scavenging of UN-PI, UN-AM and their EPHs. b ß-carotene bleaching of UN-PI, UN-AM and their EPHs. c Reducing power of UN-PI, UN-AM and their EPHs. d Iron chelating activities of UN-PI, UN-AM and their EPHs. UN-MP and UN-PI represent undigested muscle and protein isolate prepared at the same conditions without proteases addition respectively. EPHs: European eel protein hydrolysates. MPH-P MPH-G and MPH-A: protein hydrolysates obtained from A. anguilla muscle by Purafect®, goby proteases and B. invictae proteases respectively. PIH-P, PIH-G and PIH-A: protein hydrolysates produced from protein isolate with the same proteases respectively
ß-Carotene Bleaching Method
The ability of both undigested proteins and their hydrolysates to scavenge the free radical of system linoleic acid-β-carotene system was also investigated and results are reported in Fig. 3b. In this test, ß-carotene undergoes rapid discoloration in the absence of antioxidant, which reduced the absorbance of the test solution with increasing reaction time. All samples inhibited considerably the oxidation of ß-carotene at dose dependent manner. Among undigested protein, UN-PI showed significantly (p < 0.05) the highest ability to prevent bleaching of ß-carotene (99.07%). Moreover, muscle protein hydrolysates (MPH) give the best ability to inhibit ß-carotene oxidation compared to protein isolate hydrolysates (PIH). This finding could be related to the hydrolysate composition as MPH had higher lipid content compared to PIH (Qian et al. 2008). Besides, MPH had higher hydrophobicity compared to PIH it means that the higher activity is related to presence of hydrophobic peptide responsible for the neutralization of free radicals derived from linoleic acid and therefore prevent the oxidation and bleaching of ß-carotene. Furthermore, protein hydrolysates obtained using B. invectea proteases had the highest ability to scavenge the free radical of system linoleic acid-β-carotene system at a concentration of 4 mg/ml (96.68% for MPH-A and 93.66% for PIH-A). Thus, the free radical-mediated lipid peroxidation of these hydrolysates system may be related to the pronounced level of hydrophobic peptides compared to the hydrophilic peptides (Qian et al. 2008).
These results demonstrated that both undigested proteins and their EPHs prevent ß-carotene bleaching by potentially donating hydrogen atoms to peroxyl radicals of linoleic acid. The DPPH radical scavenging activity and the inhibition of oxidation of ß-carotene assay showed that the intact proteins, especially protein isolate, and their derivates exhibited an interesting ability to stabilize free radical in emulsion system that in aqueous solution.
Reducing Power Assay
The reducing powers of UN-PI, UN-MP and their EPHs, as well as BHA, as a function of their concentrations, are shown in Fig. 3c. All samples showed reducing power ability in a dose dependent manner. Besides, among undigested protein, UN-PI displayed higher reducing power (DO700 nm = 1.59) compared to UN-MP (DO700 nm = 1.16), at 5 mg/ml. Furthermore, protein hydrolysates using Purafect had the highest reducing power reaching (DO700 nm = 1.33) for MPH-P followed by PIH-P (DO700 nm = 1.12). The same hydrolysates had higher hydrophilic peptide with the higher content (17.18) on peptide with HMW (>10 kDa) compared to MPH-G and MPH-A which could be responsible for the higher reducing power capacity. In addition, MPH and PIH obtaining by means of gobie and B. invectea proteases had significantly close ability for reducing power (0.97–1.04). Wu et al. (2003) reported that the higher reducing potential might be also credited to the charge and amino acid composition of proteins hydrolysates. However, all hydrolysates and undigested proteins showed a lower reducing power than BHA at the same concentrations.
Ferrous Ion Chelating Activity
The Ferrous ion chelating activity of different samples was evaluated by the disruption of the formation of the complex Fe2+-ferrozine, resulting in the decrease of the purple color development. Figure 3d shows that all samples exhibited potential chelating activity with dose dependent manner. Undigested proteins exhibited the strongest metal chelating ability (100%, at a concentration of 200 μg/ml), which is comparable to the EDTA, used as chelating agent. Among EPHs, at 500 μg/ml, MPH-G and PIH-G and PIH-A had highest chelating effects reaching significantly a close value of 97.95, 96.24 and 95.76% respectively, followed by MPH-A (92.31%). This finding could be correlated to the higher hydrophobicity of the hydrolysates by means of goby and B. invectea proteases compared to that with Purafect proteases. The important chelating metal ions higher chelating metal effect may also attributed to the presence of acid and basic amino acids with carboxyl and amino groups in the side chains (Wiriyaphan et al. 2015). Thus, results demonstrated that protein hydrolysis could be an alternative to improve the antioxidant potentialities of the molecules depending on the mechanisms of action of the applied test.
