Abstract
The contractile performance of mammalian fast twitch skeletal muscle is history dependent. The effect of previous or ongoing contractile activity to potentiate force, i.e. increase isometric twitch force, is a fundamental property of fast skeletal muscle. The precise manifestation of force potentiation is dependent upon a variety of factors with two general types being identified; staircase potentiation referring to the progressive increase in isometric twitch force observed during low frequency stimulation while posttetanic potentiation refers to the step—like increase in isometric twitch force observed following a brief higher frequency (i.e. tetanic) stimulation. Classic studies established that the magnitude and duration of potentiation depends on a number of factors including muscle fiber type, species, temperature, sarcomere length and stimulation paradigm. In addition to isometric twitch force, more recent work has shown that potentiation also influences dynamic (i.e. concentric and/or isotonic) force, work and power at a range of stimulus frequencies in situ or in vitro, an effect that may translate to enhanced physiological function in vivo. Early studies performed on both intact and permeabilized models established that the primary mechanism for this modulation of performance was phosphorylation of myosin, a modification that increased the Ca2+ sensitivity of contraction. More recent work from a variety of muscle models indicates, however, the presence of a secondary mechanism for potentiation that may involve altered Ca2+ handling. The primary purpose of this review is to highlight these recent findings relative to the physiological utility of force potentiation in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1993 two review articles were published that addressed functional, mechanistic and biochemical features of myosin phosphorylation mediated force potentiation in vertebrate skeletal muscle (Grange et al. 1993; Sweeney et al. 1993). These comprehensive reviews provided important theoretical and practical information for future investigations. Work performed in the past 20 years has greatly expanded our knowledge concerning myosin phosphorylation and force potentiation. Thus, the purpose of this review is to highlight both functional and mechanistic aspects of force potentiation, focusing on mechanistic studies on animal muscle models. This article includes recent evidence from both wildtype and transgenic mouse as well as rat skeletal muscle models expanding the physiological utility of this mechanism for contraction. Existing cross-bridge models for the influence of myosin phosphorylation on mechanical function will be modified to account for these results. The genetic, enzymatic and regulatory aspects of RLC phosphorylation have been recently reviewed by Stull et al. (2011) and will not be detailed in this article.
First described in the literature over 100 years ago (see Lee 1907) force potentiation has long been recognized as a fundamental property of fast twitch skeletal muscle. Potentiation is generally expressed as an increase in isometric twitch force independent of change to peak tetanic force, thus increasing the twitch to tetanus ratio (e.g., Bagust et al. 1974; Ramsey and Street 1941). In contrast, slow twitch skeletal muscle displays a posttetanic depression of isometric twitch force (Buller et al. 1981; Close and Hoh 1969). A list of studies describing potentiation of fast twitch vertebrate striated muscle is compiled in Table 1. Potentiation is readily induced in most fast twitch skeletal muscles studied and as such may be a normal operating feature of these muscles (Brown and Loeb 1998). In the laboratory setting, the precise manifestation of potentiation is highly paradigm dependent, however (Fig. 1). For example, staircase potentiation describes the progressive increase in isometric twitch force observed during a period of low frequency stimulation (e.g., Colomo and Rocchi 1965; Isaacson 1969).
Examples of potentiation in fast twitch skeletal muscle. Typical experimental paradigms producing posttetanic potentiation (PTP) (a) or staircase potentiation (b). In a a single control or unpotentiated isometric twitch is elicited under resting conditions prior to the delivery of a brief, but high frequency conditioning stimulation. The precise time course of PTP depends on the frequency and duration of stimulation; in mouse EDL muscle at 25 °C the peak tends to occur shortly after the conditioning stimulus before decaying over several minutes to pre-stimulus response. Twitch duration may or may not be abbreviated compared to the unpotentiated twitch. In b a series of evenly spaced isometric twitches is elicited at a fixed low frequency (typically 5–10 Hz). In most species, stimulation rates below 5 Hz do not produce staircase while stimulation rates above 10 Hz produce fused contractions. During staircase, an initial modest decrease in twitch force is often observed before progressively increasing. As for PTP, the precise magnitude of staircase observed depends on stimulation rate, species, temperature and muscle length. Note that in both the top and bottom panels the time scale is variable
During staircase potentiation, isometric twitch amplitude may initially decrease before increasing to a peak that is dependent upon both stimulus rate and number; upon the cessation of stimulation, twitch force dissipates slowly at first and then with a more rapid time course (Krarup 1981a). On the other hand, posttetanic potentiation (PTP) describes the acute increase in isometric twitch force observed following high (or sometimes low) frequency stimulation (e.g., Close and Hoh 1968b). Like staircase, the posttetanic response is also stimulus frequency and duration dependent, often displaying an initial increase to a peak shortly after the cessation of stimulation before dissipating, rapidly at first and more slowly thereafter, over the course of several minutes (e.g., Krarup 1981a). In addition to stimulus parameters, both staircase and PTP are highly sensitive to experimental factors such as temperature (high>low), muscle length (short>long) and contraction type (concentric>isometric). Even though a single mechanism for staircase and PTP is often assumed, the existence of divergent mechanisms now seems probable.
Although much has been learned regarding how potentiation benefits the mechanical performance of isolated muscles, a definitive teleological role in vivo has not yet been identified. Interesting in this regard is evidence suggesting that the potentiated state is the normal operating state of fast twitch skeletal muscle in vivo (Brown and Loeb 1998). In addition, work on isolated mouse fast twitch skeletal muscles shows that beta-adrenergic stimulation prolongs the potentiated state, an outcome that may indicate a role for potentiation during the fight or flight response of mammals (Decostre et al. 2000). Thus, much remains to be discovered regarding how potentiation integrates into overall organismal function when extra-muscular signaling is intact.
Many early studies attempted to identify an ionic origin for potentiation, with mostly equivocal results (e.g., Brown and von Euler 1938; Bernhard et al. 1941; Walker 1948). The demonstration by Ramsey and Street (1941) that potentiation is a property of isolated skeletal muscle fibers indicated a muscle origin for this phenomenon. Indeed, by the 1960s both staircase and PTP were considered to be intrinsic properties of skeletal muscle (e.g., Standaert 1964) although the precise mechanism was still not known. Interestingly, the observation that the magnitude of PTP was inversely related to the twitch:tetanus ratio was seen as evidence that alterations to the “active state” were responsible (Close and Hoh 1968a; Ritchie and Wilkie 1955). It was not until the demonstration that the myosin II molecule of vertebrate striated muscle contained a phosphorylatable light chain subunit (Perrie et al. 1973) that a viable intracellular mechanism reconcilable with contractile data was available, however. Two small protein subunits, the essential light chain and regulatory light chain (RLC) wrap around the α-helical neck or light chain binding domain of the myosin heavy chain, providing mechanical stability (Rayment and Holden 1994; Trybus 1994). Although skeletal muscle contraction results from the Ca2+ —regulated formation of force-generating actomyosin complexes and the linking of myosin ATPase activity to structural changes in this complex (reviewed by Geeves and Holmes 2005; Vale and Milligan 2000), phosphorylation of the RLC subunit may modify these unitary reactions to enhance muscle cell force and shortening (Sweeney and Stull 1990).
