Abstract
Context
The replacement of native vegetation by exotic grasses for livestock production is driving landscape homogenization, habitat fragmentation and reducing connectivity between habitat patches in floodplains ecosystems.
Objective
In this context we examined how changes in native and exotic vegetation cover, connectivity and water depth affect the attributes of the small [standard length (SL) < 80 mm as adults] and large-sized fish assemblages (SL ≥ 80 mm as adults).
Method
We assessed the effects of water depth, exotic and native vegetation cover and habitat connectivity on the abundance, species richness, body size and biomass of fish assemblages in a 25 km2 area of the seasonal habitats of the Pantanal wetland over 5 years.
Results
We showed that fish assemblage response to meso-scale variation in water depth, vegetation cover and habitat connectivity in seasonal habitats is size-dependent. The gradient from exotic to natural vegetation cover did not affect the assemblages of small-sized fish, which were mostly regulated by water depth, habitat connectivity and the gradient from grassland to forest. However, besides being affected by water depth and habitat connectivity, large-sized fish were also affected by the gradient from exotic to natural vegetation cover.
Conclusion
Our results indicate that transformations in the landscape and changes in the dynamics of inundation may have negative consequences for the long-term persistence of fish assemblages in the Pantanal wetlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floodplains are among the most diverse and productive natural systems in the world (Tockner et al. 2000). These systems are periodically inundated by the overflow of water from the main river, lateral channels and floodplain lakes, and they also receive significant inputs from direct precipitation and groundwater. Inundated lowlands form ephemeral aquatic habitats that are colonized by aquatic organisms from nearby permanent water bodies (Henning et al. 2007) and by individuals who emerge from the resting eggs, seeds or propagules that were left in the soil during the previous inundation (Heckman 1994; Shröder 2001; Brock et al. 2003). During the dry season, precipitation is low and evapotranspiration is high, so these systems return to their terrestrial phase and become occupied by plants and animals that are not adapted to aquatic conditions. At this time, the aquatic organisms become stranded, die or are forced to return to permanent waterbodies (Penha et al. 1998; Rebellato and Nunes da Cunha 2005; Jenkins and Boulton 2007; Junk et al. 2014). These characteristics make the floodplain a very productive system and have encouraged humans to establish growing settlements along the margins of large river systems. As a consequence, the anthropogenic pressure on these ecosystems has increased with the need for more energy, agriculture and livestock production. These pressures cause habitat loss and fragmentation (Samson and Knopf 1994; Steinman and Rosen 2000; Walters et al. 2006), the two major threats to terrestrial biodiversity (Prugh et al. 2008).
The majority of studies that assess the spatial distribution of species in the floodplain have focused on the scale of the sampling unit (Baber et al. 2002; Steinman et al. 2003; Babbitt et al. 2009; Fernandes et al. 2010), but few studies have assessed both local and broad scale process (Scherer et al. 2012; Fernandes et al. 2014). Biological diversity in temporary wetlands is highly dependent on the dynamics of colonization and extinction, which maintain the habitat connectivity that enables the dispersal and recolonization of unoccupied habitat patches (Gilpin 1980; Loehle 2007), seasonal migration among populations, and metapopulation persistence at the landscape scale (Hanski 1998; Jacobson and Peres-Neto 2010; Rayfield et al. 2011). In addition to the factors at broad (i.e., dispersal) and intermediate scales (i.e., landscape composition), local habitat characteristics (i.e., water depth, hydroperiod, patch area) and biological interactions (e.g. competition and predation) are important to the structure and composition of local species (Power 1984; Harvey and Stewart 1991; Englund and Krupa 2000).
Abundance and species richness are among the most common descriptors of community structure (Magurran 2004), though body size and biomass provide information about the system productivity in aquatic habitats. These data have been used to guide fisheries management decisions. In river-floodplain systems such as the Brazilian Pantanal, fish species depend heavily on the resources available in the temporary habitat formed during the wet season (Agostinho et al. 2001; Cucherousset et al. 2007). Therefore, when the connectivity between permanent water bodies and the temporary habitats is compromised by the replacement of the native vegetation with exotic grasses (Brooks et al. 2004; Hoffmann et al. 2004; Hejda et al. 2009; Alho et al. 2011; Junk and Nunes da Cunha 2012; Simberloff et al. 2013), there are negative effects on the fish use of the floodplain and fishery productivity in subsequent years (Sommer et al. 2001; Opperman et al. 2010).
The Brazilian Pantanal, one of the biggest wetlands of the world, is mainly threatened by landscape changes caused by livestock production and dams that were built for flood control (Zeilhofer and Moura 2009; Girard 2011). Alterations of the spatial configuration of habitat and the characteristics of areas between patches in the landscape may reduce the connectivity between temporary and permanents aquatic habitats (Prugh et al. 2008; Zhao et al. 2012) and may prevent their use by aquatic organisms. To improve our understanding about how landscape changes affect the dynamics of fish in wetlands and to increase our ability to guide management, we evaluated how changes in water depth, native and exotic vegetation cover and connectivity affect the abundance, species richness, body size and the biomass of fish assemblages. Based on previous studies (Harvey and Stewart 1991; Kodric-Brown and Brown 1993; Englund and Krupa 2000; Taylor and Warren 2001; Casatti et al. 2009), we hypothesize that water depth and connectivity have a positive effect on these assemblages attributes. Moreover, we hypothesized that diverse, complex habitats of native vegetation would increase the abundance, species richness, body size and biomass compared to exotic pastures, which are dominated by few plant species and provide a less complex habitat.
Materials and methods
Study area
The Pantanal is a seasonally flooded wetland that ranges over an area of approximately 160,000 km2. It is in the central region of South America between parallels 16° and 22°S and 55° and 58°W, which includes portions of Brazil, Paraguay and Bolivia. In the late eighteenth century to the early nineteenth centuries, livestock production became important in Brazilian territories (Silva et al. 2000). Until 1970, the natural grasslands were used to feed cattle, and there were low-intensity farming practices (Seidl et al. 2001). Due to the low nutritive value of natural grasslands (carrying capacity = 0.81 individuals ha−1), farmers have been replacing the natural grasslands by an exotic African grass (Urochloa humidicola (Rendle) Morrone & Zuloaga, carrying capacity = 1.02 individuals ha−1) (Seidl et al. 2001; Junk et al. 2006), resulting in the loss of 17.5 % of the original vegetation (Harris et al. 2005).
