Abstract
In the manuscripts dealing with thermoanalytical kinetics, many flaws, mistakes, and misconceptions are encountered repeatedly. In this paper, frequent flaws encountered in manuscript of kinetic papers are reviewed, mainly those originating in the false interpretation of the general rate equation, improper employment of integral isoconversional methods, conclusions drawn from the values of a single kinetic parameter, absence of error estimation and application of single-heating rate methods. Assessment of the quality of kinetic treatment is also noticed. Some experimental imperfections that could lead to incorrect values of kinetic parameters are mentioned.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Refereeing is a volunteer activity of scientists who review the manuscripts written by their known or unknown colleagues. Collectively, the authors of this paper have completed more than 300 review assignments in Journal of Thermal Analysis and Calorimetry and Thermochimica Acta, mostly of kinetic studies. In the manuscripts dealing with thermoanalytical kinetics, many flaws, mistakes, and misconceptions are encountered repeatedly.
In this paper, we would like to call the attention to some flaws in order to avoid the “thermokinetic misconduct” and some recommendations are suggested to facilitate the publication of kinetic manuscripts. The background of flaws is outlined and reasoned. We limit ourselves here primarily to the methods based on the general rate equation (GRE) since these methods are the most popular and most flaws are encountered in their application, mainly to the model-free or isoconversional methods. The model-fitting methods and methods not based on GRE will be tackled only occasionally.
Discussion
General rate equation and its physical meaning
Processes in condensed phase are routinely studied by thermoanalytical techniques. Mechanisms of these processes are often unknown or too complex to be described by a simple kinetic model as they tend to proceed in multiple steps and also involve physical phenomena such as diffusion or adsorption. For the description of their kinetics, various methods based on the so-called single-step approximation are used.
The kinetic treatment of condensed-phase processes almost universally starts with the general (or generalized) rate equation (GRE), usually expressed as
where k(T) and f (α) are functions depending solely on temperature T and the degree of conversion α of the process, respectively. GRE has become so ubiquitous that it is often perceived as axiomatic law that is valid universally. However, GRE is just a greatly simplified form of a much more general empirical law which states that the rate of the condensed-state process is a function of temperature and conversion [1,2,3]:
GRE can be obtained from Eq. (2) by assuming that the function Φ can be expressed as a product of two functions independent of each other:
Combining Eqs. (2) and (3), the rate of a complex condensed-phase process can be formally described by Eq. (1). The distinction between GRE and Eq. (2) may seem trivial; however, it has serious consequences.
Mathematically, GRE resembles a rate equation of a single-step process, even though it is a representation of the kinetics of a complex multistep process. In theory, each elementary step of a complex process should be described by a separate rate equation with corresponding rate constants. The meaning of the single-step approximation resides in replacing a set of rate equations with the sole GRE [2, 3]. From the point of view of probability theory, GRE implies that temperature and conversion affect the reaction rate independently, i.e., without mutual interaction. Additional terms can be added to the right-hand side of Eq. (1) to account for the effect of other factors on the reaction rate such as the effect of pressure of reactants or products [4,5,6], effect of radiation [7], effect of mechanical stress, electric field, etc. The separability of temperature and conversion function indicated in Eq. (1) is crucial for performing kinetic analysis of thermoanalytical data and historically there were practically no attempts to include a conversion–temperature cross term.
The temperature function k(T) in Eq. (1) is usually interpreted as the rate constant and the conversion function is thought to reflect the mechanism of the process. However, as shown in [2], such simple interpretation of both functions may be incorrect. In [3] it has been shown that the reaction rate cannot be expressed in the form of Eq. (1) neither for the very simple case of two consecutive reactions nor for the two opposing reactions under non-isothermal conditions. The two cases mentioned in [3] represent the simplest cases of non-elementary processes. It can be thus concluded that the kinetics of a general complex process cannot be expressed by Eq. (1) when describing the true mechanism of the process. GRE could be a true reaction rate equation only in special cases such as when the process is really elementary or when the process involves an elementary step limiting the overall reaction rate. These possibilities should always be proven or at least, soundly reasoned.