Potentiation by RLC phosphorylation in rodent muscle
Work performed on isolated rat and mouse skeletal muscle demonstrates that isometric twitch potentiation is temporally correlated with RLC phosphate content following various stimulus regimes. A comprehensive list of studies examining the phosphorylation-potentiation relationship in different rodent muscle models is presented in Tables 2 and 3. In studies where multiple measurements of isometric twitch potentiation and RLC phosphorylation have been made, both non- linear (Klug et al. 1982) and linear relationships (Manning and Stull 1979; Moore et al. 1990; Palmer and Moore 1989; Vandenboom et al. 1995, 1997; Xeni et al. 2011) have been documented. However, when data from these respective studies are pooled according to species and temperature in Fig. 2, the resultant scatter plot depicting twitch potentiation versus RLC phosphorylation are decidedly linear. The robustness of these relationships is particularly striking given the inevitable differences in methodology between the different studies used to construct these plots. This analysis thus indicates that RLC phosphorylation has a direct and predictable influence on the isometric twitch force of unfatigued fast twitch rodent skeletal muscle, an effect that increases as muscle temperature approaches the physiological range. Because these results were obtained from mostly unfatigued muscle it is possible that more extreme stimulus regimes causing more severe fatigue will disrupt the linearity of these relationships, especially at high phosphorylation levels. In addition, experimental interventions that alter thin filament activation level may also alter the slope of any of these relationships (Krarup 1981b, c; MacIntosh and Gardiner 1987; MacIntosh and Kupsh 1987; Palmer and Moore 1989; Vandenboom and Houston 1996).
Quantitative relationship between stimulation induced isometric twitch potentiation and RLC phosphorylation in rodent skeletal muscle. a Data from studies employing rat gastrocnemius and EDL muscle with separate linear fits to results obtained at 23 and 35–37 °C (r value = 0.79 and 0.82, respectively). b Data from studies employing mouse EDL muscle with separate linear fits to data obtained at 25 and 30 °C (r value = 0.89 and 0.90, respectively). Although the scatter of data is increased, there is a clear effect of increasing temperature on the slope of the RLC phosphorylation vs isometric twitch force potentiation relationship in both species. Note that in both panels we have pooled data from experiments in which the conditioning stimulus was fixed and the decay in RLC phosphorylation and twitch force over time was tracked and in which different conditioning stimuli were used and RLC phosphorylation and twitch force were assessed at a fixed time point. Data in panel A taken from Klug et al. 1982; Manning and Stull 1979, 1982; Moore and Stull 1984. Data in panel B taken from Moore et al. 1990; Palmer and Moore 1989; Vandenboom et al. 1995, 1997; Xeni et al. 2011. Although not included, data from Moore et al. (1990) for mouse EDL muscle at 35 °C also shows a linear response
The first direct evidence of a causal relationship between RLC phosphorylation and isometric force potentiation was provided by studies utilizing permeabilized skeletal muscle fibers. A list of these studies is compiled in Table 4. Persechini et al. (1985) were the first to show that the addition of exogenous skeletal myosin light chain kinase (skMLCK) to the media bathing rabbit psoas fibers both phosphorylated the RLC and increased steady state tension at submaximal, but not maximal, Ca2+ activation. Subsequent studies by this and other goups have elegantly documented how the potentiation of steady state force by RLC phosphorylation appears to be inversely related to thin filament activation levels (e.g., Davis et al. 2002; Metzger et al. 1989; Patel et al. 1996, 1998; Sweeney and Kushmerick 1985; Stephenson and Stephenson 1993; Sweeney and Stull 1986, 1990; Szczesna et al. 2002). An important feature of many of these studies is the increase in the rate constant for steady state force development (i.e. the kTR) that occurs following RLC phosphorylation, an effect that may be the mechanical basis for twitch potentiation in intact muscle. These studies have thus provided important mechanistic links between skMLCK catalyzed phosphorylation of the RLC and isometric twitch force potentiation in intact skeletal muscle models. A model for the influence of RLC phosphorylation on cross-bridge kinetics, and interactions with Ca2+ activation of the thin filament, is detailed below.
RLC phosphorylation and cross-bridge structure
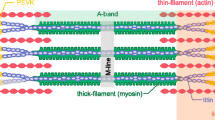
Skeletal muscle force is regulated by Ca2+ ion binding to regulatory proteins on the thin filament (Ebashi and Endo 1968). Although the ligand binding role of Ca2+ and regulatory protein conformation on the thin filament have been studied extensively (e.g., Lehman et al. 2009) there may be, in addition, thick filament structural constraints that participate in force regulation. For example, reconstructions of the thin filament of rested skeletal fibers from arthropods and mammals show that myosin head distribution is highly ordered with close proximity to the thick filament surface (Woodhead et al. 2005; Zhao et al. 2009).
Head to head interactions via highly conserved motifs present in both regulated and unregulated myosins may be responsible for this constraint, a profile that may minimize myosin head interactions with inactivated thin filaments (Jung et al. 2008). Interestingly, structural studies employing a variety of fiber types show that the addition of a negative charge to the RLC via phosphorylation disrupts these interactions to displace myosin heads away from the thick filament (Alamo et al. 2008; Brito et al. 2011; Craig et al. 1987; Hidalgo et al. 2001; Levine et al. 1991, 1996, 1998; Padrón et al. 1991; Ritz-Gold et al. 1980; Sweeney et al. 1994; Yang et al. 1998). It is proposed that these phosphorylation-induced changes to myosin head position on the thick filament surface increase the Ca2+ sensitivity of force development by promoting the formation of the actomyosin complex achieved by Ca2+ signalling alone (see below). These structure–function relations are consistent with findings from intact rat skeletal muscle showing that potentiation is greatest at short and minimized at long sarcomere lengths, respectively (e.g., Rassier et al. 1997, 1998; Rassier and MacIntosh 2000) (c.f. Moore and Persechini 1990). Finally, findings from experiments using in vitro motility assays showing that phosphorylation of the RLC does not improve intrinsic myosin motor function in isolation from the thin filament supports these arguments (Greenberg et al. 2009).
RLC phosphorylation and cross-bridge cycling kinetics
Huxley (1957) was the first to provide an analytical framework for understanding cross-bridge cycling in skeletal muscle. In his original scheme Huxley proposed that muscle force was regulated by the cyclic attachment and detachment of “side pieces” between the myofilaments. These rate constants, known as F and G, respectively, were considered intrinsic properties of independently operating force generators within the sarcomere; these structures were, in time, recognized as the actomyosin cross-bridge. Brenner and Eisenberg (1986) and Brenner (1988) advanced this two state model for cross-bridge action by incorporating the regulatory influence of Ca2+ binding to troponin C (TnC) on force. In their modern scheme, Ca2+ binding to TnC controls the rate constant describing the transition of cycling cross-bridges from non-force to force generating states (i.e. f app ). In contrast, the reverse rate constant describing the transition of cycling cross-bridges from force generating back to non-force generating states (i.e. g app ) is mostly unregulated and not under the influence of Ca2+. Thus, although only a fraction of the total available cross-bridge population may be involved, force is proportional to the fraction of cycling cross-bridges able to attain the force generating state (αFS). In turn, this term is determined by the balance between f app and g app according to the equation αFS = f app /(f app + g app ). Within this scheme the influence of RLC phosphorylation on force may be understood by noting that RLC phosphorylation increases f app at all Ca2+ activation levels with little or no effect on g app (Sweeney et al. 1993). Thus, at low levels of myofilament activation where f app is small relative to g app , the RLC phosphorylation-mediated increases in f app greatly enhance cross-bridge formation (i.e. increase αFS). Conversely, at high levels of myofilament activation where f app is large relative to g app , crossbridge formation is little affected as αFS is already near unitary (Sweeney and Stull 1990). Moreover, the absence of any effect on g app accounts for why unloaded shortening velocity of permeabilized skeletal fibers (Persechini et al. 1985; Sweeney and Stull 1990) or intact skeletal muscle (Butler et al. 1983; Decostre et al. 2000; Gittings et al. 2011; Palmer and Moore 1989) are not altered.
