Abstract
Contemporary landscape ecology continues to explore the causes and consequences of landscape heterogeneity across a range of scales, and demands for the scientific underpinnings of landscape planning and management still remains high. The spatial distribution of resources can be a key element in determining habitat quality, and that in turn is directly related to the level of heterogeneity in the system. In this sense, forest habitat mosaics may be more affected by lack of heterogeneity than by structural fragmentation. Nonetheless, increasing spatial heterogeneity at a given spatial scale can also decrease habitat patch size, with potential negative consequences for specialist species. Such dual effect may lead to hump-backed shape relationships between species diversity and heterogeneity, leading to three related assumptions: (i) at low levels of heterogeneity, an increase in heterogeneity favours local and regional species richness, (ii) there is an optimum heterogeneity level at which a maximum number of species is reached, (iii) further increase in spatial heterogeneity has a negative effect on local and regional species richness, due to increasing adverse effects of habitat fragmentation. In this study, we investigated the existence of a hump-shaped relationship between local plant species richness and increasing forest landscape heterogeneity on a complex mosaic in the French Alps. Forest landscape heterogeneity was quantified with five independent criteria. We found significant quadratic relationships between local forest species richness and two heterogeneity criteria indicators, showing a slight decrease of forest species richness at very high heterogeneity levels. Species richness–landscape heterogeneity relationships varied according to the heterogeneity metrics involved and the type of species richness considered. Our results support the assumption that intermediate levels of heterogeneity may support more species than very high levels of heterogeneity, although we were not able to conclude for a systematic negative effect of very high levels of heterogeneity on local plant species richness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many ecological phenomena are sensitive to spatial heterogeneity and fluxes within spatial mosaics. Landscape ecology, which concerns with spatial dynamics (including fluxes of organisms, materials, and energy) and the ways in which fluxes are controlled within heterogeneous mosaics, has provided new ways to explore aspects of spatial heterogeneity and to discover how spatial pattern wheels ecological processes (Pickett and Cadenasso 1995). One of the basic assumptions in ecology is that there are positive relationships between landscape heterogeneity and species richness at different spatial scales (Tews et al. 2004; Storch et al. 2007; Lundholm 2009). This assumption is related to niche theory, which links the fitness of individuals to their environment (Hutchinson 1957; Tamme et al. 2010). It states that each species is adapted to a particular set of abiotic conditions and biotic interactions, which determine the long-term persistence of populations. Niche divergence between species is one of the most important factors determining species coexistence in heterogeneous landscapes (Lundholm 2009). For instance, two categories of species might be distinguished according to their niche width: generalist species, which can benefit from different habitats in heterogeneous environments, and specialist species, which are dependent on a restricted range of resources or habitats and are more frequent in homogeneous environments (Clavel et al. 2010; Devictor et al. 2010). Thus, in a given area, a diversity of habitats favours the coexistence of specialist species that are linked to each of these habitats and generalist species that may use several habitats (Holt et al. 1999; Fahrig et al. 2011). This statement has led to the assumption that an area providing a wide amount and variety of resources or habitats (high heterogeneity) might support more species than a homogeneous area (Rosenzweig 1995; Fraser 1998; Polechová and Storch 2008). In heterogeneous areas, species richness at a local scale also appears to be directly affected by surrounding landscape heterogeneity, as it can be enriched by species or seed dispersal from neighbouring habitats (Steiner and Köhler 2003; Kumar et al. 2006; Zelený et al. 2010).
Relationships between species richness and landscape heterogeneity have been largely investigated, particularly since the 1970s (e.g. Roff 1974). Numerous studies have highlighted positive relationships for different aspects of spatial heterogeneity in relation to local and regional plant species richness (Skov 1997; Pausas et al. 2003; Dufour et al. 2006). For instance, some of the findings showed that high diversity of forest structural components at the stand level favours local undergrowth plant species richness and abundance (Brosofske et al. 2001; Bagnaresi et al. 2002; Macdonald and Fenniak 2007); while a diversified mosaic of stands with different structural characteristics might shelter more plant species at the landscape scale (Battles et al. 2001; Thysell and Carey 2001; Chávez and Macdonald 2010) as compared to homogeneous forest landscapes. Nevertheless, these relationships appear more complex than generally expected. While numerous studies have revealed positive effects of increasing landscape heterogeneity on species richness, others have found neutral or negative effects, depending on the metrics used to quantify heterogeneity, the taxonomic group considered (Wilson 2000) or the scale of analysis (Steiner and Köhler 2003; Tamme et al. 2010). At a given spatial scale, increasing spatial heterogeneity may reflect an increase in patch number and patch type diversity, but could also lead to a decrease in patch size and an increase in patch isolation (Mladenoff et al. 1993; Fahrig 2003; Dufour et al. 2006). Hence, it appears that high heterogeneity levels may lead to habitat patch fragmentation with negative consequences for specialist species adapted to homogeneous environments (Harrison 1999; Devictor et al. 2008; Zelený et al. 2010). This duality in the effects of landscape heterogeneity on biodiversity corresponds to the intermediate heterogeneity hypothesis proposed by Fahrig et al. (2011). In all, we can assume three general phases: (i) at low levels of heterogeneity, an increase in heterogeneity favours local and regional species richness, (ii) there is an optimum heterogeneity level at which a maximum number of species is reached, (iii) further increase in spatial heterogeneity has a negative effect on local and regional species richness, due to increasing adverse effects of habitat fragmentation.
