Abstract
The enthalpies of l-carnosine interaction with solutions of hydrochloric acid have been measured by calorimetric method in the presence of NaCl at 298.15 K and ionic strength as high as 0.25, 0.50 and 0.75 mol dm−3. The enthalpies of acid dissociation have been determined from the obtained data. The effect of a background electrolyte concentration on the dissociation enthalpy of peptide has been considered. Standard thermodynamic quantities (ΔdisH°, ΔdisG°, ΔdisS°) of the acid dissociation of the dipeptide in aqueous solutions have been determined on the basis of the obtained thermochemical results and available data on the acid dissociation constants corrected for the zero-order ionic strength. Ability to acid dissociation of amino acids in a free state is compared with those for amino acid residues involved in the dipeptide linkage. The alteration of acidity has been connected with variation in hydration of reactivity centers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
L-carnosine (dipeptide 3-alanyl-l-histidine, C9H14O3N4) is available in high concentrations in both muscular and brain tissues. It acts as a specified pH buffer; as a consequence, metabolic processes are unaffected by protonation and deprotonation of its molecule. The influence of acid dissociation of biologically active molecules on processes of binding with other molecules and ions has been demonstrated in number of works [1, 2].
The constants of 3-alanyl-l-histidine acid dissociation have been studied in the literature [3,4,5,6,7,8,9]. The authors of these works used various ionic strength values and different in nature supporting electrolytes. To compare the values of the constants for a stepwise dipeptide dissociation obtained by different authors under various experimental conditions, we have calculated standard pК°1, pК°2 and pК°3 values specific to a zero-order ionic strength.
Dissociation constants were corrected for the zero-order ionic strength by using Davies Eq. (1) at I < 0.5 and Eq. (2) at I > 0.5 mol dm−3
where pKc = − lgKc and pK° = − lgK°; Kc and K° are apparent and thermodynamic dissociation constants; ΔZ2 is the difference of charge squares of the reaction products and the starting reagents; A is a limiting Debye law constant equal to 0.5107 at 25 °C; δ is an empirical coefficient; I is ionic strength of solution. The correction of the data presented in the literature [3,4,5,6,7,8,9] permits to obtain the following most probable values for the thermodynamic constants of acid dissociation of 3-alanyl-l-histidine at 298.15 K: pК°1 = 2.59 ± 0.05, pК°2 = 6.77 ± 0.05 and pК°3 = 9.37 ± 0.05. On the basis of these values of pK°, we calculated distribution of ion forms of 3-alanyl-l-histidine in the water solution with use of “KEV” software [10] accounting for the processes of acid–base interaction and water dissociation. The equilibrium diagram in Fig. 1 is plotted for the composition of peptide solutions at various pH values. Here, the fraction of L−, HL±, H2L+ and H3L+2 particles in the solution is presented as a function of pH conditions.
Equilibriums of 3-alanyl-l-histidine dissociation in aqueous solution may be presented in the form of the following scheme:
The data on the enthalpies of the stepwise reactions of the formation of complexes between 3-alanyl-l-histidine and protons are given in the literature [9]. The authors highlight the following values: \(\Delta_{\text{r}} H\left( {{\text{H}}_{3} {\text{L}}^{2 + } } \right) = \, - 49.0\,{\text{kJ}}\,{\text{mol}}^{ - 1} ,\)\(\Delta_{\text{r}} H\left( {{\text{H}}_{2} {\text{L}}^{ + } } \right) = \, - 32.3\,{\text{kJ}}\,{\text{mol}}^{ - 1} ,\)\(\Delta_{\text{r}} H({\text{HL}}^{ + } ) = \, - 0.8\,{\text{kJ}}\,{\text{mol}}^{ - 1}\) at 298.15 K and ionic strength of the solution as high as 0.1 M, a background electrolyte being potassium nitrate. The enthalpies of the processes were evaluated through a temperature dependence of the constants of the peptide complex formation on the basis of potentiometric measurements. Values of dissociation enthalpies determined by direct calorimetric method are absent in the literature. The effect of the ionic strength on enthalpies of 3-alanyl-l-histidine dissociation has not been investigated previously.