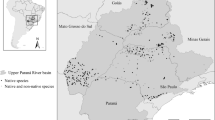
This study was performed in the Pantanal long-term sampling sites (PLTSS) located in the northern portion of the Brazilian Pantanal (Fig. 1). The PLTSS occupies an area of 25 km2 (56°21′W, 56°18′ E, 16°19′N, 16°22′S) throughout three private properties where the main activity is livestock production. The PLTSS consists of a square grid containing 30 plots (250 m length × 1 m width) that follow the topographic gradient and are located 1 km apart (for more details see Fernandes et al. 2010; Signor and Pinho 2011). In this region, the wet season is from December to June and the dry season is from July to November. Situated approximately 10 km from Cuiabá River, the research site is characterized by a highly heterogeneous landscape with different types of vegetation cover (Fantin-Cruz et al. 2010a). Within and nearby the PLTSS, there are 25 small permanent ponds (mean 0.1 ha and range 0.01–0.36 ha) and one lake (222 ha) that fish use as refuges during the dry season and that serve as a source of colonization of the floodplain during the wet season.
Fish sampling
Fish were sampled between March and April of each year across all flooded plots (2006—22 plots, 2008—22 plots, 2009—21 plots, 2010—18 plots and 2011—22 plots). These are the months in which flood peaks usually happen, and most of the plots are flooded for at least 2 months (Fantin-Cruz et al. 2010b). Fish were collected by two methods: throw traps and gill nets. Throw traps consist of metal cubes (1 m3) that are covered by a 1.5 mm nylon mesh, and they were employed six times per plot (every 50 meters). The individuals enclosed by the trap were retrieved with a triangular fish trap until no additional specimen was collected after 10 consecutive sweeps. In addition, seven gill nets (20.0 × 1.5 m; mesh size: 12, 15, 18, 20, 25, 30 and 50 mm between opposing knots) were distributed along each plot. In 2006, the gill nets were set between 0700 and 0800 h and removed between 1800 and 1900 on the same day; in other years, the gill nets they were set between 1600 and 1700 h and removed on the following day between 0800 and 0900 h. The change in sampling period improved sampling during the sunset and sunrise when fish are more active. As a consequence of this change, catfish became more abundant than cichlids in the samples, though the dominance of the characids was maintained. The methods were not employed on the same day to minimize the effects of the disturbance on fish assemblage. The combination of active and passive sampling methods allows the capture of both mobile and sedentary species, as well as individuals of a large range-size (Weaver et al. 1993; Jackson and Harvey 1997; Lapointe et al. 2006). All individuals captured were euthanized with Eugenol, fixed in 10 % formalin solution, preserved in 70 % ethanol and identified to the species level. Finally, each individual was weighed and measured by standard length (SL) (details in Fernandes et al. 2010).
Environmental variables
For each plot, altitude was recorded by a simple frequency geodetic global positioning system that tracked for 10 min, or until the error was <50 mm. As each plot has the same topographic altitude from beginning to the end, one measurement was enough to represent the entire plot (see details in Magnusson et al. 2005). The water depth in each plot was the average of six measurements that were taken where the throw trap was launched. Each year during the sampling, we visited higher altitude plots that were presumably dry to ensure that they did not become inundated.
A rectangular area of approximately 35 km2 that includes the PLTSS area and its surroundings was extracted from Google Earth™ images and transformed into a shapefile. The vegetation patches were then manually marked as polygons. The vegetation on each polygon was later categorized based on field information into one of five vegetation classes: wet grassland, wet pasture, dry pasture, wet forest and dry forest. Grasslands include mostly native grasses species and aquatic macrophytes (submerged, emergent and floating), pastures are composed mainly of exotic African grass [Urochloa humidicola (Rendle) Morrone & Zuloaga], and forests are composed of shrubs and trees without detectable undergrowth plant species (Rebellato et al. 2012; Nunes da Cunha et al. 2010). Although we used an image from 2003, all of the areas were visited annually from 2004 to 2011 and neither natural nor human landscape changes were observed.
The percentage of each vegetation class was calculated in a 450 m circular buffer around the center of each plot. The percentage or proportion of vegetation cover in any buffer is a type of data that are highly correlated. Therefore, we applied a correspondence analysis (CA) to reduce the dimensionality of the data (Jackson 1997), and these CA axes were used to represent vegetation cover in all subsequent analyses.
Connectivity metric
Landscape connectivity is the extent to which the landscape facilitates or prevents movement of organisms among patches (Taylor et al. 1993). Connectivity can be measured in different ways (Prugh 2009), and it has been commonly classified into two main types, namely functional and structural. Functional connectivity incorporates data about individuals’ movements throughout the landscape. On the other hand, measures of structural connectivity express how the spatial arrangement of different habitat and potential barriers in the landscape may affect species dispersion (Theobald et al. 2011).
Here, we used the probability of connectivity index (PC), which is based on the habitat availability concept, the probabilities of dispersal among patches and graph structure (Saura and Pascual-Hortal 2007). It measures the probability of two animals randomly placed within the landscape falling into habitat areas that are reachable from each other (interconnected) (Bodin and Saura 2010). As we are interested in local connectivity, we used a version of the probability of connectivity index that is based on patches (PCflux) and permits the measurement of the local contribution of each patch to the global PC index (Foltête et al. 2014):
where \(\varvec{n}\) is the number of habitat patches in the landscape, \(\varvec{ai}\) and \(\varvec{aj}\) are the volumes of the patches \(\varvec{i}\) and\(\varvec{j}\) and \(\varvec{A}\) is the total landscape area (both habitat and non-habitat); \(\varvec{p}_{{\varvec{ij}}}^{\varvec{*}}\) is the probability that an individual in patch \(\varvec{i}\) will disperse to patch \(\varvec{j}\). The dispersal probability \(\varvec{p}_{{\varvec{ij}}}^{\varvec{*}}\) was computed using a negative exponential function (Urban and Keitt 2001; Saura and Pascual-Hortal 2007): \(p_{ij}^{*} = exp\left( { - \alpha d_{ij} } \right)\); \(\alpha\) was determined so that \(p_{ij}^{*} =\) 0.05 when \(d_{ij}\) is a maximum distance, and \(d_{ij}\) is a least-cost distance between patch \(\varvec{i}\) and patch \(\varvec{j}\). Thus, the parameter \(p_{ij}^{*}\) expresses a greater or lesser decrease in the probability of flux (p) with distance (d). Our 30 plots, 25 small permanent ponds, and one lake were considered as preferential habitat and were the nodes of the connectivity index.
