Abstract
There is growing interest worldwide in the use of camelina oil for food as well as for biofuel purposes. For both of these applications, oxidative stability is an important feature of the oil. Therefore, the aim of this study was to test the thermal resistance to oxidation of three different cultivars of camelina oil i.e., Omega, Luna and Śmiłowska by means of isothermal and non-isothermal differential scanning calorimetry (DSC) oxidation measurement. For isothermal DSC analysis, different temperatures were tested (120, 140, 160 °C) and in the non-isothermal mode different scanning rates (1, 2, 5, 10, 15 °C min−1) were used. To support the DSC data, chemical analyzes were also performed i.e., fatty acid composition, peroxide value, p-anisidine value, acid value and radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (RSA DPPH). The isothermal test indicated that for all camelina oils the oxidation induction time (OIT) decreased with an increase in temperature on average from 69.83 min for 120 °C to 5.13 min for 160 °C. The OIT values corresponded very well with non-isothermal DSC results, for which the onset temperatures (Ton) increased with the increase of heating rate on average from 142.15 °C for 1 °C min−1 to 185.75 °C for 15 °C min−1. The parameters of DSC oxidative stability i.e., OIT as well as Ton values were negatively correlated with some unsaturated fatty acids content e.g., α-linolenic acid (C18:3, n-3) and positively with yellowness b* and RSA DPPH. Oil from camelina seeds of Śmiłowska cultivar, which was characterized by the lowest content of α-linolenic acid and the highest b* value of color and RSA DPPH, was the most thermally stable oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent research concerning fats and oils with diverse compositional characteristics and usage suggested a revised approach toward the Camelina Sativa (L.) oil crop. This is due to its unique agronomic features, promising and sustainable oilseed quality and it is being a condensed source of nutritive fatty acids. In the literature, Camelina Sativa (L.) has been described mostly as an ancient crop, hence it has been highlighted by the same authors as a good source of edible vegetable oil, which for decades was neglected in industrial and commercial usage [1,2,3,4,5]. As early as 3000 years ago the cultivation of this crop was documented in Europe [6], and in his early study on camelina, Budin et al. [1] mentioned its cultivation in central Europe as an oil-bearing crop from 600 B.C. onwards. This annual flowering oilseed plant belongs to the Cruciferae (Brassicaceae) family, which appears in spring and winter varieties [2, 5,6,7]. Other names of camelina that have been mentioned are dodder oil, German sesame oil [8] or, false flax, gold of pleasure, Siberian oilseed or wild flax [7, 9]. This yellow flowering plant exhibits similarities with rapeseed plants' generic characteristics, since they belong to the same family, and is similar to flaxseed oils in terms of two highly enriched essential n-3 fatty acids (C18:3, α-linolenic acid). Although studies have suggested that this plant is indigenous to Central European plain, it was replaced by the initiation of rapeseed plants after the 1940s [2]. Therefore, interest of camelina oil was been renewed, which resulted from the urge to find new potential uses for this oil, such as e.g., biofuels, jet fuel, bio based-products, and feed [10]. Moreover, the following two beneficial features of this plant contribute to the growing interest in it. The first of these is its very favorable composition of fatty acids (high content of unsaturated fatty acids, about 90%), and the content of antioxidant compounds that affect high stability [1], 7, 11, 12. The second feature is the unique ability of camelina to adapt to various climatic and soil conditions [10]. Camelina is most often cultivated in poor and very poor soils, probably the least sensitive to soil water deficiency of all the plants in the Brassicaceae [13] family. With its being one of the richest vegetal sources of n-fatty acids, the nutritional values of camelina oil have been studied and evaluated by scientists in recent years. It has been shown that daily consumption of this oil brings remarkable health benefits, which include the prevention and treatment of hypertension, cardiovascular disease, obesity and controlling blood glucose levels, LDL (low-density lipoprotein) oxidation, neurological dysfunction [7, 14,15,16]. At the same time, apart from its utility in the formulation of functional foods, and in nutraceuticals, pharmaceuticals and cosmetics industries, the raw camelina plant has been reported by many authors as being used as an excellent source of biodiesel compared to canola and soybean biodiesels for its unique fatty acid composition and low energy requirements during its agro life cycle [5, 15,16,17,18]. However, the fatty acid composition of this oil depends on the cultivation conditions [14, 18, 19] or extraction methods [13, 20, 21]. Among the different extraction methods, cold pressing is now relatively popular due to its ability to retain the bioactive compounds in the oils, which is of great importance from the nutritional point of view [14]. Several studies have compared the stability of cold-pressed camelina oils with other oils, where it has been found to be more stable than flaxseed oil and less stable than others [22,23,24]. Among the many different thermal analyzes used for oils and fats [25], differential scanning calorimetry (DSC) is very popular for testing the thermo-oxidative stability of oils under isothermal and non-isothermal conditions. Oxidative stability is one of the crucial parameters determining oil quality. Most methods for testing oxidative stability are based on analyzing the oil at room temperature and many studies have been performed using camelina based on the traditional chemical methods (i.e., p-anisidine value, peroxide value and acid value determination) [4, 14, 22, 25, 26]. Due to the wide range of applications of camelina oil, it is also important to investigate the thermal oxidation stability of this oil by means of differential scanning calorimetry in various temperature conditions. There is little research on this topic in the literature, and existing studies are either incomplete [20, 22, 27] or concern the conditions of altered pressure (pressure differential scanning calorimetry, PDSC) [5, 6, 23], which do not reflect the actual conditions of using the oil at high temperatures. The novelty of these studies consists in the investigation of the properties of oxidative stability of camelina varieties originating from the Wielkopolska region in Poland, which have not been tested in this respect so far using the DSC technique. In this part of Poland, camelina oil had been a traditional product since the ancient time, which is now registered under the name "Olej rydzowy tradycyjny" as a Traditional Speciality Guaranteed product in the European Union and the United Kingdom. Hence, the objective of this study was to determine the comprehensive characteristics of the thermo-oxidative stability of cold-pressed camelina oils procured from five different suppliers in Poland, and of various cultivars. The DSC isothermal and non-isothermal experiments in various conditions were performed and the oxidation kinetic parameters were calculated and compared with the results of peroxide value, p-anisidine value, acid value, RSA DPPH, color and fatty acids composition.
