Abstract
Oxidative stress is one of the factors associated with decline in fertility and betaine has been shown to bear antioxidant and methyl donor properties in our recent studies. Thus, we designed the present study to examine antioxidant and methyl donor abilities of betaine in oxidative stress induced by ethanol in the rat testes. The adult male Sprague-Dawley rats were divided into four experimental groups and treated daily for 2 months as follows: control, ethanol (4 g/kg, orally), betaine (1.5 % of total diet, orally), and betaine plus ethanol (betaine, 1.5 % of total diet and after 120 min, ethanol 4 g/kg). Sperm motility and concentration significantly increased in betaine group when compared to the ethanol–treated rats. The main antioxidant enzyme (GPx) activity significantly increased (in order compensatory) in ethanol-treated rats when compared to betaine group while, antiperoxidative enzyme (CAT) activity significantly increased in betaine plus ethanol group as compared to ethanol-treated rats. Total homocysteine (tHcy) and TBARS concentration (as a lipid peroxidation marker) also significantly decreased in betaine and betaine plus ethanol groups as compared to ethanol-treated rats. Overall, methyl donor and antioxidant properties of betaine are promising and reduce the elevated tHcy and TBARS concentrations in betaine plus ethanol group. Therefore, betaine might be used as a potential therapy in hyperhomocysteinemia and oxidative stress induced by ethanol in alcoholism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic ethanol consumption causes sexual dysfunction and impaired sperm production in humans and in animal models (Wallock-Montelius et al. 2007), and it negatively affects all levels of the male hypothalamic–pituitary–gonadal axis (Van Thiel 1983; Emanuele and Emanuele 2001; Wallock-Montelius et al. 2007). Proposed mechanisms of ethanol-induced testicular damage include oxidative stress (Turner and Lysiak 2008; Alirezaei et al. 2012), changes in nitric oxide or opioid metabolism, and defective steroid hormone metabolism secondary to altered liver metabolism (Emanuele and Emanuele 2001; Wallock-Montelius et al. 2007). Chronic alcoholism and its associated liver disease are frequently coincident with circulating and hepatic indicators of folate deficiency (Herbert et al. 1963; Leevy et al. 1965; Eichner and Hillman 1971; Wu et al. 1975; Cravo et al. 1996; Wallock-Montelius et al. 2007; Alirezaei et al. 2010, 2011b). In addition to low dietary intake, folate deficiency in chronic alcoholism can be attributed to secondary causes such as decreased intestinal absorption, decreased hepatic uptake, and increased renal excretion (Halsted et al. 1971; Romero et al. 1981; Tamura et al. 1981; Tamura and Halsted 1983; McMartin et al. 1989; Alirezaei et al. 2010, 2011b). Chronic alcoholism reduces hepatic methionine synthase activity in the rat (Wallock-Montelius et al. 2007; Alirezaei et al. 2010, 2011b), since folate deficiency limits the availability of substrate 5-methyltetrahydrofolate for hepatic methionine synthase activity, and leads to other disturbances in methionine metabolism (Halsted et al. 2002; Villanueva and Halsted 2004; Wallock-Montelius et al. 2007; Alirezaei et al. 2010, 2011b). The effects of ethanol feeding on hepatic methionine synthase activity are attenuated by the presence of the accessory enzyme, betaine-homocysteine methyltransferase (BHMT; E.C. 2.1.1.5), which remethylates homocysteine using the substrate betaine (Finkelstein 2007; Alirezaei et al. 2010, 2011a, b).
Betaine (N,N,N-trimethylglycine) is an important methyl donor in one-carbon metabolism, where BHMT the only known enzyme which uses betaine as a substrate, mediates the transfer of a methyl group from betaine to homocysteine, forming methionine and N,N-dimethylglycine (Sunden et al. 1997; Millian and Garrow 1998; Slow et al. 2009; Alirezaei et al. 2011b). Ethanol feeding can affect several hepatic enzymes in animals, including decreasing methionine synthase activity (Barak et al. 2002; Alirezaei et al. 2011b). This leads to increased BHMT activity to maintain hepatic S-adenosyl methionine (SAM) at normal concentrations (Craig 2004; Alirezaei et al. 2010, 2011a, b). BHMT activity plays a key role in regulating betaine concentrations and determines the fate of betaine between its two competing biological functions; stored to control cellular osmolarity or metabolized to provide a methyl group for homocysteine methylation (Slow et al. 2009; Alirezaei et al. 2010, 2011b). However, BHMT expression is influenced by various hormones, including corticosteroids, insulin, estradiol and testosterone (Finkelstein et al. 1971; Ratnam et al. 2006; Slow et al. 2009) (Supplementary file).
Regarding to male fertility, the high level of betaine was observed in the testes is interesting (Slow et al. 2009). There is evidence to suggest that betaine, acting as a methyl donor, may have an important role in maintaining male fertility (Kelly et al. 2005; Slow et al. 2009). It seems that metabolism of betaine via BHMT can ultimately lead to the production of SAM, which is crucial in the testes for the synthesis of creatine, that is important for sperm motility and function (Lee et al. 1998; Kelly et al. 2005). To the best of our knowledge, the members of the solute carrier 6 family, responsible for betaine transport have been identified within the testis (Chen et al. 2004). Therefore, it is possible that specific cell types within testis such as sertoli cells produce BHMT activity. In this regards, many studies demonstrated that Sertoli cells within testes express BHMT mRNA, and activity (Edgar et al. 2002; Slow et al. 2009), and therefore, this indicates the possibility that at least some of the accumulated betaine in the testes could be utilized as a source of methyl groups, which may contribute to testicular creatine production and thus aid in maintaining sperm motility and function (Slow et al. 2009).