Results from skMLCK knockout models
Perhaps the most compelling evidence from intact skeletal muscles for a causative relationship between RLC phosphorylation and isometric twitch force potentiation comes from experiments using muscles from mice devoid of the skMLCK enzyme and which, as a consequence, do not display stimulation-induced elevations in RLC phosphorylation. In this regard, Zhi et al. (2005) showed that brief tetanic stimulation of EDL muscles from wildtype mice increased both RLC phosphorylation and twitch force by ~4 and 1.8-fold, respectively, but did not elevate either of these parameters in EDL muscles from skMLCK deficient or skMLCK−/− mice. Examples of posttetanic responses from wildtype and skMLCK−/− muscles is shown in Fig. 3. Interestingly, however, low frequency repetitive stimulation of skMLCK−/− muscles still produces a significant staircase potentiation, albeit attenuated by ~50 % compared to wildtype muscles. Indeed, this remnant potentiation in skMLCK−/− muscles accords with the presence of potentiation in muscle disease or atrophy models (MacIntosh et al. 2008a; Rassier et al. 1999). Interestingly, Ryder et al. (2007) showed that overexpression of skMLCK in mouse EDL muscle enhanced the rate of RLC phosphorylation and staircase potentiation relative to wildtype muscles. The absence of PTP but continued presence of staircase potentiation in this genotype suggests that although skMLCK catalyzed phosphorylation of the RLC is the primary mechanism for PTP, additional mechanisms may contribute to staircase potentiation (Zhi et al. 2005; Gittings et al. 2011).
Genotype dependence of potentiation in mouse fast skeletal muscle Comparison of posttetanic potentiation (PTP) of EDL muscles from and wildtype (WT) (a) and skeletal myosin light chain kinase (skMLCK) knockout (KO) (b) muscles (in vitro 25 °C). The influence of a tetanic conditioning stimulus (four-volleys of 100 Hz stimulation, each lasting 400 ms, in a 20 s time window) on isometric twitch force is shown. Note that although still present, the potentiation of twitch force was much greater in WT than skMLCK KO muscles. For example, in the WT muscle the post-twitches were potentiated by up to 22 % relative to the pre-twitch and were potentiated for more than 180 s. In the skMLCK KO muscle, post-twitches were potentiated by only 5 % relative to the pre-twitch and this potentiation lasted for only 30 s. Twitch time course was similar for each genotype regardless of differences in peak force. The time interval between tetani has been compressed for clarity. In both genotypes peak tetanic force was decreased similarly from the first to last tetanic volley. Previously unpublished data
The influence of RLC phosphorylation on skeletal muscle mechanics may apply to both force development and relaxation. For example, evidence from skMLCK−/− muscles suggests that, in addition to increases in the rate and/or extent of force development, RLC phosphorylation may influence force relaxation kinetics as well. Gittings et al. (2011) compared high frequency tetanic contractions of EDL muscles from skMLCK−/− and wildtype mice following a stimulus protocol that elevated RLC phosphorylation in wildtype muscles only. They found that, despite no changes in peak tetanic force in either genotype, the relaxation rate of wildtype muscles was significantly slowed relative to skMLCK−/− muscles (in vitro, 25° C). This outcome corroborates findings from both intact cat (Brown and Loeb 1999) and permeabilized rabbit psoas skeletal fibers (Patel et al. 1998) but the functional significance of this effect is unknown. Moreover, although an increased rate of relaxation of potentiated twitch contractions is often observed, this effect may not be attributable to RLC phosphorylation as skMLCK−/− muscles also show this effect (Gittings et al. 2011).
An interesting corollary to the results from skMLCK−/− mice are results from insect flight muscle in which mutations to the serine residue have rendered the RLC unphosphorylatable. In these experiments, the power and flight characteristics of Drosophila melanogaster were attenuated related to wildtype flight characteristics (Dickinson et al. 1997; Miller et al. 2011; Tohtong et al. 1995).
Stimulus frequency and contraction type dependence for potentiation
Studies performed prior to the 1990s tended to study potentiation using single pulse, twitch contractions. Although single pulse contractions have been shown to represent ~30 % of all motor unit discharge events in hindlimb muscles of freely moving rats (Hennig and Lømo 1987), an influence of potentiation on higher frequency, multiple pulse (i.e. tetanic) contractions would greatly extend its physiological utility. The first to systematically study the stimulus frequency dependence for isometric force potentiation was Vandenboom et al. (1993) using a mouse EDL in vitro (25 °C) muscle model. These investigators showed that although a low frequency conditioning stimulus that elevated RLC phosphorylation to near maximal levels increased the rate of isometric force development at all frequencies between 1 and 200 Hz, the potentiation of peak force was restricted to frequencies below 20 Hz. Indeed, peak tetanic forces measured at higher frequencies were decreased rather than increased. It is important to point out that any threshold that is identified for peak force potentiation must be highly model dependent. As an example of this, more recent work from rat skeletal muscle in situ (35 °C) showed that potentiation of peak isometric force may in fact be observed at high stimulus frequencies (MacIntosh and Bryan 2002; MacIntosh and Willis 2000). This apparent difference may be related to the tetanus duration used to examine high frequency force; the enhanced +dF/dt that is a general characteristic of potentiated rodent muscle (Grange et al. 1995) may be able to enhance the peak force that is attained during brief, but not necessarily long, duration tetani.
Another vital mechanical factor that modulates the ability of RLC phosphorylation to potentiate muscle function is that of contraction type, i.e. isometric versus concentric. A host of studies performed on rat and mouse skeletal muscle demonstrates that the same stimulus regimes that potentiates isometric function also potentiate dynamic function (Abbate et al. 2000; Caterini et al. 2011; Gittings et al. 2012; Grange et al. 1995, 1998; MacIntosh et al. 2008b; Xeni et al. 2011). Evidence from these studies indicates that muscle shortening during concentric force development may actually sensitize the contractile apparatus to potentiation; as a result, dynamic force levels observed during shortening are potentiated to a much greater extent than might be predicted based on isometric responses alone. Representative force records depicting the potentiation of concentric forces during tetanic stimulation of wildtype and skMLCK−/− muscles are shown in Fig. 4. The first study showing this effect was Grange et al. (1995) who used mouse EDL muscles (in vitro, 25 °C) to show that work and power during after—loaded twitch contractions were enhanced more than isometric twitch force levels (see also Grange et al. 1998). Many years later, the sensitizing influence of muscle shortening was confirmed by Xeni et al. (2011) who showed that concentric twitch force was potentiated more than isometric twitch force when RLC phosphorylation levels were similar. Subsequent work from our laboratory has shown that the influence of muscle shortening speed on potentiation is progressive, i.e. twitch forces during fast, moderate and slow shortening were increased more than isometric twitches for the same increase in RLC phosphorylation (Caterini et al. 2011) an effect that also applies to higher frequency forces observed during partially fused tetani (Gittings et al. 2012). A speed-dependent increase in potentiation has also been found in rat gastrocnemius muscle studied in situ, suggesting that this response is a general feature of muscle function rather than just a species specific response (Abbate et al. 2000).
Influence of muscle shortening on potentiation in mouse muscle. Comparison of concentric force potentiation of EDL muscles from WT (a) and skMLCK knockout (b) mice. Each panel shows concentric force during shortening at 25 % Vmax while stimulated at 45 Hz for 100 ms before (thick trace) and after (thin trace) a tetanic conditioning stimulus (4 volleys of 100 Hz stimulation each lasting 400 ms). This maneuver increased mean concentric force by ~40 and by 20 % in the WT and skMLCK knockout muscles, respectively. In each panel, the horizontal line depicts peak force level during the tetanus before the conditioning stimulus. Previously unpublished data
Work on intact frog skeletal fibers by Piazzesi et al. (2007) examining the influence of filament sliding on cross-bridge cycling kinetics (i.e. f app and/or g app ) may be able to account for the speed dependence for potentiation at the sarcomeric level. For example, their experiments show that while filament sliding may increase both fapp and gapp relative to isometric, the increase in gapp much exceeds the increase in fapp at both moderate and high, but not slow, speeds of shortening. Thus, the effect of shortening to decrease the number of attached cross-bridges (i.e. αFS) is consistent with a speed dependence for concentric force potentiation. Indeed, the relationship between fapp and gapp shown in Fig. 4d of Piazzesi et al. (2007) may be able to account for why although very slow shortening does not greatly increase the potentiation of concentric compared to isometric force, moderate speeds of shortening do greatly increase concentric compared to isometric force at most activation levels (i.e. Gittings et al. 2012).