Hence, GRE is not a true reaction rate equation in general; it is a mathematical tool enabling to describe the thermoanalytical kinetic data. In general, the functions k(T) and f (α) only represent the temperature and conversion components of the kinetic hypersurface where the hypersurface is dependence on the degree of conversion on time and temperature [8]. Consideration of the influence of individual independent factors on the description of complex processes can be recognized not only in thermoanalytical kinetics. We encountered this approach applied in the theory and transformations in metals and alloys [9], in the prediction of microbial spoilage in foods [10], and in modeling of concrete curing [11, 12].
Flaws originating in the false interpretation of GRE
Many mistakes in manuscripts of kinetic papers originate in the false understanding of GRE as being a true reaction rate equation.
Activation energy
Perhaps due to the prevailing notion of k(T) as the reaction rate constant, the temperature function is almost universally expressed by the Arrhenius equation:
where A and Ea are the preexponential factor and the activation energy, respectively, T is the absolute temperature and R stands for the universal gas constant. Both kinetic parameters, A and Ea, are apparent in the case of a complex process and they may not have any clear physical meaning. However, the simple form of GRE represented by Eq. (1) is very enticing to interpret A and Ea as parameters of an elementary reaction. It is also quite mystifying to express the activation energy in kJ mol−1. For example, the activation energy of polyethylene thermooxidation is 164 ± 8 kJ mol−1 [13]. The question is: 164 kJ per mole of what? Per mole of polyethylene? What are the entities the activation energy is related to?
Thus, the concept of activation energy and preexponential factor in the case of complex processes is hazy. The kinetic parameters are “apparent” or “averaged” quantities. It should be stressed again that the temperature function, k(T), is not the rate constant in general. To demystify the activation energy and to avoid blunders and overinterpretation, it would be advisable to introduce the definition of temperature coefficient, B, as
and the Arrhenius-like temperature function would be then written as
Since it has been shown that k(T) cannot be interpreted as the rate constant in general, there is no sound reason to limit ourselves to the Arrhenius equation and use of alternative temperature functions was proposed [1, 8, 14]. Perhaps the most sophisticated method based on GRE, the nonparametric kinetic (NPK) method [15], does not make any assumptions on the functional forms of the temperature and conversion functions.
Thermodynamic parameters of activation
In many manuscripts, the Gibbs energy, enthalpy, and entropy of activation are calculated from the values of rate constant, activation energy, and preexponential factor. This flaw again originates in the false understanding of GRE as being a true reaction rate equation. The thermodynamic parameters of activation are related to the activated complex, i.e., to the elementary reactions. Again, the question arises: what is the structure of the activated complex in case of complex processes? In this case, the theory of activated complex is inapplicable and the calculated parameters ΔG#, ΔH#, and ΔS# provide no additional insight into the process under study.
Mechanistic conclusions
The conclusions on the mechanism of the process are often drawn in manuscripts dealing with model-fitting methods based on GRE. Thermoanalytical methods are “gross” methods, they measure the envelope signal of all the processes occurring in the sample. The complex nature of condensed-phase processes brings about a difficulty not encountered in classical kinetics: the term conversion is defined operationally as a normalized change of the physical property measured by the thermoanalytical instrument and only rarely can be associated with a unique chemical species. Various f (α) models often describe the kinetic data equivalently at the expense of dramatic changes in kinetic parameters [16, 17].
It is again to remind that the function f (α) may not reflect the mechanism of the process since GRE is not a true reaction rate equation. Any mechanistic conclusion should be confirmed by an independent method such as X-Ray, FTIR, Raman, optical microscopy, evolved gas analysis, mass spectrometry, etc. Conclusions on the mechanism cannot be made just from the plausible agreement between the experimental and calculated thermoanalytical curves.