The effective distance (least-cost distance) was calculated using data about vegetation cover, water level and altitude. We used a previously constructed raster grid (Fernandes et al. 2014) to represent the vegetation cover data. The 30 plots’ water depth and altitude data were interpolated to build the water level and elevation layers by means of ordinary kriging, with the assumption of a spherical model to build the semivariogram (Zimmerman et al. 1999). Because altitude and vegetation cover did not change throughout the study, only one layer was built to represent these variables in the analyses, while for water level we used one layer for each year. Effective distance was calculated as a function of the factors that facilitate individual movement across the landscape (flooded areas such as wet forest, wet grassland and wet pasture) or restrict it (dry forest and dry pasture). Therefore, the effective distance between two patches represents the minimum cumulative effort (least-cost distance) of moving across the resistance layer (Theobald et al. 2011). To create the resistance layer, we assigned a resistance value cost of 1 to wet forest, 2 for wet grassland, 3 for wet pasture and 100 for dry forest and dry pasture (PC index 4, see more details in the supplementary material). We chose 1 for wet forest because this vegetation class occurs in areas of low altitude that are the first to be inundated, so it may form important dispersion corridors; 2 for grassland because despite being an important habitat, they are in patches with lower hydroperiods (the number of days that a wetland holds water during the wet season), and they are shallower than wet forests (Fantin-Cruz et al. 2010a); wet pasture was scored 3 because these are patches where wet grassland and wet forest were replaced by exotic pasture; dry forest and dry pasture was scored 100 because these habitats are permanently dry and represent permanently impassable barriers to fish. The water level and altitude layers were reclassified with resistance values ranging from 1 to 10, and higher resistance values were imposed for high elevations and shallower regions. After reclassification, the three layers were combined to build the resistance layer. Given its greater accuracy, vegetation data were given a higher weight (0.5) on the final resistance layer. Water level and elevation, which were estimated by interpolation, were given lower weights (0.4 and 0.1, respectively) (see Adriaensen et al. 2003 for more details about the methods). Recognizing that our resistance value costs for vegetation cover are arbitrary, we assess the sensibility of our results given this decision. To perform the sensibility analysis, we calculated the least-cost distance using different resistance values cost to vegetation classes and used these value to calculate connectivity. However, our primary results did not change (see supplementary material). All the steps were performed in ArcGIS (ESRI 2006). The least-cost layer was calculated among plots and permanent ponds using the “costDistance” function of the ‘gdistance’ package (Etten 2012) in the R 2.15.3 Statistical Software (R Core Team 2013). Because water level changed throughout the sampling period but vegetation cover (or altitude) did not, the changes among years in the least-cost-distances were solely due to the water level. The variables ‘vegetation cover type’ and ‘altitude’ only affected the spatial variability in the least-cost metric.
Data analysis
We separated small-sized and large-sized fish based on published data on the maximum adult body size (SL) from each species (Reis et al. 2003). Small-sized fish (SL < 80 mm as adults) are numerically dominant taxa, whereas large-sized fish (SL ≥ 80 mm as adults) are less abundant but dominant in biomass. This threshold length was the same as that used by Chick et al. (2004) to separate small and large fish species of the Florida Everglades, and we adopted it here due to the similarity between the two systems (both are extensive shallow wetlands). We combined data from the two types of sampling gear to compute abundance (number of individuals captured), and species richness (total number of species) for each size class (i.e., small and large-sized fish) in each plot and year. Body size was calculated as the mean SL of all individuals and fish biomass as the sum of the weight of all individuals. To evaluate whether abundance, species richness and fish biomass differ among size class, we applied a Kruskal–Walllis test (Sokal and Rohlf 1995).
We used information theoretic approach to model selection in order to assess the importance of the connectivity, water depth and vegetation cover on fish community attributes (Burnham and Anderson 2002). Twenty different models were built for each dependent variable (i.e., abundance, species richness, body size and biomass). These models contained different combinations of the independent variables [water depth, vegetation cover (represented by CA1 and CA2) and connectivity (PCflux)]. The models were constructed using the generalized additive model for location, scale and shape (GAMLSS, Rigby and Stasinopoulos 2005) because the relationship between dependent and independent variables was not always linear. The GAMLSS is a semi-parametric regression-type model introduced by Rigby and Stasinopoulos (2005) to overcome some limitations of generalized linear models (GLMs) and generalized additive models (GAMs). It is parametric in that it requires a parametric distribution assumption for the response variable, and “semi” in the sense that the modelling of the parameters of the distribution may involve the use of non-parametric smoothing functions. GAMLSS is flexible enough to address linear and non-linear relationships between the response and predictor variables in the same model because the exponential family assumption for the response variable (Y) can be relaxed and replaced by a general distribution family (Landi et al. 2014).
The effects of water depth and connectivity (PCflux) on fish community attributes were modeled with a cubic spline smoothing function (cs). The cs function is based on the smooth. spline function from stats package of R and can be used for univariate smoothing (Rigby and Stasinopoulos 2005). The year of sampling was modeled as random-effect. The best distribution of each response variable was chosen from among Normal, Gamma and Poisson distributions for abundance and species richness and Normal or Gamma distributions for body size and biomass based on the Akaike information criterion (AIC) (Zuur et al. 2009).
We also used AIC to compare the 20 models and select the best model (Burnham and Anderson 2002). In addition to the AIC value, where the lower values indicate the best models, we used another two metrics to visualize the differences between models. These were ∆i values, which are used to evaluable the acceptability of each model (∆i < 2 = strong support in the data; ∆i > = 2 and < 7 = little support in the data; ∆i > 10 = without support in the data) and AIC weight (wi), which is the probability of a given model in the cases of re-sampling the available data (Burnham and Anderson 2002).