Potentiation and locomotion
Although potentiation of isometric contractions may be physiologically relevant, the stimulus frequency and shortening speed dependence for potentiation described above has important ramifications for skeletal muscle function in vivo. During locomotion, for example, skeletal muscle must generate concentric work and absorb eccentric work on a cyclic basis (reviewed by Josephson 1993). Because concentric forces are potentiated in a speed dependent manner it seems possible to suggest that faster speeds of locomotion benefit in two ways: i.e., motive forces produced by agonists may be increased at each stimulus frequency and/or the stimulus frequency range over which motive forces produced by agonists is greatly increased relative to that observed during wholly isometric contractions. This effect would both increase work and power output of potentiated muscles under a wide variety of activation envelopes and also decrease the neural input required to achieve a given submaximal level of muscle work and power (as demonstrated in human skeletal muscles during isometric contractions by Klein et al. 2001; Inglis et al. 2011). On the other hand, because eccentric forces are largely unaffected, the forces required by antagonists may be largely unchanged during cyclic muscle activity typical of locomotion. Interesting in this regard are the results of Childers and McDonald (2004) who used permeabilized rabbit psoas skeletal fibers to show that RLC phosphorylation exacerbated damage caused by active lengthening despite the fact that eccentric forces were not increased.
Fiber phenotype and potentiation
The fiber type dependence for potentiation was first demonstrated by elegant studies showing reduced PTP in muscles exposed to chronic stimulation (Close and Hoh 1969). This fundamental difference is accounted for, at least in part, on the basis of differences in skMLCK and myosin light chain phosphatase (MLCP), the enzyme responsible for dephosphorylating the RLC. In general, skMLCK content is highest in fast-glycolytic and lowest in slow-oxidative fiber types of rodents; on the other hand, MLCP content is highest in slow-oxidative and lowest in fast- glycolytic fibers (Stull et al. 2011). Moreover, chronic stimulation of rabbit tibialis anterior muscle reduces skMLCK expression in a time dependent manner that anticipates attendant changes in myosin heavy chain isoform expression, indicating that the skMLCK enzyme is part of the fast, but not slow, muscle genetic program (Klug et al. 1986, 1992). Phenotypical differences in contractile response to stimulation, highlighting differences of fast and slow muscles from mouse from our laboratory, are shown in Fig. 5.
Phenotype dependence of potentiation in mouse fast and slow skeletal muscle. a Comparison of posttetanic responses of EDL (left) and soleus (right) muscles. (Top panels) The effect of a brief tetanic conditioning stimulus (four volleys of 100 Hz stimulation, each lasting 400 ms, in a 20 s time window) to cause posttetanic potentiation of EDL and posttetanic depression of soleus muscles from wildtype mice. (Bottom panels) The effect of the same conditioning stimulus on EDL and soleus muscles from skMLCK KO mice. In this case PTP of EDL is not evident but PTD of soleus is still present. b Western blots showing phosphorylation of RLC in wildtype EDL and soleus muscles before and after a tetanic conditioning stimulus. Note that in these examples, the RLC phosphate content of wildtype (WT) EDL muscles was increased significantly from rest by stimulation and PTP was evident. In contrast, the RLC phosphate content of WT soleus muscles was similar before and after stimulation and PTP was absent. The RLC phosphate content of skMLCK KO muscles was not determined in these experiments. Horizontal arrows at blots depict unphosphorylated (skRLC) and phosphorylated blots (skRLCP), respectively. Previously unpublished data
Myosin heavy chain isoform expression may not singularly predict the capacity for potentiation. For example, although prolonged, high frequency stimulation may produce moderate elevations in RLC phosphorylation, little or no twitch potentiation is observed in rat soleus muscle (Manning and Stull 1982; Moore and Stull 1984). Consistent with this, Ryder et al. (2007) showed that overexpression of skMLCK in soleus muscle did not lead to twitch force potentiation despite stimulation induced phosphorylation of both slow and fast RLCs. The reason for the lack of twitch potentiation with substantial RLC phosphorylation in soleus muscles from transgenic mice is not clear. C57BL/6 mouse EDL muscle contains 70 % type IIb fibers (Gorselink et al. 2002). In contrast, the mouse soleus muscle contains as little as 6 % type IIb fibers with 59 % type IIa and 35 % type I fibers (Totsuka et al. 2003). Thus, the potentiation of contraction is correlated to type IIb fibers. Potentially, the sarcomeric interfilament spacing of type I and type IIa fibers may be sufficiently small for optimal force development even if RLC is not phosphorylated. It may also be possible to hypothesize that myosin light chain (MLC) isoform expression provides additional regulation. Up to five distinct MLC isoforms are present in mammalian skeletal muscle with the expression of slow (MLC1s, MLC2s) or fast (MLCf, MLC1f, MLC2f, MLC3f) isoforms generally mirroring myosin heavy chain profile (Bicer and Reiser 2004; Gonsalez et al. 2002; Schiaffino and Reggiani 1996, 2011). Interestingly, although it is unclear if alterations to MLC expression can occur independent of myosin heavy chain expression, skeletal muscle plasticity includes the MLC isoform. For example, the slow to fast phenotype transformation of rat muscle shown with hindlimb suspension or clenbuterol administration includes a change in the relative expression of slow to fast MLC isoforms (Bozzo et al. 2003). Moreover, the complementary, reverse, pattern of change to MLC and myosin heavy chain isoform was observed when a fast to slow phenotype transformation was induced (Bozzo et al. 2005). Similar results have been presented by Stevens et al. (2000, 2004). These studies suggest that, although the slow MLC isoforms may still be phosphorylatable, alterations to MLC isoform may participate in the fiber type dependence for RLC phosphorylation—mediated force potentiation. The mechanistic details for this regulation are unknown but it may be possible that RLC isoform composition differentially influences head to head interactions and displacements in the absence and presence of serine phosphorylation, respectively.
Alternate Ca2+ based mechanism for potentiation?
Results from skMLCK−/− muscles indicates the presence of a secondary mechanism for potentiation that is highly stimulus regime dependent. In principal there are several mechanisms that may be able to account for this RLC phosphorylation-independent increase in isometric twitch force; clearly, the contribution of these mechanisms to potentiation may be obscured by RLC phosphorylation influences in wildtype muscles. Recent work using mouse lumbrical has provided evidence that stimulation-induced alterations to resting Ca2+ homeostasis can account for isometric twitch potentiation in the absence of stimulation-induced elevations in RLC phosphorylation (Smith et al. 2013). Indeed, the mouse lumbrical thus appears to be a unique fast-muscle in that it does not appear to contain the enzymatic apparatus for RLC phosphorylation (Ryder et al. 2007; Smith et al. 2013). In these experiments, stimulation did not produce any apparent change to the amplitude or kinetics of the intracellular [Ca2+]i transient itself but a short lived (20−30 s) increase in resting myoplasmic [Ca2+]i was observed, an increase that correlated temporally with the relatively small and short lived isometric twitch potentiation that was observed. The short lived nature of the increase in [Ca2+]i observed in these experiments could explain why staircase potentiation is less affected than PTP by the absence of RLC phosphorylation (MacIntosh et al. 2008a; Rassier et al. 1999). Although the mechanism of action remains unknown, stimulation induced increases in resting [Ca2+]i could enhance force by increasing the Ca2+ occupancy of troponin C or other Ca2+ buffers (e.g., parvalbumin) prior to twitch stimulation (e.g., Barclay 1992). On the other hand, the effect of increased resting [Ca2+]i could be more complex and be mediated at the level of the thick filament, perhaps by increasing the population of weakly-bound cross-bridges, a necessary precursor to the attainment of the strongly-bound, force generating state in some cross-bridge models (Kraft et al. 1999). Clearly, more work is needed to establish how muscle force is potentiated in the absence of RLC as well as the relative contribution of secondary mechanisms to potentiation in muscles with RLC phosphorylation.