Experimental
Materials
Three cultivars of Camelina Sativa L. seeds were collected during 2019 from Greater Poland region in Poland from different suppliers i.e., SEMCO manufactory (Śmiłowo, Poland) and Poznan University of Life Sciences (Dłoń 4, Miejska Górka). Total five oils samples were investigated from three cultivars, i.e., Omega -spring variety (CA OM), Luna -winter variety (CA LUA, CA LUB) and Śmiłowska -spring variety (CA SMA, CA SMB). Luna and Śmiłowska cultivars were obtained from two suppliers of seeds (A and B). These seeds were cold-pressed at a temperature below 50 °C. After pressing, the oils were decanted for 24 h. During subsequent laboratory storage, they were kept at freezing temperature at − 80 °C in brown glass bottles.
Fatty acid composition
To determine the fatty acid composition of the camelina seed oils, gas chromatography-Flame Ionization Detector (GC-FID) was employed. All the samples were analyzed in two replications. Two drops of fat were dissolved in 1 mL of hexane (for HPLC, Sigma Aldrich). 1 mL of 0.4 N sodium methoxide was added. The samples were stirred and left for 15 min, then 5 mL of distilled water was added and the top layer was taken off. By following the AOCS official method [29], fatty acid methyl esters were analyzed using a Trace 1300 chromatograph (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Separation was performed on a Supelcowax 10 capillary column (30 m × 0.2 mm × 0.2 μm), and injection was performed in split less mode. The sample volume was 1 µL. Hydrogen was used as the carrier gas. The initial furnace temperature was 160 °C, and was increased from 12 °C min−1 to 220 °C. The temperature of 220 °C was maintained for 20 min. Fatty acid methyl esters were identified on the basis of comparing the retention times in the sample and in the 37-Component FAME Mix (Supelco).
Color measurement
Color measurements of oils were carried out using the Konica Minolta CM-5 spectrophotometer and SpectraMagicNx software. The instrument was calibrated prior to starting the analysis transmission chamber, which can accurately measure both translucent and transparent liquid samples using a CM-A213 zero calibration plate (black calibration) followed by distilled water in a 10 mm CM-A98 glass cuvette (white calibration). The research was conducted using the Hunter Lab scale. Parameter L* was in the range from 0 to 100, and denoted the lightness of the color black to white. Similarly, the a* parameter, depending on the range, was determined by a green (below 0) and red (above 0) tinge and the b* parameter, which in the case of negative values defined the color blue, but in the case of positive values, this was yellow. The samples used were analyzed in three replications.
Determination of oxidative stability by DSC
Oxidative stability was determined by following the ISO 11357-1 [30], and also implementing the ASTM D3895-14 [31]. Oil samples were analyzed in DSC 7 Perkin Elmer along with an Intracooler II, operated with Pyris software. Both isothermal and non-isothermal protocol was followed to determine the oxidative stability characteristics of the oils. The instrument was calibrated using indium (m.p. 156.6 °C, ΔHf = 28.45 J g−1) and n-dodecane (m.p. − 9.65 °C, ΔHf = 216.73 J g−1), while 99.99% pure nitrogen gas was used as the purge gas. Oils samples of approximately 6–7 mg were weighed into open aluminum pans of 50 µL (Perkin Elmer, No. 02190041) and placed in the equipment’s sample chamber. The reference was the same open and empty aluminum pan. For the isothermal program, temperature of 120, 140 and 160 °C was maintained with a constant oxygen flow of 20 mL min−1 (purity 99.995%). Based on the obtained curves, parameters denoted as oxidation induction time (OIT), oxidation end time (OET), length of oxidation Δt = OET-OIT, and rate of oxidation were determined. Determination of OIT was done after normalization of oxidation DSC curve, as the intersection of the extrapolated baseline and the tangent line to the descending exotherm, while OET value was measured at the minimum value of the heat flow of the exotherm, which expresses the end of the propagation and beginning of the termination stage of oxidation.
The oxidation rate was calculated according to the following equation:
where Y1—heat flow at OIT point [W g−1], Y2—heat flow at OET [W g−1], Δt—length of oxidation [min].
The non-isothermal analyzes were carried out by applying the heating rate of 1, 2, 5, 10 and 15 °C min−1, maintaining the oxygen flow 20 mL min−1. From the oxidation curves the onset temperature (Ton) and the end temperature (Tend) were calculated. The value of Ton was the onset temperature determined as the intersection of the extrapolated baseline and the tangent line to the descending curve of the recorded exotherm, whilst, Tend was measured as the temperature at the minimum value of the heat flow, which represents the end of the propagation and start of the termination stage. Oils samples were analyzed in two replications for all DSC experiments.
Chemical determination of oxidative stability
Measurement of p-anisidine value (pAV) as a measure of the level of secondary oxidative products was carried out according to ISO 6885:2016 [32]. Spectrophotometric measurements were taken with a quartz cuvette with a 10 mm optical path length. Peroxide value (PV) was determined by following the ISO 3960:2007 procedure [33]. The total oxidation value (TOTOX) parameter was calculated based on the pAV and PV values by the following formula TOTOX = pAV + 2PV, expressing the overall rate of oil oxidation. Acid value (AV), as an indicator of the degree of hydrolytic changes, was measured according to the official AOCS method [34]. All chemical analyzes were done in three replications.
Radical scavenging activity by DPPH (RSA DPPH)
The DDPH (2,2-diphenyl-1-pikrylhydrazyl) method was used to evaluate the antioxidant activity of the oils. The method is based on the ability of the oil to scavenge the DPPH• radicals (0.04 mM). In brief, 10 µL of the oil was added to 990 µL of DPPH• radical in ethyl acetate (0.04 mM) and mixed. The mixture was incubated for one hour in the dark at ambient temperature. Then, the spectrophotometric measurements at 517 nm were performed with a Varian Cary 1E (Berlose, Australia) using ethyl acetate as a blanc. Results are expressed as Trolox equivalents (TE) in mmol L−1. A Trolox calibration curve in the range from 0 to 15 µM was prepared with a slope of 5.3668. All oils were analyzed in two replications.