Application of several isoconversional methods
A strange practice of evaluating experiments by several isoconversional methods and comparing the results is often encountered where all the methods are based on Eq. (1). Results from the Friedman differential isoconversional method [18] are often compared with the results obtained by the integral isoconversional methods or there are mutually compared the results from various integral methods.
Flynn–Wall–Ozawa (FWO), Kissinger–Akahira–Sunose (KAS) and Starink methods belong to the integral isoconversional methods [19,20,21,22]. All these methods are based on the treatment of kinetic results by the relationship
where a, b and c are constants independent of temperature. After differentiating ln β by the inverted temperature, one can obtain:
The activation energies from various integral isoconversional methods are thus interrelated.
If properly applied and correctly treated the same experimental data, the kinetic parameters from all the isoconversional methods should be the same from each method since they all are based on the same principle, that is, on GRE. Differences in the values of kinetic parameters are just a mathematical artifact, they have no physical reason and no conclusion on the kinetics of the process can be drawn from the comparison of the values of parameters obtained by various methods. Hence, there is no reason to use several methods and compare the results obtained since the mathematical treatment is the source of differences, not the physical reality. One isoconversional method should be applied in a manuscript.
The isoconversional methods widely applied up to now were mostly developed many decades ago when the computation facilities resided mostly in the use of a logarithmic slider. As early as 1997 Flynn wrote [23]: “in this age of vast computational capabilities, there is no valid reason not to use precise values for the temperature integral when calculating kinetic parameters”. It would be perhaps meaningful to quit the methods based on the approximate evaluation of the temperature integral and to start with its precise evaluation as demonstrated in [24].
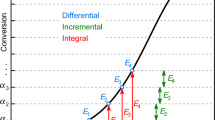
Improper use of integral isoconversional methods
The isoconversional methods can be divided into integral, incremental, and differential ones where the integral isoconversional methods are most frequently applied. The integral methods reside in separating variables in Eq. (1) and integrating the differential equation obtained within the range of conversions from 0 to α.
Isoconversional methods often lead to variable activation energy, i.e., the activation energy depends on conversion. Then we get:
As it can be seen, if the apparent activation energy depends on conversion, the variables of GRE are not properly separated since the conversion appears on both sides of Eq. (9). Hence, in case of variable activation energy, the integral isoconversional methods are mathematically incorrect and should not be applied [25]. For the treatment of thermokinetic results, the incremental methods seem to be the most suitable class of isoconversional methods [24].
Focus on E a , neglect of preexponential factor
The isoconversional methods based on Eq. (1) are primarily developed for obtaining the values of activation energy. Very often obtaining the values of activation energy is presented as the justification for publishing kinetic papers. As written in [26], “values of E should not be reported and interpreted in isolation from the other members of the kinetic triplet”. The methods for evaluation of thermoanalytical kinetic data should calculate simultaneously all the kinetic parameters describing the kinetics such as demonstrated in [24].
The isoconversional methods do not provide the whole kinetic triplet. In fact, they yield only two kinetic parameters, i.e. the activation energy and the combination of preexponential factor and the conversion function f(α). However, there is no need to separate A and f(α) since the recovery and modeling of kinetic curves are possible as shown in [27].
Conclusions based on the values of a single kinetic parameter
The kinetic parameters obtained from the treatment of thermokinetic data can be used for recovering the reaction rate, isoconversional temperature, Tα, isoconversional time, tα, and for modeling the process for other time–temperature regimes than those employed for experiments.