All of the independent variables were standardized using z-score transformations (Legendre and Legendre 2012), and the collinearity among them was tested using a variance inflation factor (VIF) (Zuur et al. 2009). GLMLSS were implemented using the gamlss package (Rigby and Stasinopoulos 2005) and values of the AIC, ∆i, wi and k were calculated using the bbmle package (Bolker 2014). All analyses were performed in R 2.15.3 Statistical Software (R Core Team 2013).
Results
Environmental characteristics
The temporary aquatic habitats in the PLTSS were shallow throughout the study period. The lowest values of mean water depth and connectivity were found in 2010, when the inundation level was atypical (18.6 cm), while the highest values were found in 2008 (28.2 cm, supplementary material).
The vegetation cover was dominated by wet grassland (36.8 %) and wet forest (30.2 %); wet pasture (14.7 %), dry forest (14.1 %) and dry pasture (4.1 %) made lower but important contributions. Two axes were extracted using the broken stick model (Jackson 1993), and they accounted for 84.9 % of the variation in vegetation cover. We performed Pearson correlation matrices between CA axes and the vegetation classes to identify which classes contributed more to axes formation. The first axis accounted for 56.3 % and showed high positive correlation with wet grassland (r = 0.69; p < 0.001), dry forest (r = 0.74; p = 0.004) and a negative correlation with dry pasture (r = −0.72; p < 0.001) and wet pasture (r = −0.84; p < 0.001). In sum, this first axis represented the gradient from exotic to natural vegetation cover. The second axis accounted for 28.6 % of the variation and was positively correlated to wet forest (r = 0.83; p < 0.001) and negatively correlated to wet grassland (r = −0.74; p = 0.001) and dry forest (r = −0.47; p = 0.02). The CA2 axis mainly represented the variation in vegetation cover from wet grassland to wet forest.
Fish community
Throughout the 5 years of sampling, a total of 6813 individuals from 70 species were collected; approximately 62 % (4220) were small-sized fishes (Mean body size = 15.08 mm and range 3.4–70.3 mm of SL) and 38 % (2593) were large-sized fishes (Mean body size = 93.20 mm and range 8.0–371.3 mm of SL). The small-sized fish (Mean = 39.11 and range 1–131) were more abundant than large-sized fish (Mean = 25.72 and range 1–143) (Kruskal–Wallis test: H = 5.16, df = 1 and p = 0.023). On the other hand, body size, species richness (Kruskal–Wallis test: H = 9.77, df = 1 and p = 0.001) and fish biomass (Kruskal–Wallis test: H = 137.13, df = 1 and p < 0.001) were greater for large-sized fish. The mean species richness was 5.6 (range 1–14) for small-sized fish and 7.27 (range 1–19) for large-sized fish, while the mean fish biomass was 8.96 g (range 0.03–151.99 g) for small-sized fish and 931.65 g (range 0.500–7222 g) for large-sized fish. Additional information such as captured species, numbers of individuals (abundance), mean body size and biomass is presented in the supplementary material.
Model selection
For small-sized fishes, the best ranked model for abundance included a non-linear effect of connectivity and linear effects of the gradient from wet grassland to wet forest (CA2) and year (wi = 0.52). A second model including the gradient from exotic to natural vegetation cover (CA1) was selected as equally plausible (∆i = 1.8; wi = 0.21; Table 1). The best ranked model for richness included a non-linear effect for connectivity and water depth and an effect of year (wi = 0.23), but an equally plausible model included an additional effect of CA2 (∆i = 1.3; wi = 0.12), and another included the effect of CA1 (∆i = 1.7; wi = 0.1; Table 1). For body size, the best model included a non-linear effect for connectivity and depth in addition to the linear effect of CA2 and year (wi = 0.65), but another plausible model included the effect of CA1 (∆i = 1.4; wi = 0.31; Table 1). The model selected for biomass had a linear effect for water depth, CA1, CA2 and year (wi = 0.19). Additional models included the linear effect of connectivity (∆i = 0.4; wi = 0.16) and a non-linear effect of connectivity and depth (∆i = 0.9; wi = 0.12; Table 1).
For large-sized fishes, the best ranked model for abundance included a non-linear effect of connectivity and depth and a linear effect of the gradient from exotic to natural vegetation cover (CA1) and year (wi = 0.54). An additional model included CA2 (∆i = 1.8; wi = 0.22; Table 2). The best ranked model for richness included a linear effect for connectivity CA1, CA2 and year (wi = 0.49; Table 2). For body size, the best model included a non-linear effect for connectivity and water depth in addition to the linear effects of CA1, CA2 and year (wi = 0.63; Table 2). The model selected for biomass had a non-linear effect for connectivity and water depth, and linear effects for CA1, CA2 and year (wi = 0.96; Table 2).
Effects of connectivity, vegetation cover and depth on fish assemblages
To understand how independent variables are affecting assemblage attributes, we inspected the beta coefficients of the best model for each dependent variable (Table 3 and supplementary material). For small-sized fish, the best model indicated that more individuals were found in patches that were more connected and fewer individuals were found in patches with more wet forest cover (i.e., more connected than patches with more wet grassland) (see supplementary material). Species richness was higher in deeper and more connected patches, while small-sized fish from deeper patches also had larger body size and biomass. Body size and biomass also increased with the amount of wet forest cover and decreased with the amount of wet grassland. In contrast to the abundance and species richness, which were higher on the more connected patches, body size was greater in less connected patches (Table 3).
For large-sized fishes, we found that abundance, species richness, body size and fish biomass were higher in deeper and more connected patches than in shallow and less connected patches (Table 3). Patches dominated by native vegetation cover (wet grassland and dry forest) had more individuals, higher species richness, more larger-bodied individuals and higher biomass than those with exotic grass (dry and wet pasture). Furthermore, patches where wet forest was dominant had larger individuals and higher fish biomass (Table 3) than patches with wet grassland (see supplementary material).
In summary, both small and large-sized fish were affected by connectivity, water depth and the gradient from wet grassland to wet forest (Table 3). However, the effect of the predictor variables changed between the two groups as reflected by the different slopes, an effect that was strongest for abundance and body size (see estimates of beta and standard error in Table 3). Finally, size-classes differences were remarkable in that large-sized fish respond positively to native cover and negatively to exotic cover, while small-sized fish were not affected by these environmental variables.