Statistical analysis of results
The results were presented in the form of mean and standard deviation. The first stage in the statistical analysis consisted in verifying variance homogeneity using the Hartley-Cochran-Bartlett test. In the case of variance homogeneity, one-way analysis of variance (ANOVA) was used and Tukey's test was applied to create statistically homogeneous groups. In turn, when variances were not homogeneous, non-parametric tests were used, i.e., ANOVA and the Kruskal–Wallis rank test. Additionally, principle component analysis (PCA) was performed to show the relationships between variables and detect some patterns between variables and objects. The analysis also enables the dataset to be reduced from a higher to a lower dimensional level. Statistical analysis of recorded results was performed using Statistica 13.3 software (TIBCO Software Inc. USA) at a significance level of α = 0.05.
Results
Chemical characteristics of cold-pressed camelina oils
Fatty acid (FA) composition analysis is one of the most important for the characterization of edible oils. The FA profile of cold-pressed camelina seed oils is presented in Table 1, which shows its very high content of unsaturated fatty acids (UFA), around 90%, and low content of saturated fatty acids (SFA), varying between 8.73% to 10.36% for different cultivars. These results are in a similar range to those obtained by other authors [18, 23]. Among the UFAs, α-linolenic acid, C18:3, n-3 (ALA) showed prepotent presence, around 30–37%, which is consistent with other studies [5, 11, 28]. The highest amount of ALA (37.17%) was determined in the Omega cultivar. Similar content of ALA (36.88%) in this cultivar was also found by Kurasiak-Popowska et al. [30]. The next most abundant UFAs were linoleic acid, C18:2 (LA), ranging from 15.31 to 20.97% and oleic acid, C18:1 (15.16–18.29%). Comparing camelina cultivars, Śmiłowska had the highest content of LA, while other authors reported lower or a similar content of LA, for example, 16.1–18.6% was mentioned by Hrastar et al. [29], and 18.5% by Berti et al. [10]. Among the other unsaturated fatty acids, the eicosenoic acid C20:1 was found in a range from 13.26 to 15.83%, which is a specific fatty acid for camelina oil. At a relatively low level, the saturated fatty acids (SFA) were detected: palmitic acid, C16:0 with around 5% and stearic acid, C18:0 with around 2%. Another important parameter calculated from the results of FA composition is the ratio of fatty acids n-3 to n-6. In our study, it was found to be within the range from 1.4 to 2.4, where Ratusz et al. [6] reported the ratio in the range from 1.79 to 2.17. Figure 1 shows the results of peroxide (PV), p-anisidine (pAV), acid (AV) and TOTOX values determination in camelina oils. These chemical analyses confirmed the good quality of the freshly pressed camelina oil samples. As the oil samples are fresh, the pAV is expected to be low, since there was not enough time to form secondary oxidation products in the oil. For the camelina oil varieties, pAV ranged from 0.20 to 0.28, which is comparatively lower than the values obtained by Ratusz et al. [6] (0.22–1.48) and Symoniuk et al. [24] (0.45–0.67). Similarly, the peroxide values for all camelina cultivars did not exceed the required limits by the Codex Alimentarius standards [31], which states that the values for PV cannot be higher than 15 meq O2kg−1. All the camelina oil varieties were quite consistent with the PV value and ranged from 2.63 to 4.31, which is a slightly higher range than that obtained by Ratusz et al. [6] (0.89–3.49 meq O2kg−1), Ratusz et al. [5] (0.79–2.04 meq O2kg−1) and Symoniuk et al. [24] (2.37–3.00 meq O2kg−1). On the other hand, Raczyk et al. [23] obtained a similar range (1.20 to 4.88 meq O2kg−1), and Hrastar et al. [29] obtained an even higher range by experimenting with oils from different growing seasons (0.74 to 8.85 meq O2kg−1). For this experiment, though all the oils PV values were relatively low, the SM B variety had the lowest value (2.63 meq O2 kg−1) and the highest was for the Omega (CA OM) variety (4.31 meq O2 kg−1). The next measured parameter was the acid value (AV), ranging from 0.4 to 1.90 mg KOH kg−1, which obviously indicates the low content of free acids in the oil, as they were freshly pressed. These values are similar to those obtained by Raczyk et al. [23] (0.53–0.89 mg KOH kg−1). Among the oils, LU A had the lowest value at 0.43 and LU B had the highest value at 1.90 mg KOH kg−1. The total oxidation state (TOTOX) of these oils was also calculated and the range of values differed significantly between the varieties, ranging from 5.43 to 8.89. An almost similar range was obtained by Ratusz et al. [6] (2.40–7.83). Another study revealed values from 3.38 to 10.22 [23], and Symoniuk et al. [24] obtained results from 5.28 to 6.45. Among the varieties investigated in this study, the OM variety exhibited the highest TOTOX value (8.89) and the SM B variety the lowest (5.43).