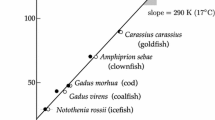
In manuscripts, mainly in those dealing with the stability of materials, quite often the conclusion can be read that the higher activation energy invariably implies the higher material stability. Figure 1 depicts an Arrhenius plot for three processes with lower activation energy and a process with a higher activation energy. It can be clearly seen that the processes with lower activation energy can be either slower or even more rapid than the process with the higher activation energy. For a certain position of both Arrhenius lines, for lower temperatures, the process with lower activation energy is more rapid than the one with the higher activation energy and for the higher temperatures, it is vice versa. Interpretation of the activation energy in isolation from the preexponential factor is further invalidated by their mutual interdependence (kinetic compensation effect) which stems from ill-conditioning of regression models based on the Arrhenius equation and inevitable presence of both random [28] and systematic errors [29] in kinetic data. Hence, reliable conclusions can never be based on the values of a single kinetic parameter. In particular, no conclusion should be reached by merely comparing the values of activation energy. The conclusions concerning the stability, rate of the processes, etc. should be drawn from the values of experimentally accessible parameters, i.e., from the values of the reaction rate, Tα, tα, etc.
The conclusion that the higher activation energy, the higher material stability could only be valid for a quite uncommon case when the preexponential factor is the same for all the processes.
Absence of error estimation
It is peculiar that statistical treatment of the kinetic results is quite rare in kinetic manuscripts. For the isoconversional methods, the activation energy and preexponential factor are presented mostly without any error estimation. As pointed out by Brown [26], “realistic uncertainties in the calculated values should be reported”. For the model-fitting methods, it should be taken into account that also the parameters occurring in the conversion function convey uncertainties that should be reported. The same is valid for the parameters obtained from the methods that are not based on GRE. The statistical treatment should be obligatory for any treatment of experimental data, not only for the kinetic data.
Another strange practice is reporting the values of activation energy in excessive number of significant figures (significant digits), six or seven significant figures can frequently be seen. Our experience is that the standard error of the activation energy is between 5 and 10% which corresponds to two or three significant figures. The number of significant figures should be reported correctly also for the other kinetic parameters.
Quality of the kinetic treatment
Quality of the kinetic treatment is often assessed, for example, from the linearity of the FWO or KAS plots. This is not satisfactory. As written in [26], “the final test of every kinetic analysis should be to use the parameters determined to construct calculated curves for comparison with the experimental results over a wide and representative range”.
Application of single-heating rate methods
Although these methods are not recommended [4, 26], they are still occasionally encountered in manuscripts. Figure 2 depicts the kinetic hypersurface as a function of rate versus conversion and temperature. It shows that any linear-heating run represents just a single path on the kinetic hypersurface. It is not trustworthy to assess the shape of the whole hypersurface just from the course of a single line on its surface. The usual consequence of single-heating rate methods is the dependence of kinetic parameters on the heating rate.
Rate of a hypothetical first-order process (Ea = 100 kJ mol−1, A = 109.3 min−1) as a function of conversion and temperature. The embedded lines correspond to α–T paths followed during a measurement at constant heating rate. Each measurement explores only a small portion of the kinetic surface; hence, reliable parameters cannot be obtained by any method based on just a single run
Experimental imperfections
There are some experimental imperfections often met in manuscripts that worsen the quality of kinetic studies. The most serious defect is employment of high heating rates in case of the materials with low thermal conductivity. Very often the heating rates between 20 and 50 °C min−1 are applied. The low thermal conductivity, high heating rates, and high sample mass may lead to the formation of thermal gradients within the sample [30]. Hence, instead of the pure transformation kinetics of the process, an undefined mixture of the transformation kinetics and heat transfer kinetics is measured. For the materials with low thermal conductivity, we would recommend applying the heating rates 10 °C min−1 and below. Higher heating rates are justified for materials with high thermal conductivity such as metals, metallic glasses, etc.
Another error is the use of insufficient number of heating rates in isoconversional studies. For statistical reasons, the least number of independent measurements should be three. Each kinetic parameter represents a bounding condition. Hence, in the case of isoconversional methods with two adjustable parameters, the minimum number of heating rates should be five.
A common mistake, perhaps originating in forgetfulness, is incomplete description of experimental conditions, such as omitting to report the purge gas used, calibration procedure applied, sample geometry, etc.