In light of these reports one could hypothesize that betaine might increase antioxidant status of the testis, subsequently promote parameters of sperm motility and concentration in ethanol-induced oxidative stress model. Thus, we undertook this study to examine whether exogenous betaine could exert its antioxidant and methyl donor effects on the rat testes.
Materials and Methods
Materials
GPx and SOD kits were obtained via Randox ® Company (Randox, UK). Alcohol (ethanol 95 %) and thiobarbituric acid (TBA) were supplied from Merck Chemical Company (KGaA, Darmstadt, Germany). Betaine (Betafin® 96 %) was obtained from Biochem Company (Brinkstrasse 55, D-49393 Lohne, Germany). The homocysteine kit was prepared by Axis® Homocysteine EIA (Axis-Shield AS, Germany). All chemicals used were of analytical grade.
Animals and Experimental Design
A total of 32 adult male Sprague-Dawley rats (weighting 220–250 g, supplied from Animal House Center, Shiraz, Iran) were housed in temperature-controlled conditions under a 12:12-h light/dark photocycle with food and tap water supplied ad libitum and weight gain and food consumption were determined at weekly intervals. All rats were treated humanely and in compliance with the recommendations of Animal Care Committee for the Shiraz University of Medical Sciences (Shiraz, Iran). The rats were divided into four equal groups. Ethanol 4 g/kg (soluble in distilled water) was administrated daily in ethanol group and the same volume of vehicle was given for the controls. Betaine group received betaine 1.5 % w/w of the total diet (soluble in distilled water) and betaine plus ethanol group received betaine, 1.5 % w/w of the total diet, and after 120 min, feeding with ethanol solution (4 g/kg). All of the treatment applied orally by stomach tube for 2 months and all of experimental procedures were carried out between 10.00 and 12.00 a.m. for prevention of circadian rhythm changes among days. Doses of ethanol and betaine were determined according to the previous studies (Ji and Kaplowitz 2003; Song et al. 2003; Alirezaei et al. 2011a, 2012). One day after the last gavage, the rats were sacrificed upon light diethyl ether anesthesia (Dagenham, UK) by decapitation. Immediately after rat killing, blood samples were collected via cardiac puncture, whole blood containing EDTA was centrifuged at 3,000×g for 5 min and plasma was prepared in microtubes. The right testes were removed and carefully cleaned of adhering, and then the right cauda epididymis in all groups was separated for measurement of sperm motility and concentration. Right testes and plasma samples were stored at −70 °C until antioxidant and tHcy analysis.
Sperm Evaluation
Rat spermatozoa were obtained by the method as described previously (Cancel et al. 2000; Alirezaei et al. 2012). In brief, 5 mm of right cauda epididymis was minced in 2 ml of physiological saline and incubated at 37 °C for 45 min to allow dispersion of spermatozoa. The obtained spermatozoa from the four groups were assessed for concentration and total sperm motility (TSM). The concentration of spermatozoa was determined after adding of 50 μl of sperm into the 1 ml of formalin–saline to achieve the dilution rate of 1:20. 10 μl of the diluted sperm suspension was transferred to each counting chamber of the haemocytometer and the total number of spermatozoa per ml was counted using light microscope (Kheradmand et al. 2009b; Alirezaei et al. 2012). The TSM (cells showing any kind of movement) was assessed according to the method as described previously (Sonmez et al. 2005; Alirezaei et al. 2012). The fluid obtained from cauda epididymis was diluted to 2 ml with PBS and an aliquot of this suspension was placed on the microscope slide covered with a cover slip and examined visually under a light microscope (Olympus, CX-31, Philippine) at the magnification of ×400. Motility estimations were performed from four different fields in each sample and the mean of the four estimations was used as the final motility score. Samples for motility evaluation were kept at 37 °C. All of the above examinations were performed by a same person with counting of at least 200 spermatozoa.
Measurement of tHcy Concentration
Plasma total homocysteine (tHcy), which refers to the sum of protein-bound, free-oxidized, and reduced species of homocysteine in plasma, was determined by the Axis® Homocysteine EIA kit (Golbahar et al. 2005; Karthikeyan et al. 2007; Alirezaei et al. 2011a, b). The sample volume used was 25 μl. Absorbance was measured at a wavelength of 450 nm using an ELISA reader (STAT FAX 2100, USA). All estimations were performed in duplicate and the intraassay coefficient of variation was <10 % and the detection limit of the tHcy assay was 2.0 μM. The tHcy results were expressed as micromole per liter of plasma (μmol/l).