The objectives of the present work are as follows: (1) to obtain the precise values of the enthalpies of 3-alanyl-l-histidine multistage dissociation by calorimetrical method at different ionic strength values; (2) to determine the standard thermodynamic parameters of 3-alanyl-l-histidine dissociation on the basis of the obtained thermochemical results and available data on the acid dissociation constants corrected for the zero-order ionic strength.
Experimental
The substances used in the experiments, their molecular mass, formula, source, purity, and purification methods are presented in Table 1. 3-Alanyl-l-histidine (L-carnosine) of Sigma-Aldrich (substance content 99.0%) was dried in a vacuum chamber at 333 K for 48 h, and kept over P2O5. Sodium chloride recrystallized from a pure reagent was used as the background electrolyte to maintain a constant ionic strength of the solution. Solutions of a specified concentration were prepared, using previously weighed samples. Reagent-grade samples were used to prepare HCl and carbonateless NaOH solutions. Heat effects were measured using a calorimeter with an isothermic shell and automatic recording of temperature/time curve [11]. In experiments, a glass ampoule with a certain amount of HCl solution from 0.3 to 0.50 g was placed in the vessel charged with 60 cm3 of solution of the peptide with the background electrolyte at the specified pH. The mixing process was initiated by the mechanical crushing of the ampoule, and the liberated or absorbed heat was observed as a resistance change of the semiconducting thermistor which was connected to a Wheatstone bridge. The calorimeter was electrically calibrated after each experiment. Mixing heat effects were measured by comparison of a temperature changes from the dissolution and from the calibrated Joule heating with uncertainty from 0.01 to 0.025 J. Calorimetric setup performance was checked against generally accepted calorimetric standards, namely the enthalpy of dissolution of crystalline potassium chloride in water. KCl sample was purified by double recrystallization of a chemical-grade reagent from doubly distilled water. Agreement between the resulting data on the enthalpy of KCl dissolution in water ΔsolH° = 17.25 ± 0.06 kJ mol−1 and the most reliable data given in the literature [12] signals the absence of a systematic error in calorimetric installation performance.
Equilibrium composition of the solutions was assessed before and after calorimetric test. On the basis of the diagram (Fig. 1), optimal parameters of a thermochemical experiment were chosen. The changes in the enthalpy of the processes (4) and (5) were studied in the pH range from 6.2 to 7.5 and from 10.1 to 8.9, respectively. The enthalpies of the interaction of 0.01 M solutions of 3-alanyl-l-histidine with HCl solutions, as well as the enthalpies of the mineral acid dilution in the solutions of the background electrolyte, have been determined. The ionic strength of the solutions in the processes (4) and (5) amounted to 0.25, 0.5 and 0.75 M (NaCl). All symbols used in the paper are listed in Table 2.
To determine the change in the enthalpy of the process (3), we have measured the enthalpies of interaction of the peptide solution with those of hydrochloric acid having pH values as high as 1.7–3.1. Besides, the enthalpies of peptide dilution in the background electrolyte solutions under the same concentration range have been established. The ionic strength in this series of experiments amounted to 0.25, 0.5, 0.75 (NaCl). All measurements were made at 298.15 K. The resulting values of the enthalpy changes for the processes of a stepwise 3-alanyl-l-histidine dissociation are given in Tables 3–5. Instrumental uncertainty in measurements with different masses of solute and solvent and different parameters of electrical calibration does not exceed 0.5% for enthalpies of mixing of solutions and 1.5% for enthalpies of dilution. Reproducibility of enthalpy values in series of experiments included from 3 to 5 measurements was less than 0.15%. The summarized uncertainties are given in tables of obtained values of enthalpies of mixing and enthalpies of dilution.