Oxidative stability parameters of cold-pressed camelina seed oils: peroxide value (PV), p-anisidine value (pAV), total oxidation value (TOTOX) and acid value (AV). Different superscript letters (a, b, c, d) indicate significant differences between oils (p ≤ 0.05). Camelina seed oils: CA OM (Omega cultivar), CA SMA, CA SMB (Śmiłowska cultivar) CA LUA, CA LUB (Luna cultivar). Vertical bars denote standard deviation
DSC isothermal and non-isothermal thermo-oxidative stability of camelina oil
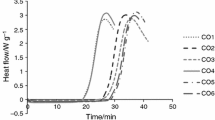
The thermo-oxidative stability of camelina oil was measured by means of the DSC technique in isothermal and non-isothermal (dynamic) mode. Figure 2 shows the curves for all camelina varieties obtained by isothermal oxidation at 120 °C (A), 140 °C (B), 160 °C (C). It can be seen that the higher the temperature, the faster the oxidation process, the start point of which is marked as the intersection of the baseline with the tangent to the descending curve (OIT). Figure 3 shows four graphs of oxidation parameters, calculated from the DSC curves: OIT (A), OET (B), the length of oxidation (C) and rate of oxidation (D). Oxidation induction time (OIT) is a parameter that shows the starting point of oxidation. Generally, for all camelina varieties, the OIT ranged between 61.38 and 76.20 min for 120 °C, between 17.02 and 20.67 min for 140 °C and between 4.48 and 5.87 min for 160 °C. This experiment revealed that for all temperatures (120, 140, 160 °C) the Śmiłowska variety turned out to be the most stable, as the highest OIT values were recorded for it. It also shows that for the OM and LU A varieties, the OIT values were the lowest, so they oxidized in the shortest time. Most of the research on the oxidative stability of camelina done by isothermal DSC was carried out under increased pressure, otherwise known as PDSC. In those conditions, lower oxidation induction times (OIT) were always recorded than in the case of DSC analysis under normal pressure. In the literature, only data on the stability of camelina oil pressed from roasted and unroasted seeds of unknown cultivars were found, which was done using the DSC technique under normal pressure. Różańska et al. [28] found as 8.96 min for unroasted seeds and 8.70 min for roasted seeds for spring camelina (SC) cultivation for 140 °C OIT values, while for winter camelina (WC) the OIT values were 7.79 min (unroasted seeds) and 9.98 min (roasted seeds). Oxidation end time (OET) parameter was also measured (Fig. 3B), which expresses the end of the propagation and beginning of the termination stage of oxidation. Data shows that for all isothermal conditions (120 °C, 140 °C and 160 °C), the lowest OET was recorded for the Omega variety and the highest for the Śmiłowska (SM B) variety, which corresponded with the OIT values. As it can be seen in Fig. 3B, for the temperature of 120 °C, the OET range was between 85.77 and 101.41 min, for 140 °C it was between 29.75 and 33.69 min, and for 160 °C between 10.36 and 12.34 min. However, Różańska et al. [28] also reported the OET for SC as 18.58 min (roasted) and 22.74 min (unroasted), in turn for WC as 14.96 min (roasted) and 17.94 min (unroasted) in a 140 °C temperature program. Ratusz et al. [5] tested samples of camelina oil obtained from the local market using the PDSC technique and several temperature programs were adopted (i.e., 90, 100, 110, 120, 130 °C) for studying oxidative stability, where for 130 °C values of OET were found between 14.08 and 16.83 min. In another experiment, Ratusz et al. [6] used the PDSC technique with 100 °C isothermal measurement, where the OET values ranged between 146.7 to 165.2 min. On the other hand, Symoniuk et al. [24] presented results obtained at 120 °C temperature using the PDSC technique for camelina oils, where the OET values were shown between 17 and 28 min. In a study of thermo-oxidative properties, along with OIT and OET parameters, the length of oxidation (Δt) parameter was calculated as a difference (Δt = OET-OIT) and presented in Fig. 3C. The values obtained for this parameter show that the lower the isothermal temperature, the longer the length of oxidation, as at 160 °C it was in the range of 5 to 6 min, at 140 °C between 12 to 13 min and at 120 °C, the range of Δt was between 22 to 27 min for different varieties. However, no significant differences (p > 0.05) were observed between varieties. Unlike these results, Różańska et al. [28] established Δt for roasted camelina seeds oils as 4.98 min (WC) and 9.88 min (SC), and unroasted samples as 10.15 min (WC) and 13.78 min (SC) at 140 °C with statistically significant differences (p ≤ 0.05). A study of the rate of oxidation was also presented in this study (Fig. 3D), which was calculated according to Eq. (1). By comparing the data for different temperature programs, oils analyzed at 140 °C were oxidized at a four times higher rate than at 120 °C. However, in the 160 °C program, the rate was also three to four times higher than in the 140 °C program. It is worth mentioning that among all the varieties, Luna (LU A) variety oxidized at a higher rate than any other varieties, however differences were not statistically significant. A comparative study for cold-pressed flaxseed oils was published by Tomaszewska-Gras et al. [32], where at 140 °C the oxidation rate was three times higher than at 120 °C for all varieties of flaxseed oils. Figure 4 shows the relationship between the DSC parameters measured (OIT, OET, Δt, oxidation rate) and the temperatures used for the analysis (120, 140, 160 °C). In Fig. 4A, the data fitted to the exponential function are shown for OIT, OET and Δt with high coefficient of determination of R2 = 0.99, and in Fig. 4B fitting of oxidation rate curve to the exponential function with R2 = 0.96. Equations are presented as they can be used for predicting the oxidation start point in different thermal conditions.