In the same vein, others have documented three-phased relationships between plant species richness and spatial heterogeneity in different landscapes, e.g. natural forests (Zelený et al. 2010) and agricultural landscapes (Fahrig et al. 2011), or for other taxa (Allouche et al. 2012). However, while some studies have demonstrated the negative effects of silvicultural systems that lead to an increasing forest matrix fragmentation (e.g. Ripple et al. 1991; Spies et al. 1994), very few studies have focused on intra-forest habitat fragmentation in forests managed within a continuous forest cover system. As a way to improve sustainable forestry, several continuous forest cover systems such as selective cutting, shelterwood and seed-tree systems are increasingly used in European mountain forests, (Buongiorno et al. 1994; Bagnaresi et al. 2002). Hence, timber harvesting has often led to a shift in forest landscape structural characteristics, resulting in a more fragmented but also more heterogeneous landscape mosaic composed of different types of more or less closed forest stands (Mladenoff et al. 1993; Ares et al. 2009). As continuous forest cover systems are expanding in several countries worldwide, particularly in Europe, it is critical to gain knowledge on the relationships between species richness and landscape heterogeneity, to be able to provide guidelines for an adaptive forest management.
In this study, we investigated trends in the relationships between local plant species richness and landscape heterogeneity in complex mountain forest mosaics in the French Alps and a predicted hump-shaped relationship between forest landscape heterogeneity and local plant species richness. We performed the analyses on two species groups: (i) all species and (ii) forest species, i.e. species linked to closed forest conditions. Forest landscape heterogeneity was quantified using five criteria that reflected two different components of spatial heterogeneity: I—spatial heterogeneity resulting from natural environmental gradients, represented by two criteria (i) spatial heterogeneity of forest habitats (SHFH) and (ii) canopy composition diversity (CCD); II—spatial heterogeneity resulting from forest management practices, represented by three criteria (iii) spatial heterogeneity of stand types (SHST), (iv) spatial complementarities of stand structures (SCS) and (v) average stand structural complexity (ASSC).
Method
Study area
This work was conducted within the Vercors Natural Regional Park (VNRP), an area located at the border between the northern and the southern French Alps (Fig. 1). The Park covers 206,000 ha of land, of which 139,000 ha are forested. The study area encompasses a wide altitudinal range (500–2,200 m), with heterogeneous mountain topography. This results in a fine-scale mosaic of soil and microclimatic conditions leading to a high diversification of natural and semi-natural habitats in the forest landscape. The most common forest habitats are European beech (Fagus sylvatica, Linnaeus), mixed beech-silver fir (Abies alba, Miller), silver fir and Norway spruce (Picea abies, Karst) forests. These tree species are often accompanied by several secondary species, such as sycamore maple (Acer pseudoplatanus, L.), Italian maple (Acer opalus, Mill.), common whitebeam (Sorbus aria, Crantz), European mountain ash (Sorbus aucuparia, L.) and mountain pine (Pinus uncinata, Ramond) at high elevations. Elevation, overstory composition and managed practices are somehow related within the study area: at low elevations (500–1,100 m), forests are dominated by coppice-like stands, generally composed of broadleaved species and silver fir standards. Intermediate elevations (1,100–1,600 m) encompass a mosaic of diversified stands with single or multi-layered canopies dominated by coniferous or mixed conifer-broadleaved species composition. At high elevations (>1,600 m), the forest structure tends to be more even-aged, with pure mountain pine or pure Norway spruce forests.
Data
Vegetation data
We used 1,472 data plots recorded by the National Alpine Botanic Conservatory. Each plot covered an area of about 1,960 m2 (25 m radius), in which all occurring species were identified. The five most frequent understory species encountered were: Prenanthes purpurea (L.), Dryopteris filix-mas (L.), Galium odoratum (L.), Vaccinium myrtillus (L.) and S. aucuparia (L.). We considered two types of species richness: (i) total species richness (TSR), which encompassed forest species, edge species and non-forest species, and (ii) forest species richness (FSR), which only included species that were highly dependent on closed forest conditions, according to Rameau et al. (1993) (see Appendix S1 for the list of forest species). Forest species were considered separately because of their particular interest for forest biodiversity conservation (Peterken 1974; Aubin et al. 2007). All of the subsequent analyses were conducted separately for the two species groups.
Local variables
In order to characterise the environmental conditions for each data plot, we extracted values from six different raster datasets: elevation [digital elevation model (DEM) from the French National Geographic Institute], pH (adapted from Gégout and Renaux (2010), slope and aspect (derived from the DEM), mean annual temperature and mean annual precipitation (both variables extracted from the French Weather Institute). Plots were also intersected with maps of stand types and forest habitats (see description hereafter).