Antibacterial Properties
The antibacterial activity of UN-MP and UN-PI and their hydrolysates (EPHs) was evaluated against Gram + and Gram− bacteria by measuring the clear growth inhibition zone (expressed in mm). As can be seen in Table 4, all samples disclosed different degrees of antibacterial activities against, at least, four bacteria. The undigested protein isolate (UN-PI) exhibited the higher inhibition effects against the 8 different Gram+ and Gram− bacteria tested compared to the undigested muscle protein. In fact, UN-PI exhibited an important antibacterial activity against M. Luteus Y. (15.75 mm), S. aureus, E. Coli and P. aeruginosa (14.5 mm) and moderate activity against the others strains. The results obtained are of a great importance, particularly in the case of S. aureus, which was well known for being resistant to a number of phytochemical compounds and for the production of several types of enterotoxins that cause gastroenteritis (Halpin-Dohnalek and Marth 1989).
In addition, among European Eel protein hydrolysates, MPH-P displayed the highest inhibition effects against the nine different bacteria tested followed by PIH-P. These hydrolysates showed a strong antibacterial activity against P.aeruginosa with close clear zone diameters for MPH-P (18 mm) and for PIH-P (19 mm) and moderate antibacterial activity for all the other samples (between 13.13 mm and 14.5 mm). The highest antibacterial activity of hydrolysates obtained by means of Purafect proteases may be related to the higher hydrophilic peptides content and to the highest solubility of MPH-P and PIH-P compared to the other hydrolysates obtained with Goby and B. invectea proteases providing lower antibacterial activity. Moreover, P.aeruginosa, as Gram-negative strain, was inhibited by all samples, which could reduce serious humane infections especially in patients hospitalized with cancer, cystic fibrosis, and burns; the case fatality is 50%. Other infections caused by P.aeruginosa species include endocarditis, pneumonia, and infections of the urinary tract, central nervous system, wounds, eyes, ears, skin, and musculoskeletal system (Iglewski 1996). Furthermore, hydrolysates from protein isolate improved the antibacterial activity compared to the muscle protein hydrolysates. Thus, PIH-P illustrated higher inhibitory activity against M. Luteus (14.5 mm) compared to MPH-P (8 mm). PIG-G showed inhibitory activity against six bacteria, while MPH-G showed inhibitory activity against four bacteria. Additionally, data unveiled that among the Gram (+) bacteria, M. luteus was most sensitive microorganism that was inhibited by all samples and the highest inhibitory activity was obtained by PIH-G (15.5 mm) and PIH-P (14.5 mm). It is important also to show that S.aureus as another Gram (+) strain was inhibited by all samples tested with highest activity obtained by UN-PI (14.5 mm). The differences detected in the efficiency of the different hydrolysates from Purafect, Goby and B. invectea could mainly related to the peptide composition, hydrophile/hydrophobe character and the water solubility which could explain the highest antibacterial activity of hydrolysates using Purafect compared the Goby and B. invectea proteases.
Therefore, the overall results prove that undigested protein and their hydrolysates exhibited not only nutritional but also biological properties for therapeutic application.
Electrosprayability of MD
The electrosprayability of maltodextrin (MD) solutions which served as a coating material for protein hydrolysates, was evaluated. This process depends on the adjustments of the dispersion properties which are required to operate the physical morphologies of the collected material through electrospraying process (Lim et al. 2019). There are some parameters related to elecrospraying process (applied potential, electric field, spinning distance, flow rate) and to polymer dispersions properties (conductivity, viscosity, surface tension) (Chronakis 2010). In the present study, the evaluation of the electrosprayability of maltodextrine and eel protein hydrolysates dispersion (MD-EPH) was carried out. MD solutions, prepared at different concentrations (10, 20 and 40%, w/v) without additives, were not electrosprayable (Table 5) which could be attributed to their physical properties. Among these parameters, the concentration, the viscosity and the surface tension of polymer dispersions were crucial as they should be high enough to boost biopolymer entanglements and the production of capsules.