Tissue Preparation
Right testes were thawed and manually homogenized using liquid nitrogen in cold phosphate buffer (0.1 M, pH 7.4, containing 5 mM EDTA), and debris were removed by centrifugation at 3,500×g for 10 min (Centrifuge 5415 R; Rotofix 32A, Germany) (Kheradmand et al. 2009a). Supernatants were recovered and used for antioxidant enzyme activities, lipid peroxidation level and protein measurement. Protein content of tissue homogenates was determined using a colorimetric method of Lowry with bovine serum albumin as a standard (Lowry et al. 1951).
Measurement of Glutathione Peroxidase (GPx) Activity
The activity of GPx was evaluated with Randox GPx detection kit according to the manufacturer’s instructions, as described previously (Alirezaei et al. 2011a, 2012). GPx catalyse the oxidation of glutathione (GSH) by cumene hydroperoxide. In the presence of glutathione reductase and NADPH, the oxidised glutathione is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance was measured spectrophotometrically (S2000 UV model; WPA, Cambridge, UK) against blank at 340 nm. One unit (U) of GPx was defined as l μmol of oxidized NADPH per min per milligram of tissue protein. The GPx activity was expressed as unit per milligram of tissue protein (mU/mg protein).
Measurement of Superoxide Dismutase (SOD) Activity
The activity of superoxide dismutase (SOD) was evaluated with Randox SOD detection kit according to the manufacturer’s instructions, as described previously (Kheradmand et al. 2009a; Alirezaei et al. 2012). The role of SOD is to accelerate the dismutation of the toxic superoxide (O2 −) produced during oxidative energy processes to hydrogen peroxide and molecular oxygen. This method employs xanthine and xanthine oxidase to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazan dye. The SOD activity is then measured by degree of inhibition of this reaction. One unit of SOD is that which causes 50 % inhibition of the rate of reduction of INT under the conditions of the assay. SOD levels were recorded at 505 nm and through a standard curve and expressed as unit per milligram of tissue protein (U/mg protein).
Measurement of CAT Activity
Tissue catalase activity was assayed using the method described by Claiborne (1985), was reported by Kheradmand et al. (2010). The reaction mixture (1 ml) consisted of 50 mM potassium phosphate (pH 7.0), 19 mM H2O2, and a 20–50 μl sample. The reaction was initiated by the addition of H2O2 and absorbance changes were measured at 240 nm (25 °C) for 30 s. The molar extinction coefficient for H2O2 is 43.6 M/cm. The CAT activity was expressed as the unit that is defined as μmol of H2O2 consumed per min per milligram of tissue protein (U/mg protein).
Measurement of Lipid Peroxidation
The amount of lipid peroxidation was indicated by the content of thiobarbituric acid reactive substances (TBARS) in the testis. Tissue TBARS determined by following the production of TBA reactive substances as described previously (Subbarao et al. 1990), was reported by Alirezaei et al. (2011a). In short, 40 μl of homogenate was added to 40 μl of 0.9 % NaCl and 40 μl of deionized H2O, resulting in a total reaction volume of 120 μl. The reaction was incubated at 37 °C for 20 min and stopped by the addition of 600 μl of cold 0.8 M hydrochloride acid, containing 12.5 % trichloroacetic acid. Following the addition of 780 μl of 1 % TBA, the reaction was boiled for 20 min and then cooled at 4 °C for 1 h. In order to measure the amount of TBARS produced by the homogenate, the cooled reaction was spun at 1,500×g in a microcentrifuge for 20 min and the absorbance of the supernatant was spectrophotometrically read at 532 nm, using an extinction coefficient of 1.56 × 105/M cm. The blanks for all of the TBARS assays contained an additional 40 μl of 0.9 % NaCl instead of homogenate as just described. TBARS results were expressed as nanomol per milligram of tissue protein (nmol/mg protein).
Statistical Analysis
All results are presented as mean ± (S.E.M.). The statistical differences were applied among the all groups by one-way analysis of variance with Tukey’s post hoc analysis. A calculated P value of <0.05 was considered statistically significant. Previously, all variables were tested for normal and homogeneous variances by Leven’s statistic test. Statistical analysis was performed using the statistical package SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA).
Results
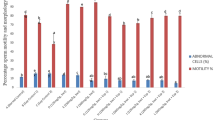
The mean values (± SEM) of the TSM (%) and sperm concentration of the four groups of rats are presented in Figs. 1 and 2. TSM was significantly lower in the ethanol group compared to the other groups, and betaine significantly increased sperm motility in betaine-treated group (P < 0.05). Sperm concentration was significantly higher in the betaine group compared to the other groups (P < 0.05), and although sperm concentration in pretreated rats with betaine was higher than to ethanol-treated rats, this enhancement was not statistically significant (P > 0.05).
In order to clarify the methyl donor effect of betaine, we measured plasma tHcy concentration. Treatment of rats with ethanol significantly increased tHcy concentration in plasma of the ethanol group compared to the betaine-treated groups. Indeed, administration of betaine to the betaine and betaine plus ethanol groups could prevent increase of tHcy concentration (P < 0.05; Fig. 3).