Cartographic data
The analysis of landscape heterogeneity was based on two 10 m spatial resolution raster maps, which represented two important drivers of forest landscape heterogeneity within the study area: (i) natural and semi-natural forest habitats (surrogate for environmental conditions and gradients) and (ii) forest stand structural diversity (surrogate for forest management).
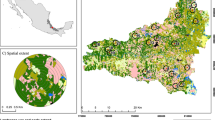
The first map was produced by classification of SPOT [© CNES (2010), distribution Spot Image S.A] images, using Définiens ®, Erdas Imagine and ArcGIS 9.3 software (see Breton et al. 2011). A hierarchical classification approach using image data and 6400 georeferenced floristic inventory points were used in tandem with environmental and ancillary information to produce the final forest habitat map. This map was classified according to CORINE Biotope European typology (Moss et al. 1990), which resulted in 10 forest habitat classes: European beech and beech-silver fir forests (41.1 and 43.1), Norway spruce forests (42.2), silver fir forests (42.1), mixed ravine and slope forests (41.4), thermophilous oak woods (41.7), mountain pine forests (42.4), Scots pine (Pinus sylvestris, L.) forests (42.5), stream European ash (Fraxinus excelsior, L.) woods (44.3) and non-forested areas (Fig. 2a).
Mapping and illustration of contrasting global heterogeneity levels: a raster maps of forest stand types to the left and forest habitats to the right within the study area (resolution 10 m), b buffer areas with contrasted spatial heterogeneity levels for forest stand types (SHST) and forest habitats (SHFH)
The second map was built in two steps using the ArcGIS 9.3 interface. We first gathered and harmonised recent (2009) detailed vector maps of stand types from the French National Forest Office (ONF). Seven stand types were delineated according to decreasing levels in forest structural diversity: multi-staged forests, two-staged forests, high forests, mixed coppice and high forests, simple coppices, young forests and young plantations (Fig. 3). All these stand types concerned mature forests (quadratic diameter >20 cm), except young forests and young plantations. Remaining forested areas matching clearings, micro-cliffs or open forests were all classified under the typological category “others”. Then, the generated map was rasterised to produce a 10 m resolution raster map with the seven forest stand types and a class for non-forest data (see Fig. 2a).
Analysis of forest landscape heterogeneity
Spatial landscape heterogeneity was computed within buffer areas of 250 m radius (19.6-ha area) created around each data plot, using ArcGIS 9.3 software. This buffer area allowed most of the spatial variability of forest habitats and stand types around the plots to be captured. It also corresponded to the scale at which management goals are implemented and, thus, influence forest spatial heterogeneity in the study area. To avoid effects of overlapping, buffers were only created for plots at least 500 m apart from each other, all other plots being discarded. In total, 313 plots were selected for the analysis.
The corresponding buffers were first intersected with the stand type and the forest habitat maps. Then, in each buffer forest landscape heterogeneity was quantified with five criteria: SHFH, CCD, SHST, SCS and ASSC. Each criterion was based on a combination of one or several quantitative indices describing forest habitats and stand type heterogeneity (Table 1a, b). SHFH and CCD were computed based on the forest habitat map. In order to consider these two criteria separately, this map was analysed at two different typological levels: (i) full ten-class typology for SHFH (i.e. European beech, beech-silver fir, Norway spruce, silver fir, mountain pine, Scots pine, mixed ravine and slope forests, thermophilous oak woods, stream European ash woods and non-forested areas) and (ii) the same typology aggregated at a three-class level for CCD (coniferous, broadleaved, mixed forests), with the non-forested areas considered as a no-data class. The three other criteria (SHST, SCS and ASSC) were based on the analysis of the stand type map.
Spatial heterogeneity of forest habitats and spatial heterogeneity of stand types
These two criteria (SHFH and SHST) were computed respectively on the forest habitat and the stand type raster maps and were based on the following four indices: area-weighted median patch size (AREA_WMD), patch richness (PR), patch equitability (SIEI) and patch continuity (MESH size). We used Fragstats 3.3 to compute these landscape metrics. For details on the mathematical expressions and further meaning of the indices, see (McGarigal et al. 2002).
The four landscape indices selected were aggregated to compute the two global indices of spatial heterogeneity (SHFH and SHST). In all, the four indices selected reflected complementary intra-forest landscape mosaic characteristics that are related to high heterogeneity levels. As a result, we depart from the assumption then, that high values favours forest biodiversity (Enoksson et al. 1995; Skov 1997; Chávez and Macdonald 2010). We therefore considered that the sum of the values measured by the four indicators was a way forward to evaluate the level of “global heterogeneity” present in a given buffer area. The spatial heterogeneity global index was calculated in two steps: we first rescaled the index ranges from 0 to 1, in order to make them commensurable. Then, we added the four corresponding values as follows:
Since each index ranged between 0 and 1 after rescaling, the spatial heterogeneity can theoretically varied continuously between 0 and 4 for a given buffer area. In this way, within the study area, which is considered as highly heterogeneous at the management unit level, SHFH varied between 0 and 2.20, while SHST varied between 0 and 2.72 (see Fig. 2b).
