Abstract
Quercetin (QCT) is a flavonoid possessing many activities, such as neuro-/cardioprotective, anti-inflammatory and anticancer, but its pharmacological application is severely curtailed by its low water solubility and in vivo bioavailability. The formation of a QCT–hydroxypropyl-β-cyclodextrin (HPβCD) host–guest complex is promising to improve QCT therapeutic potential. Therefore, here the heat effects of HPβCD solutions with QCT solutions in water–ethanol solvents at different concentrations were studied by calorimetric titration, and the stability of molecular complexes was assessed by UV–Vis spectrophotometry. Calorimetric titrations revealed the formation of a QCT/HPβCD host–guest complex with a stoichiometric ratio of 1:1 in X(EtOH) = 0.00, 0.05 and 0.10 molar fractions of solvents at pH = 7.0 and pH = 8.1. Thermodynamic parameters of the complex formation reaction (lgK; ΔrH; TΔrS) were obtained in these experimental conditions. Differently, no complex formation was noticed in water–ethanol mixed solvent when ethanol volume fraction exceeded 0.2 at neutral and alkaline pH, as well as a volume fraction higher than 0.1 at acidic pH. Furthermore, the results of differential scanning calorimetry tests run on dried HPβCD after dissolution in hydroalcoholic solutions indicated that ethanol and water compete for the complexation within the hydrophobic cavity of HPβCD. This explains the decreased QCT complexation efficacy in the presence of ethanol beyond 0.1 or 0.2 volume fraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
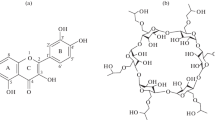
Quercetin (QCT 3,5,7,3′,40′-pentahydroxyflavone; Fig. 1a) is a flavonoid, containing a 3-hydroxyflavone backbone, that can be found in numerous fruits, vegetables and grains [1]. QCT is endowed with manifold beneficial properties, such as the reduction in systolic blood pressure [2], the inhibition of mast cell secretion [3] and in vitro production of cyclooxygenase and lipoxygenase [4], along with neuro-/cardioprotective [5, 6], antiviral [7], anti-inflammatory [8] and anticancer [9,10,11,12] activities. All these features have prompted the study of QCT as a potential molecule to be used in the pharmaceutical field. However, the bioavailability profile of QCT is very poor due to its low permeability, stability and solubility in aqueous media (approximately 1.5 and 30 μg mL−1 in water, simulated gastric fluid and simulated intestinal fluid) [13].
In general, the bioavailability of active molecules can be improved by loading them into liposomes [14], nanoparticles [15] or micelles [16] or by forming inclusion complexes with cyclodextrins (CDs) [17]. The latter are supramolecular structures commonly employed to promote the in vitro and in vivo solubility of poorly water-soluble molecules and hence their therapeutic index. CDs are cyclic oligosaccharides with a frusto-conical architecture, consisting of glucopyranose units, bound by α-(1,4) glycoside bonds. Taking advantage of their hydrophilic outer surface and lipophilic internal cavity, CDs can interact with a wide array of host molecules forming inclusion complexes through non-covalent bonds [18]. Mainly, three natural CDs exist, namely α, β and γ, which contain 6, 7 and 8 glucose units, respectively [19]. The α-CD cavity is generally too small to allow an efficient complexation of most drugs, while γ-CDs are rather expensive. Thus, β-CDs are most frequently used in pharmaceutical applications, mainly due to their prompt availability and cavity size that can fit numerous drugs [20]. Amorphous, non-crystallizable semisynthetic derivatives of β-CDs possess an enhanced physical and microbiological stability, along with a lower parenteral toxicity [21, 22].
The formation of the host–guest complex between QCT and (2-hydroxypropyl)-β-cyclodextrin (HPβCD; Fig. 1b) has been studied in detail in water [17, 23]. In a recent report, QCT/HPβCD complex has also been prepared in ethanol by the co-precipitation method, resulting in a strong enhancement of QCT aqueous solubility and photostability [24]. However, to the best of our knowledge, the stability of the complex has not been established to date in water and hydroalcoholic solutions.
In a previous work, we found that the addition of a non-aqueous substance to water promotes the stability of the molecular complexes of crown ethers and amino acids/peptides, leading to an increase in the exothermicity of the reactions of their formation due to a change in the solvation features of the complexes [25]. Thus, here we used a thermodynamic approach to quantitatively analyze the influence of individual solvation factors on the stability of the complexes as previously reported [26]. More specifically, the processes of solubilization of water-insoluble compounds by the formation of the host–guest inclusion complex with CDs can be considered as processes of competition–substitution of water molecules in the CD cavity by a guest molecule [27]. Hence, in the presence of co-solvent molecules, water content in the internal cavity of macrocycle will depend on the competition between the “guests” and co-solvent molecules of a mixed solvent in the CD cavity. For instance, it has been established that some minimal amount of methanol or ethanol facilitates the binding of large hydrophobic “guests” to β-cyclodextrin [28]. In this regard, the use of non-aqueous solvent additives to water is expected to help in creating optimal conditions for the solubilization of hydrophobic molecules by CDs. Starting from these considerations, herein we have studied the formation of QCT/HPβCD inclusion complex in water–ethanol mixtures at different concentrations of ethanol so as to assess the effect of solvent composition on the formation of QCT/HPβCD complex.
To this aim, the thermodynamic parameters involved in the complex formation have been determined starting from microcalorimetry experiments carried out on the different hydroalcoholic mixtures and at different pH values.
Materials and methods
Materials
All substances were obtained from Sigma-Aldrich. QCT and HPβCD (both ≥ 99% purity) were used as received, without further purification. Rectificate grade ethyl alcohol was purified by distillation before use. The amount of water in EtOH (≤ 5% w/w) was determined from the density by accurately weighing using a pycnometer, preliminarily calibrated with ethanol on an analytical scale balance AUX220D, and taking into account these measurements in the preparation of aqueous ethanol solvents. Mixed solvents were prepared with bidistilled and deaerated water by a gravimetric method.
Methods
Isothermal titration calorimetry
The heat effects of mixing HPβCD solutions with QCT were determined by isothermal titration calorimetry with the TAM III (TA Instruments, USA) microcalorimeter in water–ethanol mixed solvents containing X(EtOH) = 0.00, 0.05, 0.10, 0.20, 0.50 and 0.95 molar fractions in phosphate buffer at pH = 3.6, 7.0 and 8.1 and at T = 298.15 K.
The optimal concentration conditions for the experiments, limited by the low solubility of the reagents in the water–ethanol mixtures, were previously calculated according to the RRSU program [29] for each solvent composition. The yield of the QCT/HPβCD complex varied in the widest range of values (3–50%).
The initial concentration of HPβCD in the syringe ranged from 1.45 10−2 to 1.59 10−2 mol L−1. The initial concentration of QCT in the cell ranged from 1.19 10−4 to 2.25 10−4 mol L−1. The HPβCD/QCT molar ratio was in the 3–9 range. In all experiments, the composition of the solvent in the syringe and in the cell was the same.
The heat effect of mixing for solutions of HPβCD with solutions of QCT (Qmix) is contributed by the heat of QCT/HPβCD complex formation (Qcompl), the heat of dilution of HPβCD solution (Qdil 1) in a solvent in the cell and the heat of dilution of QCT solution placed in the cell in a solvent added from syringe (Qdil 2):
The last term was considered to be negligible; therefore, it follows:
The values of lgK and ΔrH for QCT/HPβCD