Fatigue and potentiation
Twitch force potentiation and fatigue have been demonstrated to exist in a variety of single fiber and whole skeletal muscle preparations (Gordon et al. 1990; MacIntosh and Gardiner 1987; MacIntosh and Kupsh 1987; MacIntosh et al. 1993; Rankin et al. 1988; Tubman et al. 1996a; Vandenboom and Houston 1996; Vergara et al. 1977). This may suggest that RLC phosphorylation offsets fatigue or that certain metabolites arising during repetitive stimulation are able to potentiate twitch force. Interestingly, the relative depression in twitch force observed during the early, but not late, stages of repetitive stimulation of mouse EDL muscle is greater in skMLCK−/− than in wildtype muscles (Gittings et al. 2011). Although this result seems to corroborate a role for RLC during moderate, but not severe fatigue, it does not eliminate the contribution of other intracellular mechanisms. As an example, Barclay (1992) used mouse EDL muscles (25 °C) to show that the enhanced rate of force development observed simultaneous with fatigue could be attributed to increases in intracellular levels for inorganic phosphate ([Pi]). Increased [Pi] may influence cross-bridge kinetics in such a way that the rate of rise of isometric force is increased coincident with fatigue (e.g., Hibberd et al. 1985). Although plausible, this mechanism is difficult to isolate experimentally from other mechanisms, including RLC phosphorylation, in fatigued wildtype muscle.
Cooke and colleagues have performed a series of studies examining the complex interactions that may occur between metabolic changes during fatigue and RLC phosphorylation (Cooke 2007). It could be noted that, in unfatigued skeletal muscle, RLC phosphorylation is expected to influence pre—but not post power—stroke steps in the cross-bridge cycle (i.e. Sweeney and Stull 1990). Work by Franks-Skiba et al. (2007) and Karatzaferi et al. (2008) both suggest, however, that when the metabolites [Pi] and/or [H+] are elevated to levels that mimic those observed in fatigued skeletal muscle, the rate of release of ADP following the power stroke is delayed in phosphorylated cross-bridges (c.f., Stewart et al. 2009). This mechanism is in fact supported by studies on individual myosin molecules using the in vitro motility assay technique under non-fatigue conditions (Greenberg et al. 2010). It is of interest that these results could account for both the augmented twitch force and/or the slowed shortening velocity sometimes observed during and attributed to fatigue. Interestingly, no differences in either peak tetanic force or unloaded shortening velocity that are expected from these interactions were noted between skMLCK knockout and wildtype mouse muscles during severe fatigue at 25 °C (Gittings et al. 2011). A possible explanation for this discrepancy is that the interaction between fatigue related changes in metabolites and RLC phosphorylation in permeabilized fibers were found to be highly temperature dependent, with little interaction noted below 30 °C. Further work on intact muscle at higher temperatures (e.g., 30 °C) is perhaps needed to clarify this issue.
RLC phosphorylation and metabolism
The physiological ability of the RLC phosphorylation mechanism to modulate skeletal muscle function in vivo may hinge upon its influences on the metabolic cost or efficiency of muscle contraction. For example, if RLC phosphorylation (or other mechanisms) increases the energetic cost of contraction, potentiated contractions may prove to be unsustainable metabolically. Unfortunately, studies in this area have been equivocal, however. For example, early studies were interpreted to suggest that phosphorylation of the RLC decreased energetic demand during sustained or repetitive contractions of mouse EDL muscle in vitro (Crow and Kushmerick 1982a, b). Subsequent work also using a mouse muscle model questioned this outcome (Barsotti and Butler 1984; Butler et al. 1983). More recently, Abbate et al. (2001) have re-examined the issue by studying the energetic cost of potentiated contractions in rat muscle. These authors reported that high energy phosphate turnover was increased to a greater extent than was force, work and power, thus decreasing muscle economy of potentiated versus unpotentiated contractions. Critically, the decreased economy was attributed to differences in force, not RLC phosphorylation, implying that potentiated contractions may not be metabolically sustainable (Abbate et al. 2001). Although this result would seem to have resounding implications for the physiological utility of RLC phosphorylation to modulate repetitive type contractions, the robustness of this decrease to different contractile conditions (i.e. temperature, species, fiber type, stimulation rate, shortening speed etc.) is as yet unknown.
Potentiation in diseased muscle
Most work examining potentiation has been performed using non-diseased, healthy animal skeletal muscles. Exceptions to this trend suggest that certain peripheral diseases may influence potentiation, however. For example, Krarup (1983) showed that both staircase and PTP were attenuated in a neuropathy rat muscle model of chronic myasthenia gravis. In addition, potentiation has been shown to be reduced in parallel with disuse and/or atrophy of rat fast twitch muscle (MacIntosh et al. 1988; Tubman et al. 1996b, 1997). Another example is work by Smith et al. (2010) who showed that a high frequency conditioning stimulus caused greater PTP in EDL muscles from mdx mice than in EDL muscles from age matched controls, despite similar RLC phosphorylation at both ages and muscle types (see also Hoekman 1977). Thus, more information regarding interactions between potentiation and peripheral diseases such as sarcopenia and/or cachexia is required before a full understanding of how this fundamental response is altered in disease is achieved.
Summary
The different fiber types comprising skeletal muscles display a range of metabolic and mechanical properties geared to optimize function. Prominent among these differences is that of force potentiation, a history dependent enhancement of dynamic force, work and power at physiological activation rates in mammalian fast twitch muscle. Evidence from a variety of sources points to stimulation-induced elevations in RLC phosphate incorporation by skMLCK as the primary mechanism for potentiation, although stimulation-induced elevations in resting [Ca2+]i may provide a secondary mechanism in some muscle fibers. Evidence from skMLCK knockout and disuse models indicates that staircase potentiation observed during prolonged low frequency stimulation may be due to both RLC phosphorylation and alterations in resting [Ca2+]i while PTP observed after brief or high frequency stimulation is predominantly due to RLC phosphorylation. Further investigations are required to resolve the relative contribution of these respective mechanisms to potentiation phenomena.