Canopy composition diversity
The CCD was calculated with the Shannon diversity index:
where n i is the number of patches for the canopy composition i, and N is the total number of patches present in a given area.
The index varied between 0, when the landscape was dominated by one type of canopy composition (i.e. coniferous, broadleaved or mixed forest), and ln (N max ) (i.e. 1.1 in our study area), when CCD was maximal (i.e. the three forest types are present) and each type of canopy composition occupied the same area.
Spatial complementarities of stand structures and average stand structural complexity
SCS reflected the number of stand structural attributes which were highly represented by adjacent stand types in a given buffer area, i.e. dead wood volume (m3/ha), heterogeneity of diameters estimated through the coefficient of variation, basal area of large trees (m2/ha) and tree species richness. SCS was a categorical criterion that varied between one and four. It was equal to one when the buffer area encompassed only one stand type (with either a diversified structure or not), or when the buffer area contained several stand types that were each characterised by the same configuration of structural attributes (e.g. all with high tree species richness and low values for the three other structural attributes). The value was maximal when the landscape encompassed at least four different stand types (generally of different diversity levels), which resulted in high values for the four structural attributes at the scale of the buffer area. In the same way, a value of two or three corresponded respectively to a buffer area with two or three different structural attributes highly represented according to the present combination of stand types (see Table A, Appendix S2 for details on the ranking of stand types according to structural attribute values).
ASSC was based on the analysis of the different levels of stand structural complexity that were present in a given buffer area, according to its composition in stand types (see Appendix S2 for details on the ranking of stand types based on structural complexity level). ASSC was computed as the area-weighted sum of stand structural complexity levels as follows:
where c i is the rank of a given stand type (i.e. seven for multi-staged and one for young forests); S ij is the area of the patch j of the stand type i; and TA equals total landscape area.
ASSC varied between zero in the case of a non-forested landscape and seven when the buffer area encompassed only multi-staged forests. Thus, although several structurally different stands had to be simultaneously present for high SCS value, ASSC could be very high when the buffer area was dominated by multi-staged forests.
Data analysis
The relationships between local floristic richness and spatial heterogeneity in buffer areas were analysed using stepwise multiple generalised linear regressions in R 2.11.1 (R core development team 2010). We first tested the effects of the five criteria of landscape heterogeneity (SHFH, CCD, SHST, SCS and ASSC). Then, for the two spatial heterogeneity criteria (SHFH and SHST), we investigated the performance of each individual index (AREA_WMD, PR, SIEI and MESH size) to predict species richness. To this aim, we built models including the three criteria CCD, SCS and ASSC, and eight indices: AREA_WMD_FH, PR_FH, SIEI_FH and MESH size_FH corresponding to SHFH, and AREA_WMD_ST, PR_ST, SIEI_ST and MESH size_ST corresponding to SHST. Spearman’s rank tests were performed in order to check for absence of collinearity among criteria and absence of collinearity among indices. No strong correlations were found in our dataset (all correlation coefficients were below 0.5).
To limit the effects of environmental conditions at the plot scale on species richness, we excluded all plots located in Scots pine forests, thermophilous oak woods and non-forested areas. This is an important factor to consider since these habitats comprised sharply different abiotic conditions when compared to the other habitats (i.e. European beech, beech-silver fir, Norway spruce, silver fir, mountain pine, mixed ravine and slope forests and stream European ash woods). After the exclusion of the plots as mentioned, we reduced the sample size to 254 plots, but we avoided important noise in the analysis. In addition, local environmental variables (e.g. elevation, pH, mean annual temperature and precipitations, slope and aspect), local forest habitat and local forest stand type were included in the models to control for a potential residual effect of abiotic conditions and particular local forest characteristics. According to the shape of the species richness distribution, we used Gaussian family models for TSR and Poisson family models for FSR, following Skov (1997). Residual distribution and homoscedasticity were visually checked.
We systematically included the landscape variables using linear and quadratic forms to detect the presence of a hump-shaped relationship. As the number of predictors was high relative to the number of observations, we used a specific R function (model.select 0.3) to include the number of observations in AIC calculation. This function also automatically estimates the consistency of all possible combinations of predictors based on a first general model that includes all the predictors (San Martin and Schtickzelle 2011). We therefore used this function to select the most parsimonious models. Then, as this function did not provide detailed model parameters, we used the GLM function in R to calculate p values and the pure part of deviance explained by each predictor.
Results
For TSR, a total of 1047 species were recorded, with an average of 27.3 species per plot (range 2–64). FSR encompassed 58 species, with a mean of four species per plot (range 0–16). Multiple generalised linear regressions showed that, despite the residual effects of local environmental variables, TSR and FSR were significantly related to different criteria of spatial heterogeneity. The type of relationship (variables involved, magnitude and direction) differed between TSR and FSR (see Tables 2, 3).