Thus, the methodology used to increase the viscosity, to reduce the surface tension and subsequently improve the electrosprayability of the MD dispersion, was to incorporate Xanthan gum (XG) (1% (w/w)) as a stabilizing agents and Span-20 (5% (w/w)) as non-anionic surfactant to ensure the stability in the electrospraying jet and then to combine both of them. Result, presented in Table 5, revealed a positive impact on the electrosprayability of MD dispersion with the addition of surfactant (Span 20), which could be mainly related to the surface tension decrease from 46 to 25 mN/m and to the decrease of conductivity from 4.10 to 3.48 (mS/cm) (Table 6). This result is in line with previous work of Pérez-Masiá et al. (2014) who reported that the electrically charged (non-ionic) surfactant (Span 20) increased the instability in the jet electrosprayability and enhanced the capsule development. Additionally, the incorporation of both surfactant Span 20 (5%) and XG (1%) had a positive impact on MD electrosprayability (Table 5). However, the incorporation of Xanthan gum (XG) alone does not improve the electrosprayability of MD solution as no material was collected (Table 5) even by modifying the process parameters (flow rate (0.10 mL/h), distance (10, 12 and 15 cm) and voltage (15 and 18 kV) and dripping is observed during electrospraying process. It could be explained by the high water holding capacity of Xanthan gums responsible for incomplete drying of the jet and the dripping in the collector (Pérez-Masiá et al. 2014).
The best conditions selected for electrospraying process, after preliminary study, were the flow rate 0.10 ml/h, the voltage 18 kV and the distance 10 cm based on the electrosprayability (data not shown) and the microstructural analysis using SEM images. Additionally, the SEM images of MD-S (5%) dispersion, presented in Fig. 4a and b, showed the ability of MD (40%, w/w) dispersed with the Surfactant Span 20 (5%, w/w) to form a small dispersed capsule and revealed that increasing the voltage from 15 kV to 18 kV improved the morphology of the MD-S (5%). Then, MD was used to evaluate the electrosprayability of Maltodextrin- European protein hydrolysates (MD-S-EPH) by dissolving protein hydrolysates (30% (w/w) respecting the maltodextrin weight) in the maltodextrin dispersion (40%, w/w) (López-Córdoba et al. 2016).
SEM images of MD-S 5% capsules obtained at different electrospraying condition (a) Flow rate = 0,10 mL/h and voltage = 15 kV; (b) Flow rate = 0,10 mL/h and voltage = 18 kV (c) SEM image of MD-S-UN-MP capsules (d), SEM images of MD-S-EPH capsules: MD-S-MPH-P (e) MD-S-PIH-P (f); MD-S-MPH-A (g); MD-S-PIH-A. All the dispersions are prepared by homogenization using Ultraturrax (5 min) and followed by 2 min Ultrasonication treatment. Electrospraying process parameters: flow rate = 0,10 mL/h, voltage = 18 kV and distance = 10 cm. MD: Maltodextrin. S (5%): Surfactant Span 20 at the concentration 5%. MD-S-MPH is the abbreviation of Maltodextrin- Surfactant Span 20 (5%, w/w) homogenized with European protein hydrolysates. MPH-P and MPH-A: protein hydrolysates obtained from A. anguilla muscle by Purafect®, and B. invictae proteases, respectively
Electrosprayability of MD-S-EPH Dispersions
The European protein hydrolysates (30%, w/w) was dissolved in MD-S (5%) and the dispersion was characterized. EPH prepared by means of Purafect and B. invectea were selected for this study as they present different hydrophilic/hydrophobic character, MPH-P, PIH-P presented the highest hydrophilic peptide content, while MPH-A and PIH-A revealed lower hydrophile peptide content (highest hydrophobicity). The stability of the MD-S-EPH dispersion was evaluated by determining the zeta potential and creaming index (CI) values.