Oxidative stability parameters of cold‐pressed camelina oils determined isothermally by DSC at 120, 140, 160 °C: oxidation induction time (OIT) (A), oxidation end time (OET) (B), the oxidation length (Δt) (C), rate of oxidation (D). Different superscript letters (a, b, c, d) indicate significant differences between oils (p ≤ 0.05). Camelina seed oils: CA OM (Omega cultivar), CA SMA, CA SMB (Śmiłowska cultivar) CA LUA, CA LUB (Luna cultivar). Vertical bars denote standard deviation
For comprehensive characteristics of thermo-oxidative stability measurements, analysis in non-isothermal (dynamic) mode was carried out at different heating rates i.e., 1, 2, 5, 10, 15 °Cmin−1 by means of DSC In Fig. 5 selected curves for 2, 5, 10 °Cmin−1 are presented. Two parameters were determined from the curves: the onset temperature (Ton), corresponding to the beginning of oxidation and the end temperature (Tend), which represents the end of the propagation stage and beginning of the termination stage, at which stable products are formed. On the basis of the obtained non-isothermal curves, it can be observed that the oxidation process proceeded in two different ways: for heating rate of 1, 2, 5 °C min−1 and for 10 and 15 °C min−1. Differences can be noticed in the region of the curves around the minimum value of heat flow after the decrease in exotherm i.e., Tend. For scanning rate 1, 2, 5 °C min−1 after reaching the Tend point, the curve rises sharply, while for scanning rate 10 and 15 °C min−1, before reaching the Tend point, it can be observed that there is a plateau after the exotherm drop. Generally, it can be seen that as the heating rate increases, the temperature at which curves go downwards (Ton) also increases. In Fig. 6A, data obtained for the Ton parameter can be compared for the scanning rates 1, 2, 5, 10 and 15 °Cmin−1. For various camelina oil cultivars, the Ton occurred at different ranges of temperatures upon exposure to various heating rates. In brief, data for scanning rate 1 °C min−1 shows that Ton was lowest for OM sample (139.64 °C) and highest for SM B sample (143.65 °C) and for 2 °C min−1, Ton ranged between 152.14 °C for OM and 155.88 °C for the SM A. Temperature resistance increased for the 5 °Cmin−1 scanning rate, where the range changed between 165.57 °C (LU A) and 169.98 °C (SM A) and for scanning rates 10 °Cmin−1, 176.95 °C (LU A) and 181.31 °C (SM A). Whilst, for scanning rate 15 °Cmin−1, temperature ranged from 183.01°C (OM) to 187.71 °C (SM A). However, for all the scanning rates (1, 2, 5, 10 and 15 °C min−1) the differences between mean values of Ton were significant (p ≤ 0.05) for the different varieties. Summarizing the results for all heating rates, the Omega and Luna (LU A) variety showed less stability, as the Ton values were lowest, and the Śmiłowska variety exhibited the highest stability with high Ton values. Values of temperatures measured at the Tend point are presented in Fig. 6B for different scanning rates. Observations from the dataset show no statistically significant differences between the varieties (p > 0.05), except for the scanning rate 1 °C min−1, for which Tend values ranged from 152.25 °C to 155.46 °C and differed significantly between varieties (p ≤ 0.05). In Fig. 7 logarithmic relationship is shown between scanning rate and parameters of Ton and Tend. It can be seen that with an increase in scanning rate, the thermal resistance of oils to oxidation increases, which can be expressed logarithmically with coefficient of determination of R2 = 0.99 (Ton) and R2 = 0.90 (Tend). The equations presented in the figure can be used for predicting the temperatures of oxidation at various scanning rates.
Oxidative stability parameters: Ton A and Tend B of cold‐pressed camelina oils determined non-isothermally by DSC at a scanning rate of Tend (1 °C min−1) Tend (2 °C min−1) Tend (5 °C min−1) Tend (10 °C min−1) and Tend (15 °C min−1). Different superscript letters (a, b, c, d) indicate significant differences between cultivars (p < 0.05). Camelina seed oils: CA OM (Omega cultivar), CA SMA, CA SMB (Śmiłowska cultivar) CA LUA, CA LUB (Luna cultivar). Vertical bars denote standard deviation.
Discussion
Kinetics oxidation analysis
The results of DSC isothermal and non-isothermal measurements show that there are differences in oxidative stability depending on the variety and conditions of analysis i.e., temperature in isothermal DSC and scanning rate in non-isothermal DSC. In order to summarize the DSC results for different cultivars, kinetic analysis was carried out, the results of which are listed in Table 2.
Results obtained during the DSC experiments are recognized as a first order reaction, which resembles those of other studies [33,34,35]. To carry out the calculation, data was analyzed by following the Ozawa-Flynn-Wall method [36] adapted by other authors [5, 33, 37, 38]. To calculate activation energy Ea, first inversed values of onset temperature (Ton) expressed in Kelvin were plotted against scanning rates (β) of 1, 2, 5, 10 and 15 °C min−1, expressed as log β. The value of the slope from the plot logβ = f (T−1) for all varieties of camelina oils was calculated, since they are needed for further calculation of the activation energy (Ea, kJ mol−1).
where β is the heating rate (K min−1), T is the onset temperature (K).
Activation energy, Ea was calculated from Eqs. (3):
and pre-exponential factor Z (min−1) was calculated from the following equation
After this, Z values was used for calculation of reaction rate constant (k, min−1):
Additionally the half-life time (t1/2) was calculated from the equation:
where R is the universal gas constant (8.31 J mol−1). By plotting the values, it was possible to compute the activation energy (Ea, kJ mol−1), reaction rate constant, (k, min−1), pre-exponential factor (Z, min−1), and half-life time as (t1/2, min).