Initially, we noticed a driving effect of local stand type. Detailed analysis of model parameters revealed that TSR and FSR were generally the lowest in simple coppices and young forests. TSR was generally maximal in plantations and mixed coppices with high forests, whereas FSR was highest in mixed coppice with high forests and in two-staged forests. Then, we observed that TSR was only related to SHST. The relationship was negative, and in fact, the analysis at the index level suggested a significant negative effect of PR on TSR.
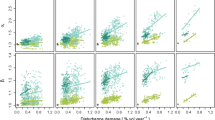
For FSR, we noticed a significant negative effect of CCD, suggesting that a mixture of coniferous, broadleaved and mixed forests adversely affects FSR. We also highlighted a significant quadratic relationship with an optimum value between FSR and SHFH (Fig. 4a). All other conditions being equal, when this criterion varied between 0.5 and 2.0 (for a maximal range of 0–4), 1.4 species were gained (with a mean of four species per plot). This effect of SHFH appeared to be due to a strong positive link with habitat patch continuity and to the quadratic effect of patch size equitability on FSR (Fig. 4b). For this last index, a variation between 0 and 1 was linked to a gain of 1.18 species. We also found a significant negative effect of forest habitat PR. However, the magnitude of this effect was low and certainly little influenced the shape of the relationship at the criteria level.
Finally, we detected a quadratic relationship with an optimum value between FSR and SCS, although the magnitude of this effect was low (Table 3; Fig. 4c). In this case, and for all other conditions being equal, 0.87 species were gained when the criterion varied between 0 and 3.2.
Discussion
As expected, we found significant quadratic relationships between local FSR and two criteria describing forest landscape composition and configuration: the SHFH and the SCS. These relationships were characterised by an optimum obtained for high values of heterogeneity. This trend was particularly pronounced in the case of SHFH, which showed the highest magnitude. Hence, global species richness would be maximised if a given landscape encompasses a diversified mosaic of habitat patches, but with patches large enough to maintain viable specialist species populations (Harner and Harper 1976; Honnay et al. 1999; Steiner and Köhler 2003). Such a result supports the intermediate heterogeneity hypothesis assuming that intermediate levels of heterogeneity may support more species than very high levels of heterogeneity as highlighted by several other studies (Holt 1997; Devictor et al. 2008; Fahrig et al. 2011) and expands its validity to managed forest within a continuous landscape matrix.
Our study also revealed that the relationship between local species richness and landscape heterogeneity varied according to the heterogeneity metrics involved and the type of species richness considered. For instance, our results showed negative relationships between species richness and some heterogeneity criteria. In particular, CCD (i.e. coniferous, broadleaved, mixed) was negatively related to FSR. As many studies have shown that a diversified overstory tree species (especially a mix of coniferous and broadleaved species) could positively influence local undergrowth species richness and composition (Saetre et al. 1997; Macdonald and Fenniak 2007; Chávez and Macdonald 2010), we expected a positive effect of CCD on local FSR. Our results did not support this assumption, although the negative effect we found was of low magnitude compared to other heterogeneity criteria. A possible explanation could be linked to the composition of the two groups of species considered (TSR and FSR), and in particular to their respective composition in specialist and generalist species. The choice of the two species groups did not consider this criterion, but both groups certainly encompassed a diversity of specialists species of different forest habitats and structures. Hence, our results may be explained by increasing evidence that species (specialisation/generalisation) patterns are dependent on spatial scale. For instance, some species can be generalists (colonise a diversity of habitats) at a given scale, while they behave as specialists (linked to only one type of habitats) at another scale (Hughes 2000; Devictor et al. 2010). In our case, the CCD was computed at a simplified three-class typological level, instead of ten-class (habitats) or eight-class (stand types) levels, as considered for the others criteria. Therefore, each forest type encompassed several forest habitats or stand types, as if it had been calculated at a larger spatial scale. Consequently, it is possible that FSR was mainly composed of species specialists of one type of canopy composition (coniferous, broadleaved, mixed). While at a lower typological scale (i.e. within each forest type), the species could be generalists where they can colonise a diversity of habitats and stand types.
Similarly, we found a significant negative relationship between TSR and SHST. This result contradicts other studies reporting positive effects of stand type diversity on large-scale species richness (Beese and Bryant 1999; Battles et al. 2001; Ares et al. 2009). Our negative effect of SHST might be related to the fact that TSR was composed of 83 % of species not strictly linked to closed forest canopy conditions. In this case, species could occupy a wide variety of habitats among which forest habitats are just one option. This may explain why TSR values were higher in landscapes characterised by low forest PR and small forest patch size embedded within large non-forested areas which have not been considered as distinct patch types in our classification (i.e. rocks, cliffs, meadows, large clearings).
Nevertheless, we should be cautious in the interpretation of these results. In the case of the quadratic relationships, the maximum values obtained were right skewed and there was a subsequent low decrease in species richness at very high heterogeneity levels. As plant species mainly depend on local abiotic conditions, they could be less sensitive to intra-forest habitat fragmentation than other mobile species (Peterken and Game 1981; Brunet 1993; Gazol and Ibáñez 2010). As a consequence, there is no reason to prejudge that species richness continues to decrease at higher heterogeneity levels than those observed in our study area. Moreover, the complexity of the forest landscape mosaic in the mountain region under study is influenced by numerous driving factors that could have influenced local floristic species richness. We considered two main heterogeneity components, natural environmental gradients and forest management practices, and variables statistically controlled for other environmental variables (i.e. elevation, pH, mean annual temperature and precipitations, slope and aspect), including local forest habitat and local forest type. But still may be other factors that may have blurred the phenomenon investigated, where further research will be worth pursuing.