Stability of MD-S-EPH Dispersion
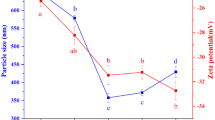
The zeta potential has been determined for MD, and EPH at different pH (Table 7). This analysis is defined as the charge developed at the interface between a solid surface and its liquid medium. The surface charge is one of the properties that transmit the stability of encapsulated products. The zeta potential values of the MD-S-MPH-P and MD-S-MPH-A mixtures were −29.33 and −42.53 mV, respectively (Data not shown). This finding is attributed to the pH of the MD-S-EPH dispersions, which was ranged between 7.8 and 8.1. The pH controls the ionization of the side chain, amino and carboxyl groups of the proteins and carboxylic groups of the polysaccharides.
These results explain the gravitational stability of dispersion, as it is widely reported that solutions with high absolute value of zeta potential (negative or positive) are electrically stabilized against aggregation and flocculation, probably leading to higher physical stability (Morales-Medina et al. 2016).
The creaming indexes (CI) are also determined to evaluate the stability of the MD-EPH dispersion. Results, shown in Table 8 revealed that MD-S-MPH had the higher stability compared to the MD-S-PIH. This finding could be related to the higher hydrophilic peptide content on MPH compared to that of PIH. Additionally, among all MD-S-EPH, MD-S-PIH-P had the lowest stability (35.7% after 24 h).
Conductivity, Surface Tension and Particles Size of MD-S-EPH Dispersion
The conductivity, surface tension and viscosity of stable MD-S-EPH mixture (MD-S-MPH-P and MD-S-MPH-A) were prepared using two homogenization methods (Table 6), homogenization with Ultraturrax (H) and the homogenization followed by ultrasonication treatment (HS), were mesured. The results showed that the conductivity of MD-S-EPH mixture was ranged from 3.56 to 5.96 mS. The highest conductivity was obtained by the dispersions obtained by the homogenization contained muscle protein hydrolysates MD-S-MPH-P (5.96 mS/cm) followed by MD-S-MPH-A (5.5 mS/cm). In addition, the surface tension of MD-S-EPH mixture was ranged between 25.40 and 25.97 mN/m. Moreover, the homogenization with Ultraturrax and Ultrasonication had no significant effect on the viscosity of MD-S-EPH dispersions.
The average particles sizes of MD-S-MPH-P and MD-S-MPH-A dispersions were determined (Table 6). The main objective of varying the homogenization conditions is to obtain homogenous dispersions with a small size and to ensure the large contact area between protein hydrolysates and MD dispersions. Indeed, the average particles sizes of MD-S-MPH-P and MD-S-MPH-A are 4.65 and 4.8 μm respectively. In addition, increasing the homogenization time from 2 min to 5 min reduced the average particles sizes to 3.72 and 3.79 μm for MD-S-MPH-P and MD-S-MPH-A, respectively. The ultrasonication treatment (cycle 0.5, amplitude 20%) reduced the average particles sizes of both MD-S-MPH-P and MD-S-MPH-A to 2.38 and 2.6 μm, respectively.
These results are in line with those reported by Mohan et al. (2015) who found that the combination of proteins and polysaccharides in encapsulation relatively large capsules, which requires the improvement of the homogenization process to reduce the average droplet size of the particles size.
In this work, electrospraying proved to be an efficient tool for MD-S-EPH microcapsules formation, as illustrated in Fig. 4. The electrospraying method allowed a good microstructure of MD-S-EPH microcapsules compared to that obtained with MD-S-UN-MP. This finding could be explained by the lowest stability of MD-S-UN-MP dispersion determined by the creaming index (22.73% after 24 h). Besides, MD dispersion prepared with undigested protein isolate which was insoluble and not stable with the creaming index test and cannot be electrosprayable. Moreover, a clear, uniform and spherical microcapsules were obtained by MD-S-MPH-P and MD-S-MPH-A compared to MD-S-PIH-P and MD-S-PIH-A. This finding is in accordance with the creaming index analysis demonstrating that the MD-MPH dispersions were totally stable (CI = 100%) after 24 h compared to MD-S-PIH dispersions. Furthermore, the best microcapsules morphology of the MD-S-MPH-A and MD-S-MPH-P approved the strong interaction between MD and MPH, boosting the chain entanglements during the electrospraying process (Pérez-Masiá et al. 2014) which is in line with the previous analysis of creaming index and the zeta potential analysis demonstrating the highest stability of MD-S-MPH-A (−42.53 mV) followed by MD-S-MPH-P (29.33 mV) dispersions.