References
Abbate F, Sargeant AJ, Verdijk PW, de Haan A (2000) Effects of high-frequency initial pulses and posttetanic potentiation on power output of skeletal muscle. J Appl Physiol 88:35–40
Abbate F, Van Der Velden J, Stienen GJ, De Haan A (2001) Post-tetanic potentiation increases energy cost to a higher extent than work in rat fast skeletal muscle. J Musc Res Cell Motil 22:703–710
Alamo L, Wriggers W, Pinto A, Bártoli F, Salazar L, Zhao FQ, Craig R, Padrón R (2008) Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol 384(4):780–797
Bagust J, Lewis DM, Luck JC (1974) Post-tetanic effects in motor units of fast and slow twitch muscle of the cat. J Physiol 237(1):115–121
Barclay CJ (1992) Effect of fatigue on rate of isometric force development in mouse fast- and slow-twitch muscles. Am J Physiol 263(32):1065–1072
Barsotti RJ, Butler TM (1984) Chemical energy usage and myosin light chain phosphorylation in mammalian skeletal muscle. J Musc Res Cell Motil 5(1):45–64
Bernhard CG, von Euler US, Skoglund CR (1941) Post-tetanic action potentials in mammalian muscle. Acta Physiol Scand 2:284–288
Bicer S, Reiser PJ (2004) Myosin light chain isoform expression among single mammalian skeletal muscle fibres: species variations. J Musc Res Cell Motil 25:623–633
Bozzo C, Stevens L, Toniolo L, Mounier Y, Reggiani C (2003) Increased phosphorylation of myosin light chain associated with slow-to-fast transition in rat soleus. Am J Physiol (Cell Physiol) 285:575–583
Bozzo C, Spolaore B, Toniolo L, Stevens L, Bastide B, Cieniewski-Bernard C, Fontana A, Mounier Y, Reggiani C (2005) Nerve influence on myosin light chain phosphorylation in slow and fast skeletal muscles. FEBS J 272:5771–5785
Brenner B (1988) Effect of Ca+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Nati Acad Sci USA 85:3265–3269
Brenner B, Eisenberg E (1986) Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83(10):3542–3546
Brito R, Alamo L, Lundberg U, Guerrero JR, Pinto A, Sulbarán G, Gawinowicz MA, Craig R, Padrón R (2011) A molecular model of phosphorylation-based activation and potentiation of tarantula muscle thick filaments. J Mol Biol 414(1):44–61
Brown IA, Loeb GE (1998) Post-activation potentiation: a clue for simplifying models of muscle dynamics. Am Zool 38:743–754
Brown IE, Loeb GE (1999) Measured and modeled properties of mammalian skeletal muscle. I. The effects of post-activation potentiation on the time course and velocity dependencies of force production. J Musc Res Cell Motil 20:443–456
Brown GL, von Euler US (1938) The after effects of a tetanus on mammalian muscle. J Physiol 93:39–60
Buller AJ, Kean CJ, Ranatunga KW, Smith JM (1981) Post-tetanic depression of twitch tension in the cat soleus muscle. Exp Neurol 73(1):78–89
Butler TM, Seigman MJ, Mooers SV, Barsotti RJ (1983) Myosin light chain phosphorylation does not modulate cross bridge cycling in mouse skeletal muscle. Science 220:1167–1169
Caterini D, Gittings W, Huang J, Vandenboom R (2011) The effect of work cycle frequency on the potentiation of dynamic function in fast mouse muscle. J Exp Biol 214:3915–3923
Childers MK, McDonald KS (2004) Regulatory light chain phosphorylation increases eccentric contraction-induced injury in skinned fast-twitch fibers. Musc Nerv 29:313–317
Close R, Hoh JF (1968a) Influence of temperature on isometric contractions of rat skeletal muscles. Nature 217(5134):1179–1180
Close R, Hoh JF (1968b) The after-effects of repetitive stimulation on the isometric twitch contraction of rat fast skeletal muscle. J Physiol 197:461–477
Close R, Hoh JF (1969) Post-tetanic potentiation of twitch contractions of cross-innervated rat fast and slow muscles. Nature 221(5176):179–181
Colomo F, Rocchi P (1965) Eserine effects on single twitches and staircase phenomenon in frog nerve-single muscle fibre preparations. Arch Fisiol 65(1):24–51
Cooke R (2007) Modulation of the actomyosin interaction during fatigue of skeletal muscle. Musc Nerv 36:756–777
Craig R, Padrón R, Kendrick-Jones J (1987) Structural changes accompanying phosphorylation of tarantula muscle myosin filaments. J Cell Biol 105(3):1319–1327
Crow MT, Kushmerick MJ (1982a) Phosphorylation of myosin light chains in mouse fast-twitch muscle associated with reduced actomyosin turnover rate. Science 217(4562):835–837
Crow MT, Kushmerick MJ (1982b) Myosin light chain phosphorylation is associated with a decrease in the energy cost for contraction in fast twitch mouse muscle. J Biol Chem 257(5):2121–2124
Davis JS, Satorius CL, Epstein ND (2002) Kinetic effects of myosin regulatory light chain phosphorylation on skeletal muscle contraction. Biophys J 83(1):359–370
Decostre V, Gillis JM, Gailly P (2000) Effect of adrenaline on the post-tetanic potentiation in mouse skeletal muscle. J Musc Res Cell Motil 21:247–254
Dickinson MH, Hyatt CJ, Lehmann FO, Moore JR, Reedy MC, Simcox A, Tohtong R, Vigoreaux JO, Yamashita H, Maughan DW (1997) Phosphorylation-dependent power output of transgenic flies: an integrated study. Biophys J 73(6):3122–3134
Ebashi S, Endo M (1968) Calcium ion and muscle contraction. Prog Biophys Mol Biol 18:123–183
Franks-Skiba K, Lardelli R, Goh G, Cooke R (2007) Myosin light chain phosphorylation inhibits muscle fiber shortening velocity in the presence of vanadate. Am J Physiol (Regul Integr Comp Physiol) 292:1603–1612
Geeves MA, Holmes KC (2005) The molecular mechanism of muscle contraction. Adv Protein Chem 71:161–193
Gittings W, Huang J, Smith IC, Quadrilatero J, Vandenboom R (2011) The effect of skeletal myosin light chain kinase gene ablation on the fatigability of mouse fast muscle. J Musc Res Cell Motil 31:337–348
Gittings W, Huang J, Vandenboom R (2012) Tetanic force potention of mouse EDL muscle is shortening speed dependent. J Musc Res Cell Motil 33(5):359–368
Gonsalez B, Negredo P, Hernando R, Manso R (2002) Protein variants of skeletal muscle regulatory myosin light chain isoforms: prevalence in mammals, generation and transitions during muscle remodeling. Eur J Physiol 443:377–386
Gordon DA, Enoka RM, Stuart DG (1990) Motor-unit force potentiation in adult cats during a standard fatigue test. J Physiol 421:569–582
Gorselink M, Drost MR, de Brouwer KF, Schaart G, van Kranenburg GP, Roemen TH, van Bilsen M, Charron MJ, van der Vusse GJ (2002) Increased muscle fatigability in GLUT-4-deficient mice. Am J Physiol Endocrinol Metab 282(2):348–354
Grange RW, Vandenboom R, Houston ME (1993) Physiological significance of myosin phosphorylation in skeletal muscle. Can J Appl Physiol 18(3):229–242
Grange RW, Cory CR, Vandenboom R, Houston ME (1995) Myosin phosphorylation augments the force-displacement and force-velocity relationships of mouse fast muscle. Am J Physiol 269:713–724
Grange RW, Vandenboom R, Xeni J, Houston ME (1998) Potentiation of in vitro concentric work in mouse fast muscle. J Appl Physiol 84:236–243
Greenberg MJ, Mealy TR, Watt JD, Jones M, Szczesna-Cordary D, Moore JR (2009) The molecular effects of skeletal muscle myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol 297(2):265–274
Greenberg MJ, Mealy TR, Jones M, Szczesna-Cordary D, Moore JR (2010) The direct molecular effects of fatigue and myosin regulatory light chain phosphorylation on the actomyosin contractile apparatus. Am J Physiol Regul Integr Comp Physiol 298(4):989–996
Guttman SA, Horton RG, Wilber DT (1937) Enhancement of muscle contraction after tetanus. Am J Physiol 119(3):463–473
Hennig R, Lømo T (1987) Gradation of force output in normal fast and slow muscles of the rat. Acta Physiol Scand 130(1):133–142