Henceforward, we found that the magnitude of the relationships between local plant species richness and landscape heterogeneity was generally weak as compared to local variables, in particular local stand type (i.e. local forest structural complexity). Contrasting effects of different stand types were found, supporting other findings that have related local stand structure to undergrowth species richness and composition (Griffis et al. 2001; Decocq et al. 2004; Moora et al. 2007). Environmental variables such as soil pH, slope and mean annual temperature also influenced plant species richness at the plot scale, although their effects were weak and depended on the type of species richness considered. These results are supported by other studies that also showed local factors as stronger determinants of plant species richness distribution as compared to landscape factors (Kolb and Diekmann 2004; Gazol and Ibáñez 2010; Costanza et al. 2011).
Conclusion
In all, this study provides insights on the complex relationships that exist between local forest plant species richness and landscape heterogeneity. In that sense, it was found that relationships were generally positive or quadratic with an optimum obtained at high heterogeneity levels. This study in complex mountain environments open possibilities for further research to gain understanding on the range of scales at which quadratic relationships between local plant species richness and landscape heterogeneity could occur, and to test whether similar relationships exist for other taxonomic groups. Additionally, our results showed that the shape and the magnitude of the relationship between local species richness and landscape heterogeneity depend on the criteria and indices used to quantify landscape heterogeneity. As an increasing number of studies are focuses on the use of landscape heterogeneity analysis as a surrogate for large-scale biodiversity assessment (Lindenmayer et al. 2000; Schindler et al. 2009), we strongly advice considering several criteria that reflect different heterogeneity components and that are carefully selected for their positive relationships with species diversity. Otherwise, there is a risk of obtaining misleading results in particular for regional biodiversity assessment.
Linear models appeared as a good compromise to take voluntary decisions regarding the inclusion of quadratic terms, while searching for quadratic relationships. Even if in the present study, the responses obtained were generally of low magnitude, they provided evidence to support our hypothesis. In addition, an advantage of using linear models is that we gained on understanding of relationships between landscape heterogeneity and local species richness. Consequently, we can directly relate an increase in the value of each heterogeneity criteria with a precise predicted variation in the number of species. This is a direct and relatively simple approach to gain understanding of complex relationships and related processes. A next step will be to consider other more complex approaches to explore further relationships that may not be so evident with this simpler approach. Still we should continue to improve our understanding of the complex relationships and responses on heterogeneous landscapes.
Spatial heterogeneity provides important insurance in the face of unpredictable changes, enhances biodiversity and affords a greater variety of future silvicultural options to address changing land use objectives and environmental conditions (Turner et al. 2012). Furthermore, as pointed out by Turner et al. (2012), spatial heterogeneity is important for sustaining forest regeneration, primary production, carbon storage, natural hazard regulation, insect and pathogen regulation, as well as timber production and wildlife habitat. Thus, understanding how spatial heterogeneity and related landscape characteristics affect biodiversity patterns at local and landscape scales is critical for a better understanding of the underpinning ecological processes that may have mitigating effects on global environmental change.
Abbreviations
- FSR:
-
Forest species richness
- TSR:
-
Total species richness
- SHFH:
-
Spatial heterogeneity of forest habitats
- CCD:
-
Canopy composition diversity
- SHST:
-
Spatial heterogeneity of stand types
- SCS:
-
Spatial complementarities of stand structures
- ASSC:
-
Average forest stand structural complexity
References
Allouche O, Kalyuzhny M, Moreno-Rueda G, Pizarro M, Kadmon R (2012) Area-heterogeneity tradeoff and the diversity of ecological communities. Proc Natl Acad Sci USA 139(43):17495–17500
Ares A, Shanti B, Puettmann KJ (2009) Understory vegetation response to thinning disturbance of varying complexity in coniferous stands. Appl Veg Sci 12:472–487
Aubin I, Gachet S, Messier C, Bouchard A (2007) How resilient are northern hardwood forests to human disturbance? An evaluation using a plant functional group approach. Ecoscience 14(2):259–271
Bagnaresi U, Giannini R, Grassi G, Minotta G, Paffetti D, Pini Prato E, Proietti Placidi AM (2002) Stand structure and biodiversity in mixed, uneven-aged coniferous forests in the easthern Alps. Forestry 75(4):357–364