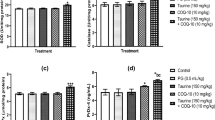
To evaluate antioxidant effects of betaine, the activities of main antioxidant enzymes including GPx, SOD, and CAT as well as TBARS concentration in the rat testicular tissue were measured (Figs. 4, 5, 6, 7). Administration of betaine in betaine and betaine plus ethanol groups significantly decreased GPx activity compared to the control and ethanol groups (P < 0.05). In contrast, SOD activity decreased insignificantly in ethanol and betaine groups compared to controls (P > 0.05). CAT activity was insignificantly higher in the betaine group compared to the ethanol group (P > 0.05). Indeed, when betaine administered prior to ethanol, it could significantly increase the CAT activity in comparison with ethanol-treated rats (P < 0.05). Treatment of rats with ethanol significantly increased lipid peroxidation (as shown by the TBARS concentration), while pretreatment of rats with betaine could suppress TBARS concentration (P < 0.05). In addition, TBARS concentrations in betaine and control group were significantly lower compared to ethanol-treated rats (P < 0.05; Fig. 7).
Discussion
The present study indicated that betaine consumption suppresses oxidative stress as monitored by elevation of sperm motility and concentration and decreasing in lipid peroxidation products in the testis, and plasma tHcy concentration of rats. Our results demonstrate that ethanol induces oxidative stress and enhances GPx activity (in order compensatory). In this context, Masella et al. (2004), have been expressed that “antioxidant responsive elements (AREs) are present in the promoter regions of many of the genes inducible by oxidative and chemical stresses”. Thus, it appears that in the present study consumption of ethanol (as an oxidative inducing agent) was able to increase activity of the antioxidant enzymes such as GPx by the compensatory mechanism via AREs. In this regard, previous studies showed that ethanol could enhance GPx activity in the kidney (Dinu et al. 2006), cerebellum (Alirezaei et al. 2011a) and testes of rats (Alirezaei et al. 2012) as well as increase in GSH-content in the developing cerebellum of neonatal rats (Smith et al. 2005). Thus, our results support and extend previous report suggesting that ethanol intoxication generally impairs the testicular antioxidant defense system and induces lipid peroxidation in experimental animals (Kasdallah-Grissa et al. 2006; Alirezaei et al. 2012). The observation for sperm kinematic parameters, antioxidant enzyme activities, TBARS level and tHcy concentration in the betaine-treated rats supports the idea that betaine is associated with antioxidant and methyl donor properties through its involvement in homocysteine remethylation and cell membrane stabilization (Ganesan et al. 2009; Alirezaei et al. 2011a, b).
During the past decade, there has been a growing body of evidence that even a moderately elevated serum homocysteine concentration is associated with an increased risk of ageing-related diseases, such as atherosclerotic, thromboembolic and neurodegenerative disorders (Bottiglieri 2005; Forges et al. 2007; Sher et al. 2007; Kim et al. 2008; Bidulescu et al. 2009; Alirezaei et al. 2011a). In this sense, hyperhomocysteinemia is associated with the production of ROS in endothelial and smooth muscle cells (Dinu et al. 2006; Alirezaei et al. 2010, 2011a, b). The mechanism of this oxidative stress returns to auto-oxidation of the highly reactive thiol group of homocysteines (Forges et al. 2007) and the formation of intracellular superoxide and peroxyl radicals with concomitant inhibition of cellular antioxidant enzymes, such as SOD and GPx (Forges et al. 2007; Alirezaei et al. 2010, 2011a, b). Homocysteine plays a crucial role in a shared biochemical cascade involving overstimulation of N-methyl-d-aspartate receptors, oxidative stress, activation of caspases, DNA damage, endoplasmic reticulum and mitochondrial dysfunction (Bleich et al. 2004; Alirezaei et al. 2010, 2011a, b). Auto-oxidation of homocysteine is known to generate ROS, whereby the prevention of homocysteine-induced toxicity by catalase suggests that hydrogen peroxide acted as a mediator of oxidative injury, leading to oxidative stress (Alirezaei et al. 2011a). In this regard, in the present study only catalase activity was insignificant higher in the betaine group compared to the ethanol group. Indeed, catalase prevented H2O2 accumulation and increased its activity in the betaine plus ethanol group. It seems, induction of oxidative stress by ethanol metabolites, subsequently AREs activity (Alirezaei et al. 2011a) with concomitant scavenging of ROS by betaine could increase significantly catalase activity in betaine plus ethanol group compared to the ethanol group.