Discussion
Our results show that the fish community response to meso-scale variation in water depth, vegetation cover and habitat connectivity in seasonal habitats of the Pantanal wetland is size-dependent. Size-dependent responses to depth and vegetation cover can arise because small organisms respond more strongly to fine scale variation in the environment than large organisms (Soininen et al. 2007) and because environmental factors act at different spatial scales (Dray et al. 2012). While water depth is a local variable and can reflect fine-scale habitat volume, vegetation cover should be an indirect measure of the habitat available at intermediate scale. Size-dependent responses to connectivity can arise because dispersal distance is a function of body size in fish (Griffith 2006). Thus, patches that are more connected are found by both small and large-sized individuals, while only large organisms can find more isolated patches.
In temporary aquatic systems such as seasonal wetlands, both shallow and deeper regions support a diversity of habitats created by the abundance and diversity of aquatic macrophytes (Barbour and Brown 1974; Schessl 1999), which add structural complexity and provide food and shelter from predators for both small and large-sized fish, increasing both abundance and species diversity (Tonn and Magnuson 1982; Kodric-Brown and Brown 1993; Mayo and Jackson 2006; Thomaz et al. 2008). Although we used patches of the same surface area, deeper patches have larger habitat volume and diversity than shallow patches, are a more effective target for both active and passive immigration (Lomolino 1990) and are less prone to extinction, while shallow patches have fewer habitat types and are subject to stochastic extinction (Miyazono and Taylor 2013). In addition, biological interactions such as predation determine the fish body size distribution patterns between shallow and deeper patches (Power 1984; Englund and Krupa 2000). This occurs because the predation pressure from terrestrial predators forces large-sized fish to seek deeper waters; piscivorous fish, which are more abundant in these habitats, force small-sized fish to find escape in shallow water (Harvey and Stewart 1991; Englund and Krupa 2000), which contributes to local assemblages composition. We think this is a plausible explanation for the water depth effect on the fish community of the Brazilian Pantanal, due to the high abundance and diversity of piscivorous birds (Signor and Pinho 2011) and predatory fish (Fernandes et al. 2010) found there. However, field experiments are necessary to support this idea.
More connected patches are more likely to be colonized by more species than less connected ones (Taylor and Warren 2001; Arrington et al. 2005; Jacobson and Peres-Neto 2010) because high connectivity allows species to colonize the habitat regardless of their dispersal ability (Baber et al. 2002). In contrast, patches with less connectivity will only be colonized by species with high dispersal ability (Fernandes et al. 2014) and are less likely to be rescued (Brown and Kodric-Brown 1977) from local extinction events. Finally, the larger body size of small-sized fish in less isolated patches can be explained by two factors that are not mutually exclusive. First, there is the aforementioned correlation between dispersal distance and body size, i.e., only larger individuals can reach patches that are more distant and less connected (Griffith 2006); and second, density-dependent growth in more connected patches, i.e., an increase in abundance, leads to a reduction in body size in floodplain habitats (Penha et al. 2015).
Landscape changes from natural to exotic grass seem to have a negligible effect on small-sized fishes because those species respond mainly to factors acting at local scales, such as the availability of shelter and food and the presence of predators (fine grained species, sensu MacArthur and Levins 1964). Another factor that can attenuate the effect of exotic pasture cover on the small-sized fish fauna is the seasonal alternation of flood and drought periods (Junk et al. 1989). These drastic environmental changes result in the temporal substitution of plant species throughout the hydrological cycle (Schessl 1999; Prado et al. 1994; Rebellato et al. 2012), mainly in wet pasture and wet grassland. When associated with the presence of cattle grazing, the seasonality prevents the dominance of exotic or arboreal species (Collins et al. 1995; Marty 2005; Questad et al. 2011; Junk and Nunes da Cunha 2012). During the dry season, the landscape is dominated by short-lived terrestrial plants that cannot endure the hydrological stress of flooding and amphibious plant species that can photosynthesize in both terrestrial and aquatic environments (Maberly and Spence 1989). With the onset of the floods, a rich assemblage of strictly aquatic plant species joins the amphibious plants (Rebellato and Nunes da Cunha 2005). Native assemblages of aquatic plants grow over both native grasslands and exotic pastures, though not in wet forest patches, so the similarity in vegetation structure across grassland habitats increases during the flood season. Thus, one should expect a high similarity in habitat structure, shelter availability and food supply between native grasslands and exotic pastures at a local scale during the flood season, which most likely explains the similarities in some attributes of the small-sized fish communities between these environments. On the other hand, the negative effect of the exotic grasses on large-sized fish may occur because at a landscape scale, differences are maintained and larger individuals respond mainly to land-use change at intermediate and broad scales (coarse grained species, sensu MacArthur and Levins 1964).
Finally, the almost total lack of aquatic macrophytes in wet forests may reduce the habitat available for small-sized fish in aquatic habitats dominated by larger fish (many of which pose a predation threat). The success of the larger fish on wet forests occurs because the lower densities of terrestrial predators makes them safer dispersal routes for larger fish (and they serve as important foraging grounds; q.v. Goulding, 1980). Thus, small individuals may be pushed to shallow habitats where the abundant vegetation decreases predation risk. This would explain the clear preference by small-sized fish for patches of shallow grass and the higher fish biomass and body size of the large-sized fish in wet forest compared to grassland habitats.
Conclusion
The results of this study support our initial hypothesis that water depth, connectivity and native vegetation cover have a positive effect on community attributes, while exotic vegetation cover has a negative effect. The increasingly frequent introduction of exotic grasses for cattle grazing threatens the native vegetation in the Brazilian Pantanal, reducing native cover and causing habitat loss and fragmentation. Replacing wet forest with wet grassland or pasture could increase the abundance of small-sized fish, which are important to biodiversity. However, these species have only a small contribution to the community biomass, so encouraging their proliferation might reduce fishery productivity and change the trophic chain including people. Approximately 17 % of the native habitat of the Brazilian Pantanal has been replaced by exotic grasses, and continued conversion or degradation could change the landscape structure and connectivity between habitats patches due to a reduction in the matrix permeability, thereby preventing the species dispersal. This, in turn, could negatively affect the dynamics of colonization and extinction of the temporary aquatic habitat during the flood season (Fernandes et al. 2014). Therefore, conservation policies should focus on the protection of all habitats (from grasslands to forests) to maintain a highly heterogeneous landscape and preserve the natural hydrological dynamics and connectivity of the floodplain, so fish species (and other organisms) could successfully complete their life-cycles and maintain the high biodiversity of the Pantanal.