Battles JJ, Shlisky AJ, Barrett RH, Heald RC, Allen-Diaz BH (2001) The effects of forest management on plant species diversity in a Sierran conifer forest. For Ecol Manag 146:211–222
Beese WJ, Bryant AA (1999) Effect of alternative silvicultural systems on vegetation and bird communities in coastal montane forests of British Columbia, Canada. For Ecol Manag 115:231–242
Breton V, Renaud J, Luque S (2011) Comment les outils de la télédétection peuvent aider à la cartographie des habitats forestiers ? Mise au point d’une méthode sur le massif du Vercors. Rendez-Vous Tec de l’ONF 31:69–73
Brosofske KD, Chen J, Crow TR (2001) Understory vegetation and site factors: implications for a managed Wisconsin landscape. For Ecol Manag 146(1–3):75–87
Brunet J (1993) Environmental and historical factors limiting the distribution of rare forest grasses in south Sweden. Forest Ecology and Management 61:263–275
Buongiorno J, Dahir S, Lu H-C, Lin C-R (1994) Tree size diversity and economic returns in uneven-aged forest stands. For Sci 40(1):83–103
Chávez V, Macdonald SE (2010) The influence of canopy patch mosaics on understory plant community composition in boreal mixed wood forest. For Ecol Manag 259:1067–1075
Clavel J, Julliard R, Devictor V (2010) Worldwide decline of specialist species: toward a global functional homogenization. Front Ecol Environ 9:222–228
Costanza JK, Moody A, Peet RK (2011) Multi-scale environmental heterogeneity as a predictor of plant species richness. Landscape Ecol 26(6):851–864
Decocq G, Aubert M, Dupont F, Alard D, Saguez R, Wattez-Franger A, de Foucault B, Delelis-Dusollier A, Bardat J (2004) Plant diversity in a managed temperate deciduous forest: understorey response to two silvicultural systems. Journal of Applied Ecology 41:1065–1079
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514
Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, Venail P, Villéger S, Mouquet N (2010) Defining and measuring ecological specialization. J Appl Ecol 47:15–25
Dufour A, Gadallah F, Wagner HH, Guisan A, Buttler A (2006) Plant species richness and environmental heterogeneity in a mountain landscape: effects of variability and spatial configuration. Ecography 29:573–584
Enoksson B, Angelstam P, Larsson K (1995) Deciduous forest and resident birds: the problem of fragmentation within a coniferous forest landscape. Landscape Ecol 10(5):267–275
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin J-L (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Fraser RH (1998) Vertebrate species richness at the mesoscale: relative roles of energy and heterogeneity. Glob Ecol Biogeogr Lett 7(3):215–220
Gazol A, Ibáñez R (2010) Variation of plant diversity in a temperate unmanaged forest in northern Spain: behind the environmental and spatial explanation. Plant Ecol 207:1–11
Gégout J-C, Renaux B (2010) Les habitats forestiers de la France tempérée : typologie et caractérisation phytoécologique. Rev For Fr 62(3–4):365–374
Griffis KL, Crawford JA, Wagner MR, Moir WH (2001) Understory response to management treatments in northern Arizona ponderosa pine forests. For Ecol Manag 146(1–3):239–245
Harner RF, Harper KT (1976) The role of area, heterogeneity, and favorability in plant species diversity of Pinyon–Juniper ecosystems. Ecology 57(6):1254–1263
Harrison S (1999) Local and regional diversity in a patchy landscape: native, alien and endemic herbs on serpentine. Ecology 80(1):70–80
Holt RD (1997) From metapopulation dynamics to community structure: some consequences of spatial heterogeneity. In: Hanski I, Gilpin ME (eds) Metapopulation biology: ecology, genetics, and evolution. Academic Press, San Diego, pp 149–165
Holt RD, Lawton JH, Polis GA, Martinez ND (1999) Trophic rank and the species-area relationship. Ecology 80(5):1495–1504
Honnay O, Endels P, Vereecken H, Hermy M (1999) The role of patch area and habitat diversity in explaining native plant species richness in disturbed suburban forest patches. Divers Distrib 5:129–141
Hughes JB (2000) The scale of resource specialization and the distribution and abundance of Lycaenid butterflies. Oecologia 123:375–383
Hutchinson GE (1957) Concluding remarks, cold spring harbor symposium. Quant Biol 22:415–427
Kolb A, Diekmann M (2004) Effects of environment, habitat configuration and forest continuity on the distribution of forest plant species. J Veg Sci 15:199–208
Kumar S, Stohlgren TJ, Chong GW (2006) Spatial heterogeneity influences native and nonnative plant species richness. Ecology 87:3186–3199
Lindenmayer DB, Margules CR, Botkin DB (2000) Indicators of biodiversity for ecologically sustainable forest management. Conserv Biol 14(4):941–950
Lundholm JT (2009) Plant species diversity and environmental heterogeneity: spatial scale and competing hypotheses. J Veg Sci 20:377–391
Macdonald SE, Fenniak TE (2007) Understory plant communities of boreal mixedwood forests in western Canada: natural patterns and response to variable-retention harvesting. For Ecol Manag 242:34–48
McGarigal K, Cushman SA, Neel MC, Ene E (2002) FRAGSTATS: spatial pattern analysis Program for Categorical Maps. University of Massachusetts, Amherst