Results and discussion
Enthalpies of the interaction of HCl solution with 0.01 M solution of 3-alanyl-l-histidine in the pH range from 8.9 to 10.1 conclude contributions from the protonation of amino group (opposite in sign to dissociation process), the dilution of reagents and the interaction between H+ and OH− ions:
whence the enthalpy of the peptide acid dissociation for the third step described by equilibrium (5) can be calculated as:
Here, ΔmixH3 and ΔdilH3 are the enthalpies of the interaction of a mineral acid solution with 0.01 M solution of 3-alanyl-l-histidine at pH as high as 10.1, and the enthalpies of HCl dilution in the background electrolyte solutions, respectively; (− α3 ΔHW) is the contribution of a heat effect of the formation of water from H+ and OH− ions to the heat effect under determination; ΔHW is the enthalpy of water dissociation; Δ[HL±] = [HL±]fin − [HL±]init is the change in equilibrium HL± particle concentration in the process of the calorimetric test; Cн+ is a total concentration of the mineral acid (with due account of its dilution up to the final volume of a calorimetric liquid). A correction for the enthalpy of the formation of water out of ions was 2.0 kJ mol−1. The resulting enthalpies of HL± particle dissociation specify splitting-out of a proton from amino group of the peptide; the values of ΔdisH3 are given in Table 3. The conversion level for reaction (5) under the calorimetric experiment is 99.9%.
Enthalpies of the interaction of HCl solution with 0.01 M solution of 3-alanyl-l-histidine in the range of neutral solution acidity (pH from 6.2 to 7.5) conclude the following contributions:
whence the enthalpy of the peptide acid dissociation for the second step described by equilibrium (4) can be calculated as:
The enthalpy changes in the process of H2L+ particle dissociation specify splitting-out of a proton from imidazole group; the values of ΔdisH2 are given in Table 4.
In the pH range from 1.7 to 3.1, enthalpies of the interaction of HCl solution with 3-alanyl-l-histidine solution conclude contributions from protonation of carboxyl group (opposite in sign to dissociation process), described by equilibrium (3), partly overlapping process (4) (HL± particle protonation) and peptide dilution:
where ΔmixH1 is the enthalpy of the interaction of 3-alanyl-l-histidine solution with those of HCl in the pH range from 1.7 to 3.1; ΔdilH1 is the enthalpy of peptide dilution against constant concentration of NaCl background electrolyte; α2 (− ΔdisH2) is the contribution of HL± particle protonation to the heat effect under determination; ΔdisH2 is the enthalpy of peptide dissociation according to the second step (4); Δ[H3L2+] = ΔH3L2+]fin − ΔH3L2+]init is the change in equilibrium concentration of H3L2+ particle during calorimetric test; CPep is a total peptide concentration with due account of its dilution up to the final volume of the calorimetric liquid. The heat effect of 3-alanyl-l-histidine dilution was calculated in this case in terms of the following ratio (8):
where ΔdilH1′ and ΔdilH3′ are the enthalpies of the mineral acid dilution in the solution of the background electrolyte at a specified ionic strength, as well as in the same solution at pH value specific to the solution of 3-alanyl-l-histidine, respectively; ΔdilH2′ is the enthalpy of peptide dilution in the solution of the background electrolyte without changing pH value. All heat effects correspond to 1 mol of 3-alanyl-l-histidine.
We proved experimentally that ΔdilH1′ and ΔdilH3′ values are quite comparable in both sign and magnitude. Thus, the following values of ΔdilH1 have been obtained: (0.62 ± 0.03, 0.83 ± 0.04, 0.96 ± 0.05) kJ mol−1 at ion strength of 0.25, 0.5, and 0.75 M, respectively. Neighbor quantities have been measured for ∆dilH3′ values: (0.59 ± 0.02, 0.86 ± 0.03, 1.00 ± 0.04) kJ mol−1 at ion strength of 0.25, 0.5, and 0.75 M, respectively. Hence, one can regard that the equality given below holds true within the limits of experimental error (11).
In accordance with equilibrium (3), degree of peptide protonation under the present experimental conditions is as high as 94–95%. Correction for the heat effect of the partly overlapping process (4) (HL ± particle protonation) was as low as 0.4 kJ mol−1. The enthalpies of 3-alanyl-l-histidine dissociation according to the first step (splitting-out of a proton from carboxyl group) are presented in Table 5.