References
Adriaensen F, Chardon JP, De Blust G, Swinnen E, Villalba S, Gulinck H, Matthysen E (2003) The application of ‘least-cost’ modelling as a functional landscape model. Landsc Urban Plan 64(4):233–247
Agostinho AA, Gomes LC, Zalewski M (2001) The importance of floodplains for the dynamics of fish communities of the upper river Paraná. Ecohydrol Hydrobiol 1:209–217
Alho CJR, Mamede S, Bitencourt K, Benites M (2011) Introduced species in the Pantanal: implications for conservation. Braz J Biol 71(1):321–325
Arrington DA, Winemiller KO, Layman CA (2005) Community assembly at the patch scale in a species rich tropical river. Oecologia 144(1):157–167
Babbitt KJ, Baber MJ, Childers DL, Hocking D (2009) Influence of agricultural upland habitat type on larval anuran assemblages in seasonally inundated wetlands. Wetlands 29(1):294–301
Baber JM, Childers DL, Babbitt KJ, Anderson DH (2002) Controls on fish distribution and abundance in temporary wetlands. Can J Fish Aquat Sci 59:1441–1450
Barbour CD, Brown JH (1974) Fish species diversity in lakes. Am Nat 108:473–489
Bodin Ö, Saura S (2010) Ranking individual habitat patches as connectivity providers: integrating network analysis and patch removal experiments. Ecol Model 221(19):2393–2405
Bolker B (2014) bblme: Tools for general maximum likelihood estimation. R package version 1.0.17
Brock MA, Nielsen DN, Shiel RJ, Green JD, Langley JD (2003) Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshw Biol 48:1207–1218
Brooks ML, D’Antonio C, Richardson DM, Grace JB, Keeley JE, DiTomasso JM, Hobbs RJ, Pellant M, Pyke D (2004) Effects of invasive alien plants on fire regimes. Bioscience 54:677–688
Brown JH, Kodric-Brown A (1977) Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58(2):445–449
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A practical information theoretic approach, 2nd edn. Springer, New York
Casatti L, Ferreira CP, Carvalho FR (2009) Grass-dominated stream sites exhibit low fish species diversity and domi-nance by guppies: an assessment of two tropical pastureriver basins. Hydrobiologia 632(1):273–283
Chick JH, Ruetz CR, Trexler JC (2004) Spatial scale and abundance patterns of large fish communities in freshwater marshes of the Florida Everglades. Wetlands 24(3):652–664
Collins SL, Glenn SM, Gibson DJ (1995) Experimental analysis of intermediate disturbance and initial floristic composition: decoupling cause and effect. Ecology 76:486–492
Cucherousset J, Paillisson JM, Paillisson A, Chapman LJ (2007) Fish emigration from temporary wetlands during drought: the role of physiological tolerance. Arch Hydrobiol 168(2):169–178
Dray S, Pélissier R, Couteron P, Fortin MJ, Legendre P, Peres-Neto PR, Bellier E, Bivand R, Blanchet FG, De Cáceres M, Dufour AB, Heegaard E, Jombart T, Munoz F, Oksanen J, Thioulouse J, Wagner HH (2012) Community ecology in the age of multivariate multiscale spatial analysis. Ecol Monogr 82(3):257–275
Englund G, Krupa JJ (2000) Habitat use by crayfish in stream pools: influence of predators, depth and body size. Freshw Biol 43(1):75–83
ESRI (2006) ArcGIS 9.2. Environmental Systems Research Institute, Redlands, California, USA
Etten JV (2012) gdistance: distances and routes on geographical grids. R package version 1.1-3. http://CRAN.R-project.org/package=gdistance
Fantin-Cruz I, Girard P, Zeilhofer P, Collischonn W, Nunes da Cunha C (2010a) Unidades fitofisionômicas em mesoescala no Pantanal Norte e suas relações com a geomorfologia. Biota Neotrop 10(2):31–38
Fantin-Cruz I, Girard P, Zeilhofer P, Collischonn W (2010b) Dinâmica de inundação. In: Fernandes IM, Signor CA, Penha J (eds) Biodiversidade no Pantanal de Poconé. Centro de Pesquisas do Pantanal, Cuiabá, pp 25–35
Fernandes IM, Machado FA, Penha J (2010) Spatial pattern of a fish assemblage in a seasonal tropical wetland: effects of habitat, herbaceous plant biomass, water depth, and distance from species sources. Neotrop Ichthyol 8(2):289–298
Fernandes IM, Henriques-Silva R, Penha J, Zuanon J, Peres-Neto PR (2014) Spatiotemporal dynamics in a seasonal metacommunity structure is predictable: the case of floodplain-fish communities. Ecography 37:464–475
Foltête JC, Girardet X, Clauzel C (2014) A methodological framework for the use of landscape graphs in land-use planning. Landsc Urban Plan 124:140–150
Gilpin ME (1980) The role of stepping-stone islands. Theor Popul Biol 17:247–253
Girard P (2011) Hydrology of surface and ground waters in the Pantanal floodplains. In: Junk WJ, da Silva CJ, Nunes da Cunha C, Wantzen KM (eds) The Pantanal: ecology, biodiversity and sustainable management of a large neotropical seasonal wetland. Pensoft Publishers, Sofia, pp 103–126
Goulding M (1980) The fish and the forests—explorations in Amazonian natural history. California Academy Press, Berkeley
Griffith D (2006) Pattern and process in the ecological biogeography of European freshwater fish. J Anim Ecol 75:734–751
Hanski I (1998) Connecting the parameters of local extinction and metapopulation dynamics. Oikos 83:390–396
Harris MB, Arcangelo C, Pinto ECT, Camargo G, Ramos Neto MB, Silva SM (2005) Estimativas de perda da área natural da Bacia do Alto Paraguai e Pantanal Brasileiro. Relatório técnico, Conservação Internacional, Campo Grande
Harvey BC, Stewart AJ (1991) Fish size and habitat depth relationships in headwater streams. Oecologia 87(3):336–342
Heckman CW (1994) The seasonal succession of biotic communities in wetlands of the tropical wet-and-dry climatic zone: I. Physical and chemical causes and biological effects in the Pantanal of Mato Grosso, Brazil. Int Revue Ges Hydrobiol 79(3):397–421