Conclusion
The present study revealed that the extraction of protein isolate from Anguilla anguilla muscle by pH-shifting process leads to reduce remarkably the fat content and to increase the yields of recuperated protein. The recovered protein isolate has interesting techno-functional and antioxidant properties. The enzymatic hydrolysis of A. anguilla muscle proteins and its protein isolate generated various protein hydrolysates dependent on the crude proteases used (Purafect, bacterial proteases or goby fish viscera proteases). The functional and antioxidant properties of the derivate peptides depend on their molecular weight and hydrophobic character. Protein hydrolysates revealed important techno-functional properties and biological activities. Therefore, the higher stability of maltodextrin-muscle protein hydrolysates explains their well electrosprayability. In fact, MPH-S-MD had the highest stability by the CI and zeta potential analysis explaining their best microcapsules formed compared to PIH-S-MD and approving the strong interaction between MD and muscle protein hydrolysates (MPH) which favored chain entanglements forming microcapsules.
References
Adler-Nissen J (1986) A review of food hydrolysis specific areas. In: Adler-Nissen J (ed) Enzymic hydrolysis of food proteins. Elsevier Applied Science Publishers, Copenhagen, pp 57–109
Ahn CB, Cho YS, Je JY (2015) Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem 168:151–156
AOAC (2000) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Washington, DC
Ben Salem I, Sghaier H, Trifi H, Héni S, Khwaldia K, Saidi M, Landoulsi A (2011) Isolation and characterization of a novel Micrococcus strain for bioremediation of strontium inradioactive residues. Afr J Microbiol Res 6:851–858
Berghe VA, Vlieinck AJ (1991) Screening methods for antibacterial and antiviral agents from higher plants. Method Plant Biochem 6:47–68
Bersuder P, Hole M, Smith G (1998) Antioxidants from a heated histidine–glucose model system I. Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Soc 75:181–187
Betty M, Awuor OL, Kirwa ME, Jackim MF (2014) Antioxidative and functional properties of Rastrineobolaargentea (Dagaa) fish protein hydrolysate. Discourse Int J Agric Food Sci 2:180–189
Chi C, Cao Z, Wang B, Hu F, Li Z, Zhang B (2014) Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. J Mol 19:11211–11230
Chronakis IS (2010) Micro-/nano-fibers by electrospinning technology: processing, properties and applications. Micro Manufact Eng Technol:264–286
Decker EA, Welch B (1990) Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem 38:674–677
Desai KGH, Park HJ (2005) Recent developments in microencapsulation of food ingredients. Dry Technol 23:1361–1394
Elavarasan K, Naveen K, V., & Shamasundar, B. A. (2014) Antioxidant and functional properties of (FPH) from fresh water carp (Catlacatla) as influenced by the nature of enzyme. J Food Process Preserv 38:1207–1214
Galla NR, Pamidighantam PR, Akula S, Karakala B (2012) Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channastriatus and Labeohorita. J Food Chem 135:1479–1484
Gbogouri GA, Linder M, Fanni J, Parmentier M (2004) Influence of hydrolysis degree on the functional properties of salmon by products hydrolysates. J Food Sci 69:615–622
Geirsdottir M, Sigurgisladottir S, Hamaguchi PY, Thorkelsson G, Johannsson R, Kristinsson HG, Kristjansson MM (2010) Enzymatic hydrolysis of blue whiting (Micromesistius poutassou); functional and bioactive properties. J Food Sci 76:14–20
Halpin-Dohnalek MI, Marth EH (1989) Growth and production of enterotoxin A by Staphylococcus aureus in cream. J Dairy Sci 72:2266–2275
Hammami A, Hamdi M, Abdelhedi O, Jridi M, Nasri M, Bayoudh A (2016) Surfactant- and oxidant-stable alkaline proteases from Bacillus invictae: characterization and potential applications in chitin extraction and as a detergent additive. Int J Biol Macromol 96:272–281
Iglewski BH (1996) Chapter 27: Pseudomonas. In: Baron S (ed) Medical microbiology, 4th edn. University of Texas Medical Branch atfdéz Galveston, Galveston