Oxidative stress is one of the factors associated with decline in fertility of spermatozoa (Turner and Lysiak 2008; Alirezaei et al. 2012). The sperm plasma membrane contains a high amount of unsaturated fatty acids and therefore is particularly susceptible to peroxidative damages with subsequent loss of membrane integrity, impaired cell function and decreased motility of spermatozoa (Aurich et al. 1997; Ball et al. 2001; Alirezaei et al. 2012). Lifestyle choices such as excessive alcohol consumption or cigarette smoking increase free-radical production in all tissues and have on multiple occasions been associated with male infertility or with conditions contributing to that infertility (Peltola et al. 1994; Wu and Cederbaum 2003; Turner and Lysiak 2008; Alirezaei et al. 2012). Although testis is endowed with sufficient antioxidant defenses (Bauché et al. 1994; Turner and Lysiak 2008; Alirezaei et al. 2012), previous studies have shown altered steroidogenesis as a consequence of increased oxidative stress with several xenobiotics and alcohol in the testis (Peltola et al. 1996; Doreswamy et al. 2004; Alirezaei et al. 2012). Various xenobiotics are known to be metabolized by cytochrome P450 enzymes, radical metabolites or ROS are known to be produced and altered steroidogenesis by itself can generate free radicals. Hence, even slight alterations in ROS levels and their detoxification can substantially affect the spermatogenetic process since germ cells are more susceptible to peroxidative damages (Hemachand and Shaha 2003; Das and Vasudevan 2007; Alirezaei et al. 2012). The results of our study showed that betaine elevated sperm motility and concentration and this enhancement is possibly due to the antioxidant and methyl donor effects of betaine which caused higher motility score in the spermatozoa. It appears the production of SAM from betaine supplementing, has a crucial role in the testes for the synthesis of creatine and affects on sperm motility (Lee et al. 1998; Slow et al. 2009). These findings confirm a fact that oxidative stress is a consistent feature of testicular physiology (Kheradmand et al. 2009a; Alirezaei et al. 2012). Our data are in accordance with previously described detrimental effects of ethanol on sperm characteristics in the treated rats (Emanuele and Emanuele 1998; Srikanth et al. 1999; Alirezaei et al. 2012).
Herein, a significant elevation in the concentration of tHcy was noted in the plasma of the ethanol group compared to the other groups (Fig. 3). The toxic accumulation of homocysteine may cause reproductive dysfunction and oxidative stress within the testis. Hyperhomocysteinemia usually occurs due to suboptimal remethylation of homocysteine to methionine by the enzyme methyl tetrahydrofolate reductatse (MTHFR) caused by a dietary deficiency of folate or a single nucleotide polymorphism in the MTHFR gene (Tremellen 2008). The suggestion that chronic ethanol consumption might interfere with homocysteine remethylation was first raised by Barak and Beckenhauer (1988), and also with chronic ethanol consumption in our recent studies (Alirezaei et al. 2010, 2011a, b). Although, in the present study we were unable to measure dimethylglycine for detection of BHMT activity in contrast, other studies have shown that feeding alcohol or methionine to rats significantly reduce the activity of methionine synthase followed by an increase in BHMT activity to maintain adequate tissue levels of SAM (Barak et al. 1985; Barak and Beckenhauer 1988; Finkelstein 2007; Alirezaei et al. 2010, 2011a, b). In this sense, methionine synthase activity was substantially higher in testis than that observed in liver of micropigs (Wallock-Montelius et al. 2007), suggesting that methionine metabolism may be especially important in this tissue. Since methionine metabolism is linked to sulfur-amino acid metabolism via the disposal of homocysteine, it may be involved with glutathione-mediated antioxidant protection to epididymal spermatozoa (Wallock-Montelius et al. 2007).
To the best of our knowledge, this study is the first in vivo experiment to show that betaine treatment results in an overall increase sperm motility and concentration in rats. Betaine is a methylating agent like SAM and it also stabilizes SAM levels via BHMT pathway (Bottiglieri 2005; Alirezaei et al. 2010, 2011a, b). Therefore, betaine may have an antioxidant effect against oxidative damage in testis. In addition, betaine may have some advantages than endogenous SAM application. Because, SAM application enhances the levels of homocysteine, which is undesirable due to the toxicity of this amino acid, whereas betaine treatment decreases homocysteine levels by directly inducing the remethylating process, which transforms homocysteine into methionine (Kanbak et al. 2008; Alirezaei et al. 2011a, b). Therefore, the beneficial properties of betaine are promising and reduce the elevated plasma homocysteine concentrations via the BHMT pathway in betaine-treated groups as compared to ethanol-treated rats (Finkelstein 2007; Bidulescu et al. 2009; Alirezaei et al. 2011a, b).
It is well known that all cells are able to defend themselves from damaging effects of oxygen free radicals by way of their own antioxidant mechanisms, including enzymatic and non-enzymatic antioxidant systems (Neamati et al. 2011; Alirezaei et al. 2012). GPx and CAT are two key antioxidant enzymes that can decompose hydrogen peroxide to water (Turner and Lysiak 2008; Alirezaei et al. 2011a, 2012; Kheradmand et al. 2010; Neamati et al. 2011). SOD, another antioxidant enzyme in cells rapidly converts superoxide anion (O2−) to less dangerous hydrogen peroxide (H2O2) and then GPx and CAT can decompose H2O2 to water (Kheradmand et al. 2009a, 2011; Alirezaei et al. 2011a, 2012). Although, H2O2 is not a particularly reactive product, it may be reduced to the highly reactive metabolites hydroxyl radicals and/or single oxygen (Peltola et al. 1992). In this sense, antioxidants play a critical role in limiting the propagation of free radical reactions, which would otherwise result in extensive lipid peroxidation (Sehirli et al. 2008; Alirezaei et al. 2011a, 2012). In the present study, betaine caused significant decrease in the activities of GPx and CAT in betaine-treated group in comparison with controls, whereas ethanol treatment increased antioxidant enzyme activities (in order compensatory) and TBARS concentration due to ROS production. On one hand, chronic ethanol feeding has caused an up-regulation of the GPx at the mRNA level (Das and Vasudevan 2007), which seems to be a protective mechanism and on the other hand, homocysteine inhibited the expression of antioxidant enzymes despite the compensatory mechanism induced by ethanol (Alirezaei et al. 2011a; Bleich et al. 2004). However, SOD activity decreased insignificantly in betaine and betaine plus ethanol groups compared to the controls and SOD results was similar to a previous study (Ganesan et al. 2009). On the other hand, testicular antioxidants was not depleted in the betaine and betaine plus ethanol group, suggesting that betaine possessed antioxidant effects and preserved the cellular antioxidant stores (Alirezaei et al. 2011a).