Hejda M, Pysek P, Jarosík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403
Henning JA, Gresswell RE, Fleming IA (2007) Use of seasonal freshwater wetlands by fishes in a temperate river floodplain. J Fish Biol 71:476–492
Hoffmann WA, Lucatelli VM, Silva FJ, Azeuedo INC, Marinho MS, Albuquerque AMS, Lopes AOL, Moreira SP (2004) Impact of the invasive alien grass Melinis minutiflora at the savanna-forest ecotone in the Brazilian Cerrado. Divers Distrib 10(2):99–103
Jackson DA (1993) Stopping rules in principal component analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214
Jackson DA (1997) Compositional data in community ecology: the paradigm or peril of proportions? Ecology 78(3):929–940
Jackson DA, Harvey HH (1997) Qualitative and quantitative sampling of lake fish communities. Can J Fish Aquat Sci 54:2807–2813
Jacobson B, Peres-Neto PR (2010) Quantifying and disentangling dispersal in metacommunities: how close have we come? How far is there to go? Landscape Ecol 25(4):495–507
Jenkins KM, Boulton AJ (2007) Detecting impacts and setting restoration targets in arid-zone rivers: aquatic micro-invertebrate responses to reduced floodplain inundation. J Appl Ecol 44:823–832
Junk WJ, Nunes da Cunha CN (2012) Pasture clearing from invasive woody plants in the Pantanal: a tool for sustainable management or environmental destruction? Wetl Ecol Manag 20(2):111–122
Junk WJ, Bayley PB, Sparks RS (1989) The flood pulse concept in river—floodplain systems. In: Dodge DP (ed) Proceedings of the international larger river symposium (LARS). Can J Fish Aquat Sci 106:110–127
Junk WJ, Nunes da Cunha C, Wantzen KM, Petermann P, Strussmann C, Marques MI, Adis J (2006) Biodiversity and Its Conservation in the Pantanal of Mato Grosso, Brazil. Aquat Sci 68:278–309
Junk WJ, Piedade MTF, Lourival R, Wittmann F, Kandus P, Lacerda LD, Bozelli RL, Esteves FA, Nunes da Cunha C, Maltchik L, Schöngart J, Schaeffer-Novelli Y, Agostinho AA (2014) Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquat Conserv 24(1):5–22
Kodric-Brown A, Brown JH (1993) Highly structured fish communities in australian desert springs. Ecology 74(6):1847–1855
Landi M, Zoccola A, Bacaro G, Angiolini C (2014) Phenology of Dryopteris affinis ssp. affinis and Polystichum aculeatum: modeling relationships to the climatic variables in a Mediterranean area. Plant Spec Biol 29:129–137
Lapointe NWR, Corrum LD, Mandrak NE (2006) A comparison of methods for sampling fish diversity in shallow offshore waters of large rivers. N Am J Fish Manag 26:503–513
Legendre P, Legendre LF (2012) Numerical ecology, vol 20. Elsevier, Oxford
Loehle C (2007) Effect of ephemeral stepping stones on metapopulations on fragmented landscapes. Ecol Complex 4(1):42–47
Lomolino MV (1990) The target area hypothesis: the influence of island area on immigration rates of non-volant mammals. Oikos 57:297–300
Maberly SC, Spence DHN (1989) Photosynthesis and photorespiration in freshwater organisms: amphibious plants. Aquat Bot 34(1):267–286
MacArthur RH, Levins R (1964) Competition, habitat selection and character displacement in a patchy environment. Proc Natl Acad Sci 51:1207–1210
Magurran AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Magnusson WE, Lima AB, Luizao RC, Luizão ]F, Costa FRC, Castilho CV, Kinupp VF (2005) RAPELD, uma modificação do método de Gentry para inventários de biodiversidade em sítios para pesquisa ecológica de longa duração. Biota Neotrop 5(2):1–6
Marty JT (2005) Effects of cattle grazing on diversity in ephemeral wetlands. Conserv Biol 19(5):1626–1632
Mayo JS, Jackson DA (2006) Quantifying littoral vertical habitat structure and fish community associations using underwater visual census. Environ Biol Fish 75:395–407
Miyazono S, Taylor CM (2013) Effects of habitat size and isolation on species immigration–extinction dynamics and community nestedness in a desert river system. Freshw Biol 58(7):1303–1312
Nunes da Cunha C, Rebellato L, Costa CP (2010) Vegetação e flora: uma experiência pantaneira no sistema de grade. In: Signor C, Fernandes I, Penha J (eds) Biodiversidade no Pantanal de Poconé, vol 01. 1ed.Manaus, Attema, pp 37–57
Opperman JJ, Luster R, McKenney BA, Roberts M, Meadows AW (2010) Ecologically functional floodplains: connectivity, flow regime, and scale. J Am Water Resour Assoc 46(2):211–226
Penha JMF, Da Silva CJ, Bianchini Júnior I (1998) Análise do crescimento da macrófita aquática Pontederia lanceolata em área alágavel do Pantanal Mato-grossense, Brasil. Braz J Biol 58(2):287–300
Penha J, Mateus L, Lobón-Cerviá J (2015) Population regulation in a Neotropical seasonal wetland fish. Environ Biol Fish 98:1023–1034
Power ME (1984) Depth distributions of armored catfish predator-induced resource avoidance. Ecology 65(2):523–528
Prado AL, Heckman CW, Martins FR (1994) The seasonal succession of biotic communities in wetlands of the tropical wet-and-dry climatic zone: II. The aquatic macrophyte vegetation in the Pantanal of Mato Grosso, Brazil. Internatertionale Revue gesamten Hydrobiologie 79(4):569–589
Prugh LR (2009) An evaluation of patch connectivity measures. Ecol Appl 19:1300–1310
Prugh LR, Hodges KE, Sinclair AR, Brashares JS (2008) Effect of habitat area and isolation on fragmented animal populations. Proc Natl Acad Sci 105(52):20770–20775
Questad EJ, Foster BL, Jog S, Kindscher K, Loring H (2011) Evaluating patterns of biodiversity in managed grasslands using spatial turnover metrics. Biol Conserv 144:1050–1058