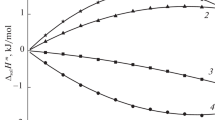
Data given in Tables 2–4 show that the enthalpies of 3-alanyl-l-histidine dissociation are considerably affected by the background electrolyte concentration, the increase of the latter giving rise to the increase in endothermicity of the processes.
Standard enthalpies of dissociation for 3-alanyl-l-histidine were found out from Eq. (14) [11, 13]
where ΔHi, ΔH° are the changes of the enthalpy at the final ionic strength value and I = 0, respectively; Ψ(I) is the theoretically calculated ionic strength function; ΔZ2 is the difference in the squares of charges of the reaction products and initial reagents; b is an empirical coefficient. The dependence of dissociation enthalpy on ionic strength is caused by variation of activity coefficients of ionic forms of the peptide. There are a number of works in which dependence of dissolution enthalpy on concentration of electrolyte additive was referred to interaction of different peptide groups with mineral ions [14]. In our work, experimental ΔdisH values are satisfactorily described by Eq. (14). The whole dataset of the standard thermodynamic values for the stepwise 3-alanyl-l-histidine dissociation is given in Table 6.
Of special interest is matching of the standard thermodynamic parameters of the stepwise dissociation of amino acids in a free state with those for amino acid residues involved in peptide linkage. The data available show that acidic properties of carboxyl and protonated imidazole group of l-histidine (\({\text{p}}K^{\circ}_{1}=-{\text{lg}}\,K^{\circ}_{1}=1.70\); \({\text{p}}K^{\circ}_{2}=-{\text{lg}}\,K^{\circ}_{2}=6.04\) [15]) become weaker in 3-alanyl-l-histidine dipeptide (\({\text{p}}K^{\circ}_{1}=2.59;\,{\text{p}}K^{\circ}_{2}=6.77\)). And conversely, dissociation constants of the protonated amino group of β-alanine (pK = 10.16 at I = 0.2, KCl) [16]) rise when the amino acid is involved in the peptide composition (\({\text{p}}K^{\circ}_{3}=9.37\)). The changes in entropy of l-histidine acid dissociation are evaluated as negative rather small values \(\Updelta_{\text{dis}}S^{\circ}_{1}=-17.6;\,\Updelta_{\text{dis}}S^{\circ}_{2}=-14.0\,{\text{J}}\,{\text{mol}}^{-1}\,{\text{K}}^{-1}\) [15]. The increase in the distance between protonated amino fragment and carboxyl or imidazole group in the dipeptide chain results in increasing negative values of the dissociation entropy: \(\Updelta_{\text{dis}}S^{\circ}_{1}=-33.3;\,\Updelta_{\text{dis}}S^{\circ}_{2}=-26.3\,{\text{J}}\,{\text{mol}}^{-1}\,{\text{K}}^{-1}\) (Table 6). When passing from l-histidine to 3-alanyl-l-histidine, the change in the enthalpy of dissociation \(\Updelta_{\text{dis}}H^{\circ}_{1}\) and \(\Updelta_{\text{dis}}H^{\circ}_{2}\) accounts to less than 1 kJ mol−1. More pronounced changes (up to 4.5 kJ mol−1) of the unfavorable entropy factor \(T\Updelta_{\text{dis}}S^{\circ}_{1}\) and \(T\Updelta_{\text{dis}}S^{\circ}_{2}\) are accountable for the lowering of the acid dissociation constants in the series. The entropy changes as being the most susceptible to structural rearrangements occurring in the solution can be attributed to the variations in hydration of reactivity centers. Results obtained by calculating and spectral methods [17, 18] suggest that the hydrogen-bonding structure of water in the solutions of amino acids is destroyed, and the maximum structure perturbation localizes around charged groups (ammonium and carboxylate groups). The hydration of multipolar ionic particles is dependent on the distance between the positively and negatively charged groups. An uncoordinated orientation water molecule in hydration shells of cation and anion strengthens the destroying effect on water structure. The destructive effect of the oppositely charged groups on the structure of their hydrated shells reduces as the distance between protonated amino fragment and carboxyl or imidazole group increases. In this, the change in dissociation entropy becomes more negative. Thus, the trend in alteration of \(\Updelta_{\text{dis}}S^{\circ}_{1}\) and \(\Updelta_{\text{dis}}S^{\circ}_{2}\) values supports that weakening ability to acid dissociation of carboxyl and imidazole groups of histidine in peptide composition is caused by the change in hydration of these reactive fragments. A similar tendency toward variation of the constants and entropies of dissociation has been observed by us earlier upon passing from glycine to triglycine [19] as well as from glutamine acid to its peptide [20]. It is interesting to note that the difference in dynamics of water molecules exchange between bound water and bulk water on amino acid sequence in peptides has been suggested by recent data [21].