By considering the data obtained in Table 2, the values of activation energy Ea ranged from 96.49 kJ mol−1 (CA LU A) to 92.17 kJ mol−1 (CA LU B), respectively. The values descended in the following order CA LU A > CA SM B > CA SM A > CA OM > CA LU B. Coherently, the pre-exponential factor (Z) followed approximately the same order, as the CA LU A showed the highest Z value 9.65 × 1010, while for the CA LU B it was 2.30 × 1010. In Table 2 it has been shown that, there were no significant differences (p > 0.05) between the camelina oils varieties concerning the activation energy values. Similar results of Ea for camelina oil were obtained by other authors, ranging between 91.9 and 122 kJ mol−1 for camelina oil extracted by different methods [21], or between 87.63 and 93.61 kJ mol−1 for six different commercial camelina oils [5]. The results of Ea obtained by Adhvaryu et al. [39] ranged from 63 to 88 kJ mol−1 for different unmodified vegetable oils, such as cottonseed, corn, canola, sunflower oil, soybean oil and genetically modified high oleic sunflower and high oleic safflower oils. Adhvaryu et al. [33] suggested that the PUFA and SFA content does not fully explain the variation in Ea values or the other kinetic behaviors. Baokun et al. [37] showed that the SFA rich refined palm oil exhibited a noticeably high Ea value (134.7 kJ mol−1), while PUFA rich safflower oil showed a lower Ea value (86.05 kJ mol−1) for all experimented scanning rates at 5, 7.5, 10, 12.5, and 15 °C min−1. Besides activation energy, it is recommended to evaluate the oxidative stability of the oils based on the other kinetic parameters. The values of the oxidation rate constant (k) and half-life time as (t1/2) were also taken into consideration, as these parameters enable the thermal behavior of the oils in other thermal conditions to be predicted. In Table 2 the k values are presented for different camelina varieties calculated for the temperature of 441 K. The rate constant ranged from 0.25 min−1 for the Śmiłowska cultivar (CA SM A) to 0.35 min−1 for Luna cultivar (CA LU A). Statistically, rate constant k did not differ significantly for samples CA LUB, CA SMA, CA SMB (p > 0.05), while for the samples OM and CA LUA mean values were significantly different (p ≤ 0.05). Similarly, the half-life time (t1/2) values were lower for the low stable oils (CA OM and CA LUA), and higher for the more stable varieties (CA LU B, CA SM A, CA SM B). Table 2 shows that, all the oil varieties were showing significant differences (p ≤ 0.05) regarding half-life time (t1/2). The values of the k constant correspond well with the rate of oxidation calculated for the isothermal test, which are shown in Fig. 3D, where it can be seen that the same three samples of camelina oil (CA LU B, CA SM A, CA SM B) were characterized by the lowest values. In other study the reaction rate constant k of camelina oils, measured at a scanning rate from 2.5 to 15 °C min−1, was reported between 0.19 and 0.41 min−1 [21]. Since the oxidation kinetic parameters presented in Table 2 show differences between the camelina cultivars, analysis of correlations between all the oxidation parameters and fatty acid composition was carried out (Table 3). As can be seen in Table 3, significant positive correlation coefficients were found between saturated and unsaturated fatty acids (C16:0, C18:0, C18:1, C18:2) and DSC oxidation parameters. In turn, between two unsaturated fatty acids (C20:1, C18:3, n-3) and DSC oxidation parameters a negative correlation was established, the higher the content of unsaturated fatty acids, the lower the OIT and Ton values and the lower the oxidative stability of the oils. Similarly, high positive correlation coefficients were found between those fatty acids (C20:1, C18:3, n-3) and oxidation rate constant k, so the higher content of unsaturated FA, the faster the oxidation reaction. It has also been proved in other studies that the oxidative properties of camelina oils rely on upon the MUFA and PUFA contents [5, 6, 23, 24, 27]. However, the question may arise as to whether differences between oxidative stability can only be explained by the fatty acid composition. The explanation for the differences in oxidative stability could also be found in the antioxidant activity of different camelina cultivars. In Table 4, the results of the RSA DPPH analysis and color measurement are presented. Color measurement can indicate the content of compounds responsible for the color of camelina oil like carotenoids, which are compounds of antioxidant activity. Color measurement was expressed as L*, a* and b*, for which significant differences (p ≤ 0.05) between the varieties were determined. The lowest values of L*, representing the lightness of oil, were measured in sample CA SM B and the highest in sample CA OM. For three samples (CA LU B, CA SM A, CA SM B) very high values of b * were observed, representing the yellowness of the oils. Among all the oils, the sample CA LU B had the highest b* value (96.07), which can indicate the highest carotenoid content. This result can be compared with the study done by Itle et al. [39], where they found high correlation between b* value with lutein carotenoid (r = 0.87), and also total carotenoids (r = 0.75). Another study has confirmed the presence of noticeable lutein content in Luna varieties of camelina oil as 9.35 mg L−1 [30]. Comparing to the results of the antioxidant activity RSA DPPH, shown in Table 4, the highest values were also observed for CA LUB and CA SM. Correlation analysis between the antioxidant activity (RSA DPPH) and DSC oxidation parameters (Table 5) revealed that for all the DSC parameters measured (OIT, Ton), high positive correlation coefficients were obtained, which means the greater the antioxidant activity, the higher the DSC parameters (time or temperature) and the more stable the oils. Significant correlation coefficients were also found between all DSC parameters measured and parameter a* and b* of color measurement. An analysis of the correlation between RSA DPPH and parameters of a*, b* revealed significant positive correlations with Pearson’s coefficients r = 0.732 and r = 0.885, respectively.
Principle component analysis (PCA)
PCA was applied to investigate the structure of the relationships between all variables i.e., OIT (120, 140, 160 °C), Ton (1, 2, 5, 10 and 15 °C min−1), fatty acids (C16:0, C18:1, C18:2, C18:3 n-3, C20:1), kinetic data parameters (Ea, k), b*, RSA DPPH and to classify the objects in terms of camelina varieties. Based on the eigenvalue plot, which showed that around 96.7% of the variation in the data can be explained by four principal components, since for the first two PCs 88.35% of total variance was explained. A projection of the variables and a projection of the cases on the principal component plane with PC1 and PC2 on the X and Y axes, respectively, are presented in Fig. 8. The plot in Fig. 8A depicts the relations between variables and the plot of scores (Fig. 8B), distribution of objects (samples). As can be seen from Fig. 8A, the first PC1 explains the observed variability in 77.76% and PC2 in 10.59%. For the first principal component (PC1), the highest correlations were observed for the variables of oxidation constant k (−0.92), fatty acid content i.e., C18:3 (− 0.89) and C20:1 (− 0.93), for which vectors were located on the negative side of X axis and on the opposite side were variables positively correlated with PC1 i.e., C16:0 (0.95), RSA DPPH (0.94), b* (0.98), Ton (0.97), OIT (0.92). The distribution of camelina samples in terms of cultivars is shown in Fig. 8B. They were divided into four clusters. The first (CA SMA, CA LUB) and second (CA SMB) clusters, located on the positive side of X axis, consist of the most stable camelina oils characterized by the highest oxidative stability by means of RSA DPPH and OIT values. Those samples were placed on the opposite side to the third and fourth cluster (CA LUA, CA OM), for which the lowest values of PC1 were obtained (the lowest oxidative stability, the highest content of unsaturated fatty acids, the lowest b* and RSA DPPH).