Hibberd MG, Dantzig JA, Trentham DR, Goldman YE (1985) Phosphate release and force generation in skeletal muscle fibers. Science 228(4705):1317–1319
Hidalgo C, Craig R, Ikebe M, Padrón R (2001) Mechanism of phosphorylation of the regulatory light chain of myosin from tarantula striated muscle. J Musc Res Cell Motil 22(1):51–59
Hoekman TB (1977) Fatigability of normal and dystrophic chicken muscle in vivo. Exp Neurol 54(3):565–578
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Inglis JG, Howard J, McIntosh K, Gabriel DA, Vandenboom R (2011) Decreased motor unit discharge rate in the potentiated human tibialis anterior muscle. Acta Physiol 201(4):483–492
Isaacson A (1969) Post-staircase potentiation, a long-lasting twitch potentiation of muscles induced by previous activity. Life Sci 8(7):337–342
Josephson RK (1993) Contraction dynamics and power output of skeletal muscle. Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol 55:527–546
Jung HS, Komatsu S, Ikebe M, Craig R (2008) Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol Biol Cell 19(8):3234–3242
Karatzaferi C, Franks-Skiba K, Cooke R (2008) Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol (Regul Integr Comp Physiol) 294:948–955
Klein CS, Ivanova TD, Rice CL, Garland SJ (2001) Motor unit discharge rate following twitch potentiation in human triceps brachii muscle. Neurosci Lett 316(3):153–156
Klug GA, Botterman BR, Stull JT (1982) The effect of low frequency stimulation on myosin light chain phosphorylation in skeletal muscle. J Biol Chem 257:4670–4688
Klug GA, Houston ME, Stull JT, Pette D (1986) Decrease in myosin light chain kinase activity of rabbit fast muscle by chronic stimulation. FEBS Lett 200(2):352–354
Klug GA, Biedermann M, Houston ME, Stuart D, Mumby M, Stull JT (1992) Chronic low frequency stimulation reduces myosin phosphorylation in rabbit fast twitch muscle. Can J Physiol Pharmacol 70(6):859–865
Kraft T, Xu S, Brenner B, Yu LC (1999) The effect of thin filament activation on the attachment of weak binding cross-bridges: a two-dimensional x-ray diffraction study on single muscle fibers. Biophys J 76(3):1494–1513
Krarup C (1981a) Enhancement and diminution of mechanical tension evoked by staircase and by tetanus in rat muscle. J Physiol 311:355–372
Krarup C (1981b) Temperature dependence of enhancement and diminution of tension evoked by staircase and by tetanus in rat muscle. J Physiol 311:373–387
Krarup C (1981c) The effect of dantrolene on the enhancement and diminution of tension evoked by staircase and by tetanus in rat muscle. J Physiol 311:389–400
Krarup C (1983) Evoked responses in normal and diseased muscle with particular reference to twitch potentiation. Acta Neurol Scand 68(5):269–315
Lee FS (1907) The cause of treppe. Am J Physiol 18:267–282
Lehman W, Galińska-Rakoczy A, Hatch V, Tobacman LS, Craig R (2009) Structural basis for the activation of muscle contraction by troponin and tropomyosin. J Mol Biol 388(4):673–681
Levine RJ, Chantler PD, Kensler RW, Woodhead JL (1991) Effects of phosphorylation by myosin light chain kinase on the structure of Limulus thick filaments. J Cell Biol 113(3):563–572
Levine R, Kensler R, Yang Z, Stull J, Sweeney H (1996) Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J 71:898–907
Levine RJ, Yang Z, Epstein ND, Fananapazir L, Stull JT, Sweeney HL (1998) Structural and functional responses of mammalian thick filaments to alterations in myosin regulatory light chains. J Struct Biol 122:149–161
MacIntosh BR, Bryan SN (2002) Potentiation of shortening and velocity of shortening during repeated isotonic tetanic contractions in mammalian skeletal muscle. Pflugers Arch 443:804–812
MacIntosh BR, Gardiner PF (1987) Posttetanic potentiation and skeletal muscle fatigue: interactions with caffeine. Can J Physiol Pharmacol 65(2):260–268
MacIntosh BR, Kupsh CC (1987) Staircase, fatigue, and caffeine in skeletal muscle in situ. Musc Nerve 10(8):717–722
MacIntosh BR, Willis JC (2000) Force-frequency relationship and potentiation in ammalian skeletal muscle. J Appl Physiol 88(6):2088–2096
MacIntosh BR, Roberge MC, Gardiner PF (1988) Absence of staircase following disuse in rat gastrocnemius muscle. Can J Physiol Pharmacol 66(6):707–713
MacIntosh BR, Grange RW, Cory CR, Houston ME (1993) Myosin light chain phosphorylation during staircase in fatigued skeletal muscle. Pflugers Arch 425:9–15
MacIntosh BR, Smith MJ, Rassier DE (2008a) Staircase but not posttetanic potentiation in rat muscle after spinal cord hemisection. Musc Nerve 38(5):1455–1465
MacIntosh BR, Taub EC, Dormer GN, Tomaras EK (2008b) Potentiation of isometric and isotonic contractions during high-frequency stimulation. Pflugers Arch 456:449–458
Manning DR, Stull JT (1979) Myosin light chain phosphorylation and phosphorylase A activity in rat extensor digitorum longus muscle. Biochem Biophys Res Commun 90(1):164–170
Manning DR, Stull JT (1982) Myosin light chain phosphorylation -dephosphorylation in mammalian skeletal muscle. Am J Physiol 242:C234–C241
Metzger JM, Moss RL (1990) Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science 247(4946):1088–1090
Metzger JM, Greaser ML, Moss RL (1989) Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibres. J Gen Physiol 93:855–883
Miller MS, Farman GP, Braddock JM, Soto-Adames FN, Irving TC, Vigoreaux JO, Maughan DW (2011) Regulatory light chain phosphorylation and N-terminal extension increase cross-bridge binding and power output in drosophila at in vivo myofilament lattice spacing. Biophys J 100(7):1737–1746
Moore RL, Persechini A (1990) Length-dependence of isometric twitch tension potentiation and myosin phosphorylation in mouse skeletal muscle. J Cell Physiol 143(2):257–262
Moore RL, Stull JT (1984) Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. Am J Physiol Cell Physiol 247(5):C462–C471
Moore RL, Houston ME, Iwamoto GA, Stull JT (1985) Phosphorylation of rabbit skeletal muscle myosin in situ. J Cell Physiol 125:301–305
Moore RL, Palmer BL, Williams SL, Tanabe H, Grange RW, Houston ME (1990) Effect of temperature on myosin phosphorylation in mouse skeletal muscle. Am J Physiol 259:C432–C438
Padrón R, Panté N, Sosa H, Kendrick-Jones J (1991) X-ray diffraction study of the structural changes accompanying phosphorylation of tarantula muscle. J Musc Res Cell Motil 12(3):235–241
Palmer BM, Moore RL (1989) Myosin light chain phosphorylation and tension potentiation in mouse skeletal muscle. Am J Physiol 257:C1012–C1019
Patel JR, Diffee GM, Moss RL (1996) Myosin regulatory light chain phosphorylation modulates the Ca2+ dependence of the kinetics of tension development in skeletal muscle. Biophys J 70(5):2333–2340
Patel JR, Diffee GM, Huang XP, Moss RL (1998) Phosphorylation of myosin regulatory light chain eliminates force-dependent changes in relaxation rates in skeletal muscle. Biophys J 74:360–368
Perrie WT, Smillie LB, Perry SB (1973) A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J 135(1):151–164
Persechini A, Stull JT, Cooke R (1985) The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem 260:7951–7954
Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V (2007) Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131(4):784–795
Ramsey RW, Street SF (1941) Muscle function as studied in single muscle fibres. Biol Symp 3:9–34
Rankin LL, Enoka RM, Volz KA, Stuart DG (1988) Coexistence of twitch potentiation and tetanic force decline in rat hindlimb muscle. J Appl Physiol 65(6):2687–2699
Rassier DE, Herzog W (2002) Effect of pH on the length dependent twitch potentiation in skeletal muscle. J Appl Physiol 92(3):1293–1299