Conclusions
In this paper, we considered the effect of a background electrolyte concentration on the dissociation enthalpy of the natural dipeptide, l-carnosine. It is found that the variation of ionic strength from 0.25 to 0.75 M results in the change in dissociation enthalpy by 4.2, 5.2 and 1.4 kJ mol−1 for amino group, imidazole fragment and carboxyl group, respectively. The correction to the zero-order ionic strength of obtained thermochemical results and available data on the acid dissociation constants permitted us to determine standard thermodynamic quantities (ΔdisH°, ΔdisG°, ΔdisS°) of the acid dissociation of the dipeptide in aqueous solution. The noticeable difference in ability to acid dissociation for amino acids in a free state and for amino acid residues involved in the dipeptide has been found. The alteration of acidity is caused by the change in dissociation entropy, and it is connected with the variation in hydration of reactivity centers.
References
Usacheva T, Kabirov D, Beregova D, Gamov G, Sharnin V, Biondi M, Mayol L, D’Aria F, Giancola C. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08136-5.
Makowska J, Wyrzykowski D, Kamysz E, Tesmar A, Kamysz W, Chmurzyński L. Probing the binding selected metal ions and biologically active substances to the antimicrobial peptide LL-37 using DSC, ITC measurements and calculations. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08310-9.
Bonomo RP, Bruno V, Conte E, De Guidi G, La Mendola D, Maccarrone G, Nicoletti F, Rizzarelli E. Salvatore Sortino and Graziella Vecchio, Potentiometric, spectroscopic and antioxidant activity studies of SOD mimics containing carnosine. Dalton Trans. 2003. https://doi.org/10.1039/b308168k.
Daniele PG, Prenesti E, Ostacoli G. Ultraviolet–circular dichroism spectra for structural analysis of copper(II) complexes with aliphatic and aromatic ligands in aqueous solution. J Chem Soc Dalton Trans. 1996. https://doi.org/10.1039/DT9960003269.
Gajda T, Henry B, Delpuech J-J. Multinuclear NM R and potentiometric study on tautomerism during protonation and zinc(II) complex formation of some imidazole-containing peptide derivatives. J Chem Soc Perkin Trans. 1994;2:157–64. https://doi.org/10.1039/P29940000157.
Gaggelli E, Valensin G. 1H and 13C NMR relaxation investigation of the calcium complex of β-alanyl-l-histidine (carnosine) in aqueous solution. J Chem Soc Perkin Trans 2. 1990. https://doi.org/10.1039/P29900000401.
Farkas E, Sovago I, Gergely A. Studies on transition-metal–peptide complexes. Part 8. Parent and mixed-ligand complexes of histidine-containing dipeptides. J Chem Soc Dalton Trans. 1993. https://doi.org/10.1039/DT9830001545.
Daniele PG, Amico P, Ostacoli G. Heterobinuclear Cu(II) L-carnosine complexes with Cd(II) or Zn(II) in aqueous solution. Inorg Chem Acta. 1982;66:65–70. https://doi.org/10.1016/S0020-1693(00)85791-6.
Daniele P, Prenesti E, Zelano V, Ostacoli G. Chemical relevance of the copper(II)–L-carnosine system in aqueous solution: a thermodynamic and spectrophotometric study. Spectrochim Acta A. 1993;49:1299–303. https://doi.org/10.1016/0584-8539(93)80037-B.