Conclusions
The aim of this study was to assess the thermal stability of cold-pressed camelina seed oils by means of DSC isothermal and non-isothermal, dynamic tests. The results of oxidation induction time (OIT) obtained in isothermal mode at 120, 140 and 160 °C clearly show that Omega and Luna (CA LU A) cultivars were the least stable, while the Śmiłowska variety was the most resistant to oxidation. Generally, for all camelina oils, oxidation induction time (OIT) decreased with an increase in temperature (isothermal test) on average from 69.83 min for 120 °C to 5.13 min for 160 °C. In addition to the results of OIT, the oxidation rate was also calculated, which showed that the oxidation is faster at higher temperatures, increasing 3–4 times with every increase of 20 degrees in temperature. Findings from isothermal measurements corresponded very well with the results of non-isothermal experiments with heating rates of 1, 2, 5, 10, 15 °C min−1. Analysis of the onset temperature showed the lowest values of Ton for Omega and Luna samples and the highest for the Śmiłowska cultivar at all heating rates. All correlation analyzes led to the conclusion that differences in oxidative stability between the cultivars were mainly caused by the content of some unsaturated fatty acids e.g., α-linolenic acid (C18:3, n-3) as well as by the antioxidative activity. Oil from camelina seeds of Omega cultivar, characterized by the highest content of fatty acid C18:3, n-3 (37.2%), was the least thermally stable oil. There was a strong and statistically significant positive correlation between this fatty acid and DSC oxidation parameters (OIT and Ton), which were also highly correlated with value b* (yellowness) and the radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (RSA DPPH). The lowest values of b* and RSA DPPH were observed for the least stable oil (Omega cultivar), and the highest for Śmiłowska cultivar. Principal component analysis confirmed all observations, namely that the DSC thermal oxidation stability of camelina oil can be explained by the unsaturated fatty acid content, yellowness b* and RSA DPPH, and can also be predicted by the oxidation rate constant k, which was negatively correlated to OIT and Ton.
References
Budin JT, Breene WM, Putnam DH. Some compositional properties of camelina (camelina sativa L. Crantz) seeds and oils. J Am Oil Chem Soc. 1995;72:309–15.
Crowley JG, Fröhlich A. (1998) Factors affecting the composition and use of Camelina. End Proj Reports, Teagasc. 1–23
Ibrahim FM, El Habbasha SF. Chemical composition, medicinal impacts and cultivation of camelina (Camelina sativa): review. Int J PharmTech Res. 2015;8:114–22.
Abramovič H, Abram V. Physico-chemical properties, composition and oxidative stability of camelina sativa oil. Food Technol Biotechnol. 2005;43:63–70.
Ratusz K, Popis E, Ciemniewska-Żytkiewicz H, Wroniak M. Oxidative stability of camelina (Camelina sativa L.) oil using pressure differential scanning calorimetry and Rancimat method. J Therm Anal Calorim. 2016;126:343–51.
Ratusz K, Symoniuk E, Wroniak M, Rudzińska M. Bioactive compounds, nutritional quality and oxidative stability of cold-pressed camelina (Camelina sativa L.) oils. Appl Sci. 2018;8:1–17.
Piravi-vanak Z, Azadmard-Damirchi S, Kahrizi D, Mooraki N, Ercisli S, Savage GP, et al. Physicochemical properties of oil extracted from camelina (Camelina sativa) seeds as a new source of vegetable oil in different regions of Iran. J Mol Liq. 2021;345:117043.
Gunstone FD, Morris LJ. Fatty acids. Part VI. The oxygenated acid present in Camelina sativa (Crantz.) seed oil. J Chem Soc. 1959. https://doi.org/10.1039/jr9590002127.
Abramovič H, Butinar B, Nikolič V. Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chem. 2007;104:903–9.
Berti M, Gesch R, Eynck C, Anderson J, Cermak S. Camelina uses, genetics, genomics, production, and management. Ind Crops Prod. 2016;94:690–710.
Zubr J, Matthaus B. Effects of growth conditions on fatty acids and tocopherols in Camelina sativa oil. Ind Crops Prod. 2002;15:155–62.
Gugel RK, Falk KC. Agronomic and seed quality evaluation of Camelina sativa in western Canada. Can J Plant Sci. 2006;86:1047–58.
Kurasiak-Popowska D, Graczyk M, Przybylska-Balcerek A, Stuper-Szablewska K. Influence of variety and weather conditions on fatty acid composition of winter and spring Camelina sativa varieties in Poland. Eur Food Res Technol. 2021;247:465–73. https://doi.org/10.1007/s00217-020-03639-0.
Moslavac T, Jokić S, Šubarić D, Aladić K, Vukoja J, Prce N. Pressing and supercritical CO2 extraction of Camelina sativa oil. Ind Crops Prod. 2014;54:122–9.
Kiralan M, Kiralan SS, Subaşi I, Aslan Y, Ramadan MF. Fatty acids profile and stability of camelina (Camelina sativa) seed oil as affected by extraction method and thermal oxidation. Riv Ital delle Sostanze Grasse. 2018;95:223–8.
Urbaniak SD, Caldwell CD, Zheljazkov VD, Lada R, Luan L. The effect of seeding rate, seeding date and seeder type on the performance of Camelina sativa L. in the Maritime Provinces of Canada. Can J Plant Sci. 2008;88:501–8.
Ciubota-Rosie C, Ruiz JR, Ramos MJ, Pérez Á. Biodiesel from Camelina sativa: a comprehensive characterisation. Fuel Elsevier Ltd. 2013;105:572–7.
Yang J, Caldwell C, Corscadden K, He QS, Li J. An evaluation of biodiesel production from Camelina sativa grown in Nova Scotia. Ind Crops Prod. 2016;81:162–8.
Obeng E, Obour AK, Nelson NO, Moreno JA, Ciampitti IA, Wang D, et al. Seed yield and oil quality as affected by Camelina cultivar and planting date. J Crop Improv Taylor Francis. 2019;33:202–22.