Mladenoff DJ, White MA, Pastor J, Crow TR (1993) Comparing spatial pattern in unaltered old-growth and disturbed forest landscapes. Ecol Appl 3(2):294–306
Moora M, Daniell T, Kalle H, Liira J, Püssa K, Roosaluste E, Öpik M, Wheatley R, Zobel M (2007) Spatial pattern and species richness of boreonemoral forest understorey and its determinants—a comparison of differently managed forests. For Ecol Manag 250:64–70
Moss D, Wyatt b, Cornaert M-H, Roekaerts M (1990) CORINE Biotopes. The design, compilation and use of an inventory of sites of major importance for nature conservation in the European Community. Directorate-General Environment, Nuclear Safety and Civil Protection, Brussels
Pausas JG, Carreras J, Ferré A, Font X (2003) Coarse-scale plant species richness in relation to environmental heterogeneity. J Veg Sci 14(5):661–668
Peterken GF (1974) A method for assessing woodland flora for conservation using indicator species. Biol Conserv 6(4):239–245
Peterken GF, Game M (1981) Historical factors affecting the distribution of Mercurialis perennis in Central Lincolnshire. J Ecol 69(3):781–796
Pickett STA, Cadenasso ML (1995) Landscape ecology: spatial heterogeneity in ecological systems. Science 269:331–334
Polechová J, Storch D (2008) Ecological niche. In: Jørgensen SE, Fath BD (eds) Encyclopedia of ecology. Elsevier, Oxford, pp 1088–1097
Rameau J-C, Mansion D, Dumé G, Lecointe A, Timbal J, Dupont P, Keller R (1993) Flore Forestière Française—Tome 2: Montagnes. Ministère de l’Agriculture et de la Pêche—Institut pour le développement forestier, Paris
R core team (2010) R: A Language and Environment for Statistical Computing
Ripple WJ, Bradshaw GA, Spies TA (1991) Measuring forest landscape patterns in the cascade range of Oregon, USA. Biol Conserv 57:73–88
Roff DA (1974) Spatial heterogeneity and the persistence of populations. Oecologia 15:245–258
Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press, Cambridge
Saetre P, Saetre LS, Brandtberg PO, Lundkvist H, Bengtsson J (1997) Ground vegetation composition and heterogeneity in pure Norway spruce and mixed Norway spruce–birch stands. Can J For Res 27:2034–2042
San Martin G, Schtickzelle N (2011) Model selection and multimodel inference in R with the model.select function. Version 2.0 edn. University of Louvain-la-Nauve, Belgium
Schindler S, Kati V, Von Wehrden H, Wrbka T, Poirazidis K (2009) Landscape metrics as biodiversity indicators for plants, insects and vertebrates at multiple scales. European landscapes in transformation: Challenges for landscape ecology and management, pp 228–231
Skov F (1997) Stand and neighbourhood parameters as determinants of plant species richness in a managed forest. J Veg Sci 8:573–578
Spies TA, Ripple WJ, Bradshaw GA (1994) Dynamics and pattern of a managed coniferous forest landscape in Oregon. Ecol Appl 4(3):555–568
Steiner NC, Köhler W (2003) Effects of landscape patterns on species richness—a modelling approach. Agric Ecosyst Environ 98:353–361
Storch D, Marquet PA, Brown JH (2007) Introduction: scaling biodiversity—what is the problem? In: Storch D, Marquet PA, Brown JH (eds) Scaling biodiversity. Cambridge University Press, Cambridge, pp 1–11
Tamme R, Hiiesalu I, Laanisto L, Szava-Kovats R, Pärtel M (2010) Environmental heterogeneity, species diversity and co-existence at different spatial scales. J Veg Sci 21:796–801
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Thysell DR, Carey AB (2001) Manipulation of density of Pseudotsuga menziesii canopies: preliminary effects on understory vegetation. Can J For Res 31:1513–1525
Turner MG, Donato DC, Romme WH (2013) Consequences of spatial heterogeneity for ecosystem services in changing forest landscapes: priorities for future research. Landscape Ecol 28:1081–1097
Wilson SD (2000) Heterogeneity, diversity and scale in plant communities. In: John EA, Stewart AJA (eds) Hutchings MJ. The ecological consequences of environmental heterogeneity Blackwell Scientific, Oxford, pp 53–69
Zelený D, Li C-F, Chytrý M (2010) Pattern of local plant species richness along a gradient of landscape topographical heterogeneity: result of spatial mass effect or environmental shift ? Ecography 33:578–589
Acknowledgments
This work was financially supported by a graduate student research fellowship to M. Redon from Grenoble University, France and by funds from the FORGECO project (ANR-09-STRA-02-01). We extend our appreciation to the National Forest Office which has provided vector maps of forest stand structures for all public forests in the study area. We also acknowledge Vincent Thierion and Vincent Breton for producing and providing the raster dataset of the Vercors mountain range natural habitats. Special appreciation goes to the National Alpine Botanic Conservatory which provided the vegetation data inventory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Redon, M., Bergès, L., Cordonnier, T. et al. Effects of increasing landscape heterogeneity on local plant species richness: how much is enough?. Landscape Ecol 29, 773–787 (2014). https://doi.org/10.1007/s10980-014-0027-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-014-0027-x