Meshkov AN, Gamov GA. KEV: a free software for calculating the equilibrium composition and determining the equilibrium constants using UV–Vis and potentiometric data. Talanta. 2019;198:200–5. https://doi.org/10.1016/j.talanta.2019.01.107.
Lytkin AI, Chernikov VV, Krutova ON, Skvortsov IA. Standard enthalpies of formation of l-lysine and the products of its dissociation in aqueous solutions. J Therm Anal Calorim. 2017;130:457–60. https://doi.org/10.1007/s10973-017-6134-6.
Archer DG. Thermodynamic properties of the KCl + H2O system. J Phys Chem Ref Data. 1999;28:1–16. https://doi.org/10.1063/1.556034.
Vasil
 ev VP. Termodinamicheskie svoystva elektrolitnykh rastvorov, Moskva, Vysshaya shkola, 1982, s.200.
ev VP. Termodinamicheskie svoystva elektrolitnykh rastvorov, Moskva, Vysshaya shkola, 1982, s.200.Piekarski H, Nowicka B. Calorimetrical studies of interactions of some peptides with electrolytes, urea and ethanol in water at 298.15 K. J Therm Anal Calorim. 2010;102:31–6. https://doi.org/10.1007/s10973-009-0547-9.
Vasil
 ev VP, Kochergina LA, Garavin VYu. Termodinamicheskie kharakteristiki dissotsiatsii l-hitidina v vodnykh rastvorakh. Zhurnal Obshei Khimii. 1985;55:2780–7.
ev VP, Kochergina LA, Garavin VYu. Termodinamicheskie kharakteristiki dissotsiatsii l-hitidina v vodnykh rastvorakh. Zhurnal Obshei Khimii. 1985;55:2780–7.Farkas E, Megyeri K, Somsak L, Kovacs L. Interaction between Mo(VI) and siderophore models in aqueous solution. J Inorg Biochem. 1998;70:41–5. https://doi.org/10.1016/S0162-0134(98)00011-7.
Ide M, Maeda Y, Kitano H. Effect of hydrophobicity of amino acids on the structure of water. J Phys Chem B. 1997;101:7022–6. https://doi.org/10.1021/jp971334m.
Lim VI, Curran JF, Garber MB. Hydration shells of molecules in molecular association: a mechanism for biomolecular recognition. J Theor Biol. 2012;301:42–8. https://doi.org/10.1016/j.jtbi.2012.02.008.
Badelin VG, Barannikov VP, Tarasova GN, Chernyavskaya NV, Katrovtseva AV, Lan FT. Thermodynamical characteristics of Acid_Base equilibria in Glycyl_Glycyl_Glycine aqueous solutions at 298 K. Rus J Phys Chem A. 2012;86:40–4. https://doi.org/10.1134/S003602441112003X.
Badelin VG, Barannikov VP, Katrovtseva AV, Tarasova GN. Dissociation constants of protolytic dissociation of glutamyl-glutamic and glycyl-glutamic acids in aqueous solution at 298 K. Rus J Gen Chem. 2013;83:945–8. https://doi.org/10.1134/S1070363213050113.
Dandurand J, Samouillan V, Pepe A, Bochicchio B. Phase behavior and chain dynamics of elastin-like peptides versus amino acid sequences. J Therm Anal Calorim. 2018;131:1323–32. https://doi.org/10.1007/s10973-017-6633-5.
Acknowledgements
The work was financially supported by the Russian Foundation for Basic Research (Project No: 18-43-370018_r_a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lytkin, A.I., Barannikov, V.P., Badelin, V.G. et al. Enthalpies of acid dissociation of l-carnosine in aqueous solution. J Therm Anal Calorim 139, 3683–3689 (2020). https://doi.org/10.1007/s10973-019-08604-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08604-y




 ev VP. Termodinamicheskie svoystva elektrolitnykh rastvorov, Moskva, Vysshaya shkola, 1982, s.200.
ev VP. Termodinamicheskie svoystva elektrolitnykh rastvorov, Moskva, Vysshaya shkola, 1982, s.200.