As previously mentioned, administration of betaine has been shown to exert a significant role within tissue as a methyl donor, which in turn may be used for the synthesis of methionine, carnithine, phosphatidylcholine, creatine, and these substances play a key role in protein and energy metabolism in the cells (Craig 2004; Alirezaei et al. 2011a, b). Betaine is believed to play a significant role in maintaining the structural and functional integrity of cell membranes. Previous studies have demonstrated that betaine through its participation in sequential methylation within the cellular membranes maintains the proper balance between phosphotidyl ethanolamine and phosphotidyl choline, thus sustaining proper membranes (Ganesan et al. 2009; Alirezaei et al. 2011a, b). In the present study betaine, a methyl donor that continuously generates SAM is shown to lead to long-term lowering of plasma homocysteine during supplementation in the dietary intake range of 1.5 % (w/w). Furthermore, since humans produce little betaine from choline due to lack of choline oxidase (Haubrich and Gerber 1981), betaine is practical for investigations regarding the treatment of hyperhomocysteinemia and oxidative stress in alcoholism (Alirezaei et al. 2011a, b). However, protective effect of betaine against ethanol-induced oxidative stress observed in this study may also be associated with the restoration of SAM, which contributes to an increase in the supply of substrate needed for the synthesis of GSH that protects the cell from reactive metabolites and ROS (Ganesan et al. 2009; Alirezaei et al. 2011a). Taken together, these results and our previous reports suggest that betaine with its methyl donor and antioxidant properties might be used as a potential therapy in hyperhomocysteinemia and oxidative stress mediated by ethanol in alcoholism.
References
Alirezaei M, Saeb M, Javidnia K, Nazifi S, Khalighyan N, Saeb S (2010) Betaine reduction of hyperhomocysteinemia and enhancement of 5-hydroxyindoleacetic acid in ethanol-induced hyperhomocysteinemia in rabbits. Afr J Biochem Res 4(11):246–254
Alirezaei M, Jelodar G, Niknam P, Ghayemi Z, Nazifi S (2011a) Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J Physiol Biochem 67:605–612
Alirezaei M, Saeb M, Javidnia K, Nazifi S, Saeb S (2011b) Hyperhomocysteinemia reduction in ethanol-fed rabbits by oral betaine. Comp Clin Pathol. doi:10.1007/s00580-010-1110-6
Alirezaei M, Kheradmand A, Heydari R, Tanideh N, Neamati S, Rashidipour M (2012) Oleuropein protects against ethanol-induced oxidative stress and modulates sperm quality in the rat testis. Mediterr J Nutr Metab :1–7. doi:10.1007/s12349-011-0079-2
Aurich JE, Schonherr U, Hoppe H, Aurich C (1997) Effects of antioxidants on motility and membrane integrity of chilled-stored stallion semen. Theriogenology 48(2):185–192
Ball BA, Medina V, Gravance CG, Baumber J (2001) Effect of antioxidants on preservation of motility, viability and acrosomal integrity of equine spermatozoa during storage at 5 C. Theriogenology 56(4):577–589
Barak AJ, Beckenhauer HC (1988) The influence of ethanol on hepatic transmethylation. Alcohol Alcohol 23(1):73–77
Barak AJ, Beckenhauer HC, Tuma DJ (1985) Ethanol feeding inhibits the activity of hepatic N5-methyltetrahydrofolate: homocysteine methyltransferase in the rat. IRCS Med Sci 13:760–761
Barak AJ, Beckenhauer HC, Tuma DJ (2002) Methionine synthase a possible prime site of the ethanolic lesion in liver. Alcohol 26(2):65–67
Bauché F, Fouchard MH, Jégou B (1994) Antioxidant system in rat testicular cells. FEBS Lett 349(3):392–396
Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G (2009) Repeatability and measurement error in the assessment of choline and betaine dietary intake: the atherosclerosis risk in communities (ARIC) study. Nutr J 8(1):14–20
Bleich S, Degner D, Sperling W, Bönsch D, Thürauf N, Kornhuber J (2004) Homocysteine as a neurotoxin in chronic alcoholism. Prog Neuropsychopharmacol Biol Psychiatry 28(3):453–464
Bottiglieri T (2005) Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry 29(7):1103–1112
Cancel AM, Lobdell D, Mendola P, Perreault SD (2000) Objective evaluation of hyperactivated motility in rat spermatozoa using computer-assisted sperm analysis. Hum Reprod 15(6):1322
Chen NH, Reith ME, Quick MW (2004) Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch Eur J Physiol 447:519–531
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research, Vol 1. CRC Press, Boca Raton, Florida, USA, pp 283–284
Craig SA (2004) Betaine in human nutrition. Am J Clin Nutr 80(3):539–549
Cravo ML, Gloria LM, Selhub J, Nadeau MR, Camilo ME, Resende MP, Cardoso JN, Leitao CN, Mira FC (1996) Hyperhomocysteinemia in chronic alcoholism: correlation with folate, vitamin B-12, and vitamin B-6 status. Am Soc Nutr 63:220–224