Conclusions
Analysis of experimental data using methods based on GRE is a feasible way to describe the kinetics of complex processes. The model-free isoconversional methods are the most popular since the treatment of experimental data is simple and easy; however, interpretation of the results may not be so simple and straightforward. The reason is that the temperature function, k(T), cannot be understood as the rate constant in general, and the conversion function, f (α), may not reflect the mechanism of the process. Both these functions enable to describe the kinetic hypersurface. The parameters of both these functions may not have any clear physical meaning; however, they can be used to recover isoconversional temperature, isoconversional time, reaction rate, i.e., they enable modeling the complex processes and making predictions. A great advantage of isoconversional methods is the description of the kinetics of the process without the necessity of knowing the detailed mechanism.
Since the physical meaning of parameters may not be clear, no mechanistic conclusions should be based solely on the values of any individual kinetic parameter. The conclusions might be done from the quantities with clear physical meaning, i.e., from the values of Tα, tα, reaction rate, etc. Any mechanistic conclusion should be verified by an independent method.
Finally, it is essential to realize that the general rate equation is just a mathematical tool, it is not a true rate equation in general. Its reliability depends on the reliability of the single-step approximation given by Eq. (1). If there exist other models and methods with better physical justification, it is advisable to apply them instead of methods based on GRE.
References
Šimon P. Isoconversional methods: fundamentals, meaning and application. J Therm Anal Calorim. 2004;76:123–32. https://doi.org/10.1023/B:JTAN.0000027811.80036.6c.
Šimon P. Considerations on the single-step kinetics approximation. J Therm Anal Calorim. 2005;82:651–7. https://doi.org/10.1007/s10973-005-0945-6.
Šimon P. The single-step approximation: Attributes, strong and weak sides. J Therm Anal Calorim. 2007;88:709–15. https://doi.org/10.1007/s10973-006-8140-y.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Kodani S, Iwasaki S, Favergeon L, Koga N. Revealing the effect of water vapor pressure on the kinetics of thermal decomposition of magnesium hydroxide. Phys Chem Chem Phys. 2020;22:13637–49. https://doi.org/10.1039/D0CP00446D.
Kreps F, Dubaj T, Krepsová Z. Accelerated oxidation method and simple kinetic model for predicting thermooxidative stability of edible oils under storage conditions. Food Packag Shelf Life. 2021;29:100739. https://doi.org/10.1016/j.fpsl.2021.100739.
Vykydalová A, Dubaj T, Cibulková Z, Mizerová G, Zavadil M. A predictive model for polyethylene cable insulation degradation in combined thermal and radiation environments. Polym Degrad Stab. 2018;158:119–23. https://doi.org/10.1016/j.polymdegradstab.2018.11.002.
Šimon P. Single-step kinetics approximation employing non-Arrhenius temperature functions. J Therm Anal Calorim. 2005;79:703–8. https://doi.org/10.1007/s10973-005-0599-4.
Christian JW. Formal theory of transformation kinetics. In: Christian JW, editor. The theory of transformation in metals and alloys. Oxford: Pergamon; 2002. p. 529–52.
Zwietering MH, Wijtzes T, Rombouts FM, Riet K. A decision support system for prediction of microbial spoilage in foods. J Ind Microbiol. 1993;12:324–9. https://doi.org/10.1007/BF01584209.
Mi Z, Hu Y, Li Q, Gao X, Yin T. Maturity model for fracture properties of concrete considering coupling effect of curing temperature and humidity. Constr Build Mater. 2019;196:1–13. https://doi.org/10.1016/j.conbuildmat.2018.11.127.
Sun B, Noguchi T, Cai G, Chen Q. Prediction of early compressive strength of mortars at different curing temperature and relative humidity by a modified maturity method. Struct Concr. 2021;22:E732–44. https://doi.org/10.1002/suco.202000041.
Šimon P, Kolman Ľ. DSC study of oxidation induction periods. J Therm Anal Calorim. 2001;64:813–20. https://doi.org/10.1023/A:1011569117198.