Rayfield B, Fortin MJ, Fall A (2011) Connectivity for conservation: a framework to classify network measures. Ecology 92(4):847–858
Rebellato L, Nunes da Cunha C (2005) Efeito do “fluxo sazonal mínimo da inundação” sobre a composição e estrutura de um campo inundável no Pantanal de Poconé, MT, Brasil. Acta Bot Bras 19(4):789–799
Rebellato L, Nunes da Cunha C, Figueira JEC (2012) Respostas da comunidade herbácea ao pulso de inundação no Pantanal de poconé, Mato Grosso. Oecol Aust 16(4):797–818
Reis RE, Kullander O, Ferraris CJ Jr (2003) Check list of the freswater fishes of South and Central America. Edipucrs, Porto Alegre, p 742
Rigby RA, Stasinopoulos DM (2005) Generalized additive models for location, scale. J Roy Stat Soc C-App 54(3):507–554
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Samson F, Knopf F (1994) Prairie conservation in North America. Bioscience 44:418–421
Saura S, Pascual-Hortal L (2007) A new habitat availability index to integrate connectivity in landscape conservation planning: comparison with existing indices and application to a case study. Landsc Urban Plan 83:91–103
Scherer RD, Muths E, Noon BR (2012) The importance of local and landscape-scale processes to the occupancy of wetlands by pond-breeding amphibians. Popul Ecol 54(4):487–498
Schessl M (1999) Floristic composition and structure of floodplain vegetation in northern Pantanal of Mato Grosso, Brasil. Phyton 39(2):303–336
Seidl AF, Silva JDSVD, Moraes AS (2001) Cattle ranching and deforestation in the Brazilian Pantanal. Ecol Econ 36(3):413–425
Shröder T (2001) Colonizing strategies and diapause of planktonic rotifers (Monogononta, Rotifera) during aquatic and terrestrial phases in a floodplain (Lower Oder Valley, Germany). Int Rev Hydrobiol 86:635–660
Signor CA, Pinho JB (2011) Spatial diversity patterns of birds in a vegetation mosaic of the Pantanal, Mato Grosso, Brazil. Zoologia 28(6):725–738
Silva JSV, Seidl AF, Moraes AS (2000) Evolucao da agropecuaria do Pantanal Brasileiro, 1975–1985. EMBRAPA-CPAP, Corumba
Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pysek P, Sousa R, Tabacchi E, Vilà M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28(1):58–66
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography 30(1):3–12
Sokal RR, Rohlf JF (1995) Biometry: the principles and practice of statistics in biological research. W.H. Freeman, New York
Sommer TR, Nobriga ML, Harrell WC, Batham W, Kimmerer WJ (2001) Floodplain rearing of juvenile Chinook salmon: evidence of enhanced growth and survival. Can J Fish Aquat Sci 58(2):325–333
Steinman AD, Rosen BH (2000) Lotic–lentic linkages associated with Lake Okeechobee, Florida. J N Am Benthol Soc 19(4):733–741
Steinman AD, Conklin J, Bohlen PJ, Uzarski DG (2003) Influence of cattle grazing and pasture land use on macroinvertebrate communities in freshwater wetlands. Wetlands 23(4):877–889
Taylor CM, Warren ML (2001) Dynamics in species composition of stream fish assemblages: environmental variability and nested subsets. Ecology 82:2320–2330
Taylor PD, Hafrig L, Henein K, Merriam G (1993) Connectivity as a vital element of landscape structure. Oikos 68(571):573
Theobald D, Crooks R, Norman J (2011) Assessing effects of land use on landscape connectivity: loss and fragmentation of western US forests. Ecol Appl 21(7):2445–2458
Thomaz SM, Dibble ED, Evangelista LR, Higuti J, Bini LM (2008) Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Fresh Biol 53(2):358–367
Tockner K, Malard F, Ward JV (2000) An extension of the flood pulse concept. Hydrol Process 14:2861–2883
Tonn WM, Magnuson JJ (1982) Patterns in the species composition and richness of fish assemblages in northern Wisconsin lakes. Ecology 63:1149–1166
Urban D, Keitt T (2001) Landscape connectivity: a graph-theoretic perspective. Ecology 82(5):1205–1218
Walters DJJ, Kotze DC, O’Connor TG (2006) Impact of land use on vegetation composition, diversity, and selected soil properties of wetlands in the southern Drakensberg mountains, South Africa. Wetl Ecol Manag 14:329–348
Weaver MJ, Magnuson JJ, Clayton MK (1993) Analyses for differentiating littoral fish assemblages with catch data from multiple sampling gears. Trans Am Fish Soc 122:1111–1119
Zeilhofer P, Moura RM (2009) Hydrological changes in the northern Pantanal caused by the Manso dam: impact analysis and suggestions for mitigation. Ecol Eng 35(1):105–117
Zhao Q, Liu S, Deng L, Dong S, Yang Z, Yang J (2012) Landscape change and hydrologic alteration associated with dam construction. Int J Appl Earth Obs Geoinf 16:17–26
Zimmerman D, Pavlik C, Ruggles A, Armstrong MP (1999) An experimental comparison of ordinary and universal Kriging and inverse distance weighting. Math Geol 31(4):375–389
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We would like to thank the Pantanal Research Center for their financial support. We would also like to thank the National Council for Scientific and Technological Development (CNPq) for productivity grants given to Jansen Zuanon (Proc. 307464/2009-1) and the Postgraduate scholarship given to Izaias Fernandes. Our sincere thanks to S. Magela, M. Bini, F. Costa, W. E. Magnusson and C. E. C. Freitas and two anonymous referees for reviews of earlier versions of the manuscript that greatly improved it.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernandes, I., Penha, J. & Zuanon, J. Size-dependent response of tropical wetland fish communities to changes in vegetation cover and habitat connectivity. Landscape Ecol 30, 1421–1434 (2015). https://doi.org/10.1007/s10980-015-0196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-015-0196-2