Das SK, Vasudevan DM (2007) Alcohol-induced oxidative stress. Life Sci 81(3):177–187
Dinu D, Nechifor MT, Movileanu L (2006) Ethanol-induced alterations of the antioxidant defense system in rat kidney. J Biochem Mol Toxicol 19(6):386–395
Doreswamy K, Shrilatha B, Rajeshkumar T (2004) Nickel-induced oxidative stress in testis of mice: evidence of DNA damage and genotoxic effects. J Androl 25(6):103–996
Edgar R, Domrachev M, Lash AE (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acid Res 30(1):207–210
Eichner ER, Hillman RS (1971) The evolution of anemia in alcoholic patients. Am J Med 50(2):218–232
Emanuele MA, Emanuele NV (1998) Alcohol’s effects on male reproduction. Alcohol Health Res World 22:195–201
Emanuele MA, Emanuele N (2001) Alcohol and the male reproductive system. Alcohol Res Health 25(4):282–287
Finkelstein JD (2007) Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med 45(12):1694–1699
Finkelstein JD, Kyle WE, Harris BJ (1971) Methionine metabolism in mammals. Regulation of homocysteine methyltransferases in rat tissue. Arch Biochem Biophys 146(1):84–92
Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL (2007) Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update 13(3):225–239
Ganesan B, Buddhan S, Anandan R, Sivakumar R, Anbinezhilan R (2009) Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol Biol Rep 37(3):1319–1327
Golbahar J, Aminzadeh MA, Hamidi SA, Omrani GR (2005) Association of red blood cell 5-methyltetrahydrfoate folate with bone mineral density in postmenopausal Iranian women. Osteoporos Int 16(12):1894–1898
Halsted CH, Robles EA, Mezey E (1971) Decreased jejunal uptake of labeled folic acid (3H-PGA) in alcoholic patients: roles of alcohol and nutrition. N Eng J Med 285(13):701–706
Halsted CH, Villanueva JA, Devlin AM, Niemela O, Parkkila S, Garrow TA, Wallock LM, Shigenaga MK, Melnyk S, James SJ (2002) Folate deficiency disturbs hepatic methionine metabolism and promotes liver injury in the ethanol-fed micropig. Proc Natl Acad Sci USA 99(15):10072–10077
Haubrich DR, Gerber NH (1981) Choline dehydrogenase. Assay, properties and inhibitors. Biochem Pharmacol 30(21):2993
Hemachand T, Shaha C (2003) Functional role of sperm surface glutathione S-transferases and extracellular glutathione in the haploid spermatozoa under oxidative stress. FEBS Lett 538(1–3):14–18
Herbert V, Zalusky R, Davidson CS (1963) Correlation of folate deficiency with alcoholism and associated macrocytosis, anemia, and liver disease. Ann Intern Med 58(6):977–988
Ji C, Kaplowitz N (2003) Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124(5):1488–1499
Kanbak G, Arslan OC, Dokumacioglu A, Kartkaya K, Inal ME (2008) Effects of chronic ethanol consumption on brain synaptosomes and protective role of betaine. Neurochem Res 33(3):539–544
Karthikeyan G, Thachil A, Sharma S, Kalaivani M, Ramakrishnan L (2007) Elevated high sensitivity CRP levels in patients with mitral stenosis and left atrial thrombus. Int J Cardiol 122(3):252–254
Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El-Fazaa S (2006) Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol 41(3):236–239
Kelly TLJ, Neaga OR, Schwahn BC, Rozen R, Trasler JM (2005) Infertility in 5,10-methylenetetrahydrofolate reductase (MTHFR)-deficient male mice is partially alleviated by lifetime dietary betaine supplementation. Biol Reprod 72(3):667–677
Kheradmand A, Alirezaei M, Asadian P, Alavi ER, Joorabi S (2009a) Antioxidant enzyme activity and MDA level in the rat testis following chronic administration of ghrelin. Andrologia 41(6):335–340
Kheradmand A, Taati M, Babaei H (2009b) The effects of chronic administration of ghrelin on rat sperm quality and membrane integrity. Anim Biol 59(2):159–168
Kheradmand A, Alirezaei M, Birjandi M (2010) Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul Pept 162(1–3):84–89
Kheradmand A, Dezfoulian O, Tarrahi MJ (2011) Ghrelin attenuates heat-induced degenerative effects in the rat testis. Regul Pept 167:97–104
Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS (2008) Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry 192(4):268–274
Lee H, Kim JH, Chae YJ, Ogawa H, Lee MH, Gerton GL (1998) Creatine synthesis and transport systems in the male rat reproductive tract. Biol Reprod 58(6):1437–1444
Leevy CM, Baker H, Tenhove W, Frank O, Cherrick GR (1965) B-complex vitamins in liver disease of the alcoholic. Am J Clin Nutr 16:339–346
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Masella R, Vari R, D’Archivio M, Di Benedetto R, Matarrese P, Malorni W, Scazzocchio B, Giovannini C (2004) Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J Nutr 134(4):785–791