Rassier DE, MacIntosh BR (2000) Length dependence of staircase potentiation: interactions with caffeine and dantrolene sodium. Can J Physiol Pharmacol 78(4):350–357
Rassier DE, MacIntosh BR (2002) Sarcomere length-dependence of activity-dependent twitch potentiation in mouse skeletal muscle. BMC Physiol 2:19
Rassier DE, Tubman LA, MacIntosh BR (1997) Length-dependent potentiation and myosin light chain phosphorylation in rat gastrocnemius muscle. Am J Physiol 273(1):C198–C204
Rassier DE, Tubman LA, MacIntosh BR (1998) Caffeine and length dependence of staircase potentiation in skeletal muscle. Can J Physiol Pharmacol 76(10–11):975–982
Rassier DE, Tubman LA, MacIntosh BR (1999) Staircase in mammalian muscle without light chain phosphorylation. Braz J Med Biol Res 32(1):121–129
Rayment I, Holden HM (1994) The three-dimensional structure of a molecular motor. Trends Biochem Sci 19(3):129–134
Ritchie JM, Wilkie DR (1955) The effect of previous stimulation on the active state of muscle. J Physiol 130:488–496
Ritz-Gold CJ, Cooke R, Blumenthal DK, Stull JT (1980) Light chain phosphorylation alters the conformation of skeletal muscle myosin. Biochem Biophys Res Commun 93(1):209–214
Ryder JW, Lau KS, Kamm KE, Stull JT (2007) Enhanced skeletal muscle contraction with myosin light chain phosphorylation by a calmodulin-sensing kinase. J Biol Chem 282:20447–20454
Schiaffino S, Reggiani C (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76(2):371–423
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91(4):1447–1531
Smith IC, Huang J, Quadrilatero J, Tupling AR, Vandenboom R (2010) Force potentiation in the MDX mouse. J Musc Res Cell Motil 31(4):267–277
Smith IC, Gittings W, Bloemberg D, Huang J, Quadrialtero J, Tupling AR, Vandenboom R (2013) Potentiation in mouse lumbrical muscle without myosin light chain phosphorylation: is resting calcium responsible? J Gen Physiol 141(3):297–308
Standaert FG (1964) The mechanisms of post-tetanic potentiation in cat soleus and gastrocnemius muscles. J Gen Physiol 47:987–1001
Stephenson GM, Stephenson DG (1993) Endogenous MLC2 phosphorylation and Ca(2+)-activated force in mechanically skinned skeletal muscle fibres of the rat. Stephenson GM, Stephenson DG. Pflugers Arch 424(1):30–38
Stevens L, Firinga C, Gohlsch B, Bastide B, Mounier Y, Pette D (2000) Effects of unweighting and clenbuterol on myosin light and heavy chains in fast and slow muscles of rat. Am J Physiol (Cell Physiol) 279(5):1558–1563
Stevens L, Bastide B, Bozzo C, Mounier Y (2004) Hybrid fibres under slow-to-fast transformations: expression is of myosin heavy and light chains in rat soleus muscle. Flugers Arch 448(5):507–514
Stewart M, Franks-Skiba K, Cooke R (2009) Myosin regulatory light chain phosphorylation inhibits shortening velocities of skeletal muscle fibers in the presence of the myosin inhibitor blebbistatin. J Musc Res Cell Motil 30:17–27
Stull JT, Kamm C, Vandenboom R (2011) Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys 510:120–128
Sweeney HL, Kushmerick MJ (1985) Myosin phosphorylation in permeabilized rabbit psoas fibers. Am J Physiol 249(3 Pt 1):C362–C365
Sweeney HL, Stull JT (1986) Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. Am J Physiol 250(4 Pt 1):657–660
Sweeney HL, Stull JT (1990) Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: Implications for regulation of actin-myosin interaction. Proc Natl Acad Sci USA 87:414–418
Sweeney HL, Bowman BF, Stull JT (1993) Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol 264:1085–1095
Sweeney HL, Yang Z, Zhi G, Stull JT, Trybus KM (1994) Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci USA 91:1490–1494
Szczesna D, Zhao J, Jones M, Zhi G, Stull J, Potter JD (2002) Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. J Appl Physiol 92(4):1661–1670
Tohtong R, Yamashita H, Graham M, Haeberle J, Simcox A, Maughan D (1995) Impairment of muscle function caused by mutations of phosphorylation sites in myosin regulatory light chain. Nature 374(6523):650–653
Totsuka Y, Nagao Y, Horii T, Yonekawa H, Imai H, Hatta H, Izaike Y, Tokunaga T, Atomi Y (2003) Physical performance and soleus muscle fiber composition in wild-derived and laboratory inbred mouse strains. J Appl Physiol 95(2):720–727
Trybus KM (1994) Role of myosin light chains. J Musc Res Cell Motil 15(6):587–594
Tubman LA, MacIntosh BR, Maki WA (1996a) Myosin light chain phosphorylation and posttetanic potentiation in fatigued skeletal muscle. Pflugers Arch 431(6):882–887
Tubman LA, Rassier DE, MacIntosh BR (1996b) Absence of myosin light chain phosphorylation and twitch potentiation in atrophied skeletal muscle. Can J Physiol Pharmacol 74(6):723–728
Tubman LA, Rassier DE, MacIntosh BR (1997) Attenuation of myosin light chain phosphorylation and posttetanic potentiation in atrophied skeletal muscle. Pflugers Arch 434(6):848–851
Vale RD, Milligan RA (2000) The way things move: looking under the hood of molecular motor proteins. Science 288(5463):88–95
Vandenboom R, Houston ME (1996) Phosphorylation of myosin and twitch potentiation in fatigued skeletal muscle. Can J Physiol Pharmacol 74(12):1315–1321
Vandenboom R, Grange RW, Houston ME (1993) Threshold for force potentiation associated with skeletal myosin phosphorylation. Am J Physiol 265:1456–1462
Vandenboom R, Grange RW, Houston ME (1995) Myosin phosphorylation enhances rate of force development in fast-twitch skeletal muscle. Am J Physiol 268:596–603
Vandenboom R, Xeni J, Bestic M, Houston ME (1997) Increased force development rates of fatigued skeletal muscle are graded to myosin light chain phosphate content. Am J Physiol 272:1980–1984
Vergara JL, Rapoprot SI, Nassar-Gentina V (1977) Fatigue and posttetanic potentiation in single muscle fibers of the frog. Am J Physiol 232(5):185–190
Walker SM (1948) Action potentials in rat muscle with twitch tension potentiated by KCI treatment, adrenalectomy, tetanus and treppe. Am J Physiol 154(1):63–72
Woodhead JL, Zhao FQ, Craig R, Egelman EH, Alamo L, Padrón R (2005) Atomic model of a myosin filament in the relaxed state. Nature 436(7054):1195–1199
Xeni J, Gittings W, Caterini D, Huang J, Houston ME, Grange RW, Vandenboom R (2011) Myosin light chain phosphorylation and potentiation of dynamic function in mouse fast muscle. Pflugers Archiv 362:349–358
Yang Z, Stull JT, Levine RJ, Sweeney HL (1998) Changes in interfilament spacing mimic the effects of myosin regulatory light chain phosphorylation in rabbit psoas fibers. J Struct Biol 122:139–148
Zhao FQ, Craig R, Woodhead JL (2009) Head-head interaction characterizes the relaxed state of Limulus muscle myosin filaments. J Mol Biol 385(2):423–431
Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT (2005) Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci USA 102:17519–17524
Acknowledgments
Work in our lab supported by the Natural Sciences and Engineering Research Council of Canada (RV) (2008–2013). The important contribution of Jian Huang to these studies is also gratefully acknowledged.
Conflict of interest
The authors do not have any conflicting interests regarding the findings or interpretations of findings reviewed in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vandenboom, R., Gittings, W., Smith, I.C. et al. Myosin phosphorylation and force potentiation in skeletal muscle: evidence from animal models. J Muscle Res Cell Motil 34, 317–332 (2013). https://doi.org/10.1007/s10974-013-9363-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-013-9363-8