Šimon P, Dubaj T, Cibulková Z. Equivalence of the Arrhenius and non-Arrhenian temperature functions in the temperature range of measurement. J Therm Anal Calorim. 2015;120:231–8. https://doi.org/10.1007/s10973-015-4531-2.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics method. Thermochim Acta. 1998;316:37–45. https://doi.org/10.1016/S0040-6031(98)00295-0.
Militký J, Málek J, Šesták J. Parameter distortion by unappropriate nonisothermal treatment. J Therm Anal. 1989;35:1837–47. https://doi.org/10.1007/bf01911671.
Koga N, Šesták J, Málek J. Distortion of the Arrhenius parameters by the inappropriate kinetic model function. Thermochim Acta. 1991;188:333–6. https://doi.org/10.1016/0040-6031(91)87091-A.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermo-gravimetry. application to a phenolic plastic. J Polym Sci C. 1965;50:183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6. https://doi.org/10.1246/bcsj.38.1881.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8. https://doi.org/10.1002/pol.1966.110040504.
Akahira T, Sunose T. Trans. joint convention of four electrical institutes, paper no. 246, 1969 research report. Chiba Institute of Technology, Sci Technol. 1971;16:22–31
Starink MJ. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim Acta. 1996;288:97–104. https://doi.org/10.1016/S0040-6031(96)03053-5.
Flynn JH. The “temperature integral”—its use and abuse. Thermochim Acta. 1997;300:83–92. https://doi.org/10.1016/S0040-6031(97)00046-4.
Dubaj T, Cibulková Z, Šimon P. An incremental isoconversional method for kinetic analysis based on the orthogonal distance regression. J Comput Chem. 2015;36:392–8. https://doi.org/10.1002/jcc.23813.
Šimon P, Thomas P, Dubaj T, Cibulková Z, Peller A, Veverka M. The mathematical incorrectness of the integral isoconversional methods in case of variable activation energy and the consequences. J Therm Anal Calorim. 2014;115:853–9. https://doi.org/10.1007/s10973-013-3459-7.
Tanaka H, Brown ME. The theory and practice of thermoanalytical kinetics of solid-state reactions. J Therm Anal Calorim. 2005;80:795–7. https://doi.org/10.1007/s10973-005-0732-4.
Matos J, Oliveira JF, Magalhaes D, Dubaj T, Cibulková Z, Šimon P. Kinetics of ambuphylline decomposition studied by the incremental isoconversional method. J Therm Anal Calorim. 2016;123:1031–6. https://doi.org/10.1007/s10973-015-4899-z.
Barrie PJ. The mathematical origins of the kinetic compensation effect: 1. the effect of random experimental errors. Phys Chem Chem Phys. 2012;14:318–26.
Barrie PJ. The mathematical origins of the kinetic compensation effect: 2. the effect of systematic errors. Phys Chem Chem Phys. 2012;14:327–36.
Šesták J, Holba P. Heat inertia and temperature gradient in the treatment of DTA peaks. J Therm Anal Calorim. 2013;113:1633–43. https://doi.org/10.1007/s10973-013-3025-3.
Acknowledgements
This work was financially supported by the Structural Funds of EU, OP Integrated Infrastructure by implementation the project “Strategic research in SMART monitoring, treatment, and prevention against coronavirus (SARS-CoV-2)”. ITMS 2014+ code: NFP313011ASS8; co-financed from the European Regional Development Fund. Financial support from the Slovak Research and Development Agency (APVV-15-0124) and from the Slovak Scientific Grant Agency (VEGA 1/0498/22) is also gratefully acknowledged. Further, we thank Ministry of Education, Science, Research and Sport of the Slovak Republic for funding within the scheme “Excellent research teams”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Šimon, P., Dubaj, T. & Cibulková, Z. Frequent flaws encountered in the manuscripts of kinetic papers. J Therm Anal Calorim 147, 10083–10088 (2022). https://doi.org/10.1007/s10973-022-11436-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11436-y