McMartin KE, Collins TD, Eisenga BH, Fortney T, Bates WR, Bairnsfather L (1989) Effects of chronic ethanol and diet treatment on urinary folate excretion and development of folate deficiency in the rat. J Nutr 119(10):1490–1497
Millian NS, Garrow TA (1998) Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch Biochem Biophys 356(1):93–98
Neamati S, Alirezaei M, Kheradmand A (2011) Ghrelin acts as an antioxidant agent in the rat kidney. Int J Pept Res Therap 17:239–245
Peltola V, Huhtaniemi I, Ahotupa M (1992) Antioxidant enzyme activity in the maturing rat testis. J Androl 13(5):450–455
Peltola V, Mantyla E, Huhtaniemi I, Ahotupa M (1994) Lipid peroxidation and antioxidant enzyme activities in the rat testis after cigarette smoke inhalation or administration of polychlorinated biphenyls or polychlorinated naphthalenes. J Androl 15(4):353–361
Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M (1996) Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology 137(1):105–112
Ratnam S, Wijekoon EP, Hall B, Garrow TA, Brosnan ME, Brosnan JT (2006) Effects of diabetes and insulin on betaine-homocysteine S-methyltransferase expression in rat liver. Am J Physiol-Endocrinol Metab 290(5):E933–E939
Romero JJ, Tamura T, Halsted CH (1981) Intestinal absorption of [3H] folic acid in the chronic alcoholic monkey. Gastroenterology 80(1):99–102
Sehirli O, Sener E, Sener G, Cetinel S, Erzik C, Yegen BC (2008) Ghrelin improves burn-induced multiple organ injury by depressing neutrophil infiltration and the release of pro-inflammatory cytokines. Peptides 29(7):1231–1240
Sher L, Oquendo MA, Grunebaum MF, Burke AK, Huang Y, Mann JJ (2007) CSF monoamine metabolites and lethality of suicide attempts in depressed patients with alcohol dependence. Eur Neuropsychopharmacol 17(1):12–15
Slow S, Lever M, Chambers ST, George PM (2009) Plasma dependent and independent accumulation of betaine in male and female rat tissues. Physiol Res 58:403–410
Smith AM, Zeve DR, Grisel JJ, Chen WJA (2005) Neonatal alcohol exposure increases malondialdehyde (MDA) and glutathione (GSH) levels in the developing cerebellum. Dev Brain Res 160(2):231–238
Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C (2003) S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J Nutr Biochem 14(10):591–597
Sonmez M, Türk G, Yüce A (2005) The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 63(7):2063–2072
Srikanth V, Malini T, Arunakaran J, Govindarajulu P, Balasubramanian K (1999) Effects of ethanol treatment on epididymal secretory products and sperm maturation in albino rats. J Pharmacol Exp Therap 288(2):509–515
Subbarao KV, Richardson JS, Ang LC (1990) Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J Neurochem 55(1):342–345
Sunden SLF, Renduchintala MS, Park EI, Miklasz SD, Garrow TA (1997) Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch Biochem Biophys 345(1):171–174
Tamura T, Halsted CH (1983) Folate turnover in chronically alcoholic monkeys. J Lab Clin Med 101(4):623–628
Tamura T, Romero JJ, Watson JE, Gong EJ, Halsted CH (1981) Hepatic folate metabolism in the chronic alcoholic monkey. J Lab Clin Med 97(5):654–661
Tremellen K (2008) Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update 14(3):243–259
Turner TT, Lysiak JJ (2008) Oxidative stress: a common factor in testicular dysfunction. J Androl 29(5):488–498
Van Thiel DH (1983) Ethanol: its adverse effects upon the hypothalamic-pituitary-gonadal axis. J Lab Clin Med 101(1):21–33
Villanueva JA, Halsted CH (2004) Hepatic transmethylation reactions in micropigs with alcoholic liver disease. Hepatology 39(5):1303–1310
Wallock-Montelius LM, Villanueva JA, Chapin RE, Conley AJ, Nguyen HP, Ames BN, Halsted CH (2007) Chronic ethanol perturbs testicular folate metabolism and dietary folate deficiency reduces sex hormone levels in the Yucatan micropig. Biol Reprod 76(3):455–465
Wu D, Cederbaum AI (2003) Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 27:277–284
Wu A, Chanarin I, Slavin G, Levi AJ (1975) Folate deficiency in the alcoholic—its relationship to clinical and haematological abnormalities, liver disease and folate stores. Br J Haematol 29(3):469–478
Acknowledgment
This research was financially supported by School of Veterinary Medicine-Shiraz University, Shiraz, Iran. We are most grateful to Saeedeh Ahmadi for the kind technical assistance; also like to thank M. Shoaei and R. Shirazi (the member and manager of Aryadalman Company, Tehran, Iran) for providing betaine (Betafine®).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alirezaei, M., Jelodar, G. & Ghayemi, Z. Antioxidant Defense of Betaine Against Oxidative Stress Induced by Ethanol in the Rat Testes. Int J Pept Res Ther 18, 239–247 (2012). https://doi.org/10.1007/s10989-012-9297-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-012-9297-9