Belayneh HD, Wehling RL, Cahoon EB, Ciftci ON. Effect of extraction method on the oxidative stability of camelina seed oil studied by differential scanning calorimetry. J Food Sci. 2017;82:632–7.
Belayneh HD, Wehling RL, Cahoon E, Ciftci ON. Extraction of omega-3-rich oil from Camelina sativa seed using supercritical carbon dioxide. J Supercrit Fluids. 2015;104:153–9.
Szterk A, Roszko M, Sosińska E, Derewiaka D, Lewicki PP. Chemical composition and oxidative stability of selected plant oils. JAOCS, J Am Oil Chem Soc. 2010;87:637–45.
Raczyk M, Popis E, Kruszewski B, Ratusz K, Rudzińska M. Physicochemical quality and oxidative stability of linseed (Linum usitatissimum) and camelina (camelina sativa) cold-pressed oils from retail outlets. Eur J Lipid Sci Technol. 2016;118:834–9.
Symoniuk E, Ratusz K, Ostrowska-Ligęza E, Krygier K. Impact of selected chemical characteristics of cold-pressed oils on their oxidative stability determined using the Rancimat and pressure differential scanning calorimetry method. Food Anal Methods. 2018;11:1095–104.
Tamási K, Marossy K. Combined thermal analysis of plant oils. J Therm Anal Calorim. 2021;147:2047–54. https://doi.org/10.1007/s10973-020-10470-y.
Eidhin DN, Burke J, O’Beirne D. Oxidative stability of ω3-rich Camelina oil and camelina oil-based spread compared with plant and fish oils and sunflower spread. J Food Sci. 2003;68:345–53.
Eidhin DN, O’Beirne D. Oxidative stability of camelina oil in salad dressings, mayonnaises and during frying. Int J Food Sci Technol. 2010;45:444–52.
Różańska MB, Kowalczewski PŁ, Tomaszewska-Gras J, Dwiecki K, Mildner-Szkudlarz S. Seed-roasting process affects oxidative stability of cold-pressed oils. Antioxidants. 2019;8:313. https://doi.org/10.3390/antiox8080313.
Hrastar R, Abramovič H, Košir IJ. In situ quality evaluation of Camelina sativa landrace. Eur J Lipid Sci Technol. 2012;114:343–51.
Kurasiak-Popowska D, Rynska B, Stuper-Szablewska K. Analysis of distribution of selected bioactive compounds in camelina sativa from seeds to pomace and oil. Agronomy. 2019;9:168.
Codex Alimentarius. (1999) Standard for named vegetable oils codex Stan 210–1999. Codex Aliment. 1–13.
Tomaszewska-Gras J, Islam M, Grzeca L, Kaczmarek A, Fornal E. Comprehensive thermal characteristics of different cultivars of flaxseed oil (Linum usittatissimum L.). Molecules. 2021;26:1–20.
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta. 2000;364:87–97.
Ostrowska-Ligeza E, Bekas W, Kowalska D, Lobacz M, Wroniak M, Kowalski B. Kinetics of commercial olive oil oxidation: dynamic differential scanning calorimetry and Rancimat studies. Eur J Lipid Sci Technol. 2010;112:268–74.
Tan CP, Che Man YB, Selamat J, Yusoff MSA. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. JAOCS, J Am Oil Chem Soc. 2001;78:1133–8.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B Polym Lett. 1966;4:323–8.
Qi B, Zhang Q, Sui X, Wang Z, Li Y, Jiang L. Differential scanning calorimetry study - Assessing the influence of composition of vegetable oils on oxidation. Food Chem. 2016;194:601–7. https://doi.org/10.1016/j.foodchem.2015.07.148.
Saldana MDA, Martinez-Monteagudo SI. (2013) Oxidative Stability of Fats and Oils Measured by Differential Scanning Calorimetry for Food and Industrial Applications. In: Amal Ali Elkordy, (Eds) Appl Calorim a Wide Context - Differ Scanning Calorimetry, Isothermal Titration Calorim. Microcalorim. IntechOpen https://doi.org/10.5772/54486
Itle RA, Kabelka EA. Correlation between lab color space values and carotenoid content in Pumpkins and Squash (Cucurbita spp.). HortScience. 2009;44:633–7.
Acknowledgements
Part of the results published in this article was presented at the conference “7th International Congress on Thermal Analysis and Calorimetry; 8th Joint Czech-Hungarian-Polish-Slovakian Thermoanalytical Conference; 14th Conference on Calorimetry and Thermal Analysis of the Polish Society of Calorimetry and Thermal Analysis” on August 29– September 2, 2021.
Funding
This research was funded by the NATIONAL SCIENCE CENTRE, POLAND, grant number: 2018/31/B/NZ9/02762.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mahbuba Islam, Jolanta Tomaszewska-Gras; methodology Mahbuba Islam, Jolanta Tomaszewska-Gras, Małgorzata Muzolf-Panek; formal analysis: Mahbuba Islam, Jolanta Tomaszewska-Gras, Małgorzata Muzolf-Panek; investigation: Mahbuba Islam, Jolanta Tomaszewska-Gras, Małgorzata Muzolf-Panek; resources: Jolanta Tomaszewska-Gras; data curation: Mahbuba Islam, Jolanta Tomaszewska-Gras; writing—original draft preparation: Mahbuba Islam, Jolanta Tomaszewska-Gras; writing—review and editing: Mahbuba Islam, Jolanta Tomaszewska-Gras, visualization, Mahbuba Islam, Jolanta Tomaszewska-Gras; supervision: Jolanta Tomaszewska-Gras, project administration: Emilia Fornal, Jolanta Tomaszewska-Gras, funding acquisition: Emilia Fornal, Jolanta Tomaszewska-Gras. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Islam, M., Muzolf-Panek, M., Fornal, E. et al. DSC isothermal and non-isothermal assessment of thermo-oxidative stability of different cultivars of Camelina sativa L. seed oils. J Therm Anal Calorim 147, 10013–10026 (2022). https://doi.org/10.1007/s10973-022-11367-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11367-8