Jafari SM, Assadpoor E, He Y, Bhandari B (2008) Encapsulation efficiency of foodflavours and oils during spray drying. Dry Technol 26:816–835
Kembhavi A, Kulkarni A, Pant A (1993) Salt-tolerant and thermostable alkaline proteases from Bacillus subtilis NCIM no. 64. J Appl Biochem Biotechnol 38:83–92
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroidesleptolepis) as influenced by the degree of hydrolysis and enzyme type. J Food Chem 102:1317–1327
Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. J Phytochem Anal 13:8–17
Kristinsson HG, Rasco BA (2000) Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J Agric Food Chem 48:657–666
Lajmi K, Gómez-Estacac J, Hammami M, Martínez-Alvarez O (2019) Upgrading collagenous smooth hound by-products: effect of hydrolysis conditions, in vitro gastrointestinal digestion and encapsulation on bioactive properties. Food Biosci 28:99–108
Lassoued I, Mora L, Nasri R, Aydi M, Toldrá F, Aristoy MC, Nasri M (2015) Characterization, antioxidative and ACE inhibitory properties of hydrolysates obtained from thornback ray (Raja clavata) muscle. J Proteome 128:458–468
Li Z, Wang B, Chi C, Gong Y, Luo H, Ding G (2013) Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatisakjei and Raja porosa. J Food Res Int 51:183–193
Lim LT, Mendes AC, Chronakis IS (2019) Electrospinning and electrospraying technologies for food applications. Adv Food Nutr Res:1–68
Lin MJY, Humbert ES, Sosulski FW (1974) Certain functional properties of sunflower meal products. J Food Sci 39:368–370
López-Córdoba A, Lagarón JM, Goyanes S (2016) Fabrication of electrospun and electrosprayed carriers for the delivery of bioactive food ingredients. Food Sci 1:–6
Mac-Connel AA, Eastwood A, Mitchell WD (1974) Physical characterization of vegetable foodstuffs that could influence bowel function. J Sci Food Agric 25:1457–1464
Mahmoud MI, Malone WT, Cordle CT (1992) Enzymatic hydrolysis of casein: effect of degree of hydrolysis on antigenicity and physical properties. J Food Sci 57:1223
Martínez-Alvarez O, Batista I, Ramos C, Montero P (2016) Enhancement of ACE and prolyl oligopeptidase inhibitory potency of protein hydrolysates from sardine and tuna by-products by simulated gastrointestinal digestion. Food Funct 7:2066–2073
Mohan A, Rajendran SRCK, He QS, Bazinet L, Udenigwe CC (2015) Encapsulation of food protein hydrolysates and peptides: a review. RSC Adv 5:79270–79278
Morales-Medina R, Tamm F, Guadix AM, Guadix EM, Drusch S (2016) Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem 194:1208–1216
Nalinanon S, Benjakul S, Kishimura H, Shahidi F (2011) Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem 124:1354–1362
Nasri R, Chataigné G, Bougatef A, Chaâbouni MK, Dhulster P, Nasri M, Nedjar-Arroume N (2013) Novel angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysates of goby (Zosterisessorophiocephalus) muscle proteins. J Proteome 91:444–452
Nasri R, Jridi M, Lassoued I, Jemil I, Ben Slama-Ben Salem R, Nasri M, Karra-Châabouni M (2014) The influence of the extent of enzymatic hydrolysis on antioxidative properties and ACE-inhibitory activities of protein hydrolysates from goby (Zosterisessor ophiocephalus) muscle. J Appl Biochem Biotechnol 173:1121–1134
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26:716–723
Pérez-Masiá R, Lagaron JM, López-Rubio A (2014) Development and optimization of novel encapsulation structures of interest in functional foods through electrospraying. Food Bioprocess Technol 7:3236–3245
Pires C, Teixeiraa B, Cardosoa C, Mendesa R, Nunes ML, Batista I (2015) Cape hake protein hydrolysates prepared from alkaline solubilised proteins pre-treated with citric acid and calcium ions: functional properties and ACE inhibitory activity. J Pro Biochem 50:1006–1015
Qian ZJ, Jung QK, Kim SK (2008) Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Ranacatesbeiana Shaw. J Biores Technol 99:1690–1698
Sanchez CC, Patino JMR (2005) Interfacial, foaming and emulsifying characteristics of sodium caseinate as influenced by protein concentration in solution. J Food Hydrocoll 19:407–416
Schmitt C, Turgeon SL (2011) Protein/polysaccharide complexes and coacervates in food systems. Adv Colloid Interf Sci 167:63–70
Shahidi F, Han XQ, Synowiecki J (1995) Production and characteristics of protein hydrolysates from capelin (Mallotusvillosus). J Food Chem 53:285–293
Sripokar P, Benjakul S, Klomklao S (2019) Antioxidant and functional properties of protein hydrolysates obtained from starry triggerfish muscle using trypsin from albacore tuna liver. Biocatal Agric Biotechnol 17:447–454
Taghvaei M, Jafari SM, Mahoonak AS, Nikoo AM, Rahmanian N, Hajitabar J, Meshginfar N (2014) The effect of natural antioxidants extracted from plant and animalresources on the oxidative stability of soybean oil. LWT Food Sci Technol 56:124–130
Taheri A, Anvar SAA, Ahari H, Fogliano V (2013) Comparison the functional properties of protein hydrolysates from poultry by-products and rainbow trout (Onchorhynchus mykiss) viscera. Iran J Fish Sci 12:154–169
Taktak W, Nasri R, Hamdi M, Gomez-Mascaraque L, Rubio AL, Li S, Nasri M, Chaâbouni MK (2018) Physicochemical, textural, rheological and microstructural properties of protein isolate gels produced from European eel (Anguilla anguilla) by heat-induced gelation process. Food Hydrocoll 82:278–287
Tanuja S, Viji P, Zynudheen AA, Joshy CG (2012) Composition, functional properties and antioxidative activity of hydrolysates prepared from the frame meat of striped catfish (Pangasianodonhypophthalmus). Egypt J Biol 14:27–35
Tsumura K, Saito T, Tsuge K, Ashida H, Kugimiya W, Inouye K (2005) Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT Food Sci Technol 38:255–261
Van der Ven C, Gruppen H, de Bont DBA, Voragen AGJ (2002) Optimization of the angiotensin converting enzyme inhibition by whey protein hydrolysates using response surface methodology. Int Dairy J 12:813–820
Wen P, Zong MH, Linhardt RJ, Feng K, Wu H (2017) Electrospinning: a novel nano-encapsulation approach for bioactive compounds. Trends Food Sci Technol
Wiriyaphan C, Xiao H, Decker EA, Yong Sawatdigul J (2015) Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi by product hydrolysates fractionated by ultrafiltration. J Food Chem 167:7–15
Wu HC, Chen HM, Shiau CY (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomberaustriasicus). J Food Res Int 36:949–957
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumexcrispus L. extracts. J Agric Food Chem 49:4083–4089
Acknowledgements
This work was funded by the Ministry of Higher Education and Scientific Research-Tunisia. Authors would also like to thank the Central Support Service for Experimental Research (SCSIE) of the University of Valencia for the electronic microscopy service.
Funding
This study was sponsored by the Ministry of Higher Education and Scientific Research- Tunisia. Authors would also like to thank the Central Support Service for Experimental Research (SCSIE) of the University of Valencia for the electronic microscopy service.
Author information
Authors and Affiliations
Contributions
Wafa Taktak conceived of the study, formulated its design, coordinated the conduct of the study including data collection, performed the statistical analysis and interpreted the data, and drafted the manuscript; Marwa Hamdi and Laura G. Gomez-Mascaraque participated in the design of the study and helped to draft the manuscript; Rim Nasri participated in the design of the study, assisted in interpreting the data, and helped to draft the manuscript. Maha Karra-Chaâbouni, Rim Nasri and Moncef Nasri are the supervisor who read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical Approval
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Research Involving Human and/or Animal Rights
This article does not contain any studies involving humain or animals performed by any of the authors.
Informed Consent
Informed Consent Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taktak, W., Nasri, R., López-Rubio, A. et al. Enzymatic Production of Novel European Eel Proteins Hydrolysates: Biological Activities, Techno-Functional Properties and Maltodextrin-Hydrolysates Efficient Electrosprayability. Int J Pept Res Ther 27, 1129–1148 (2021). https://doi.org/10.1007/s10989-020-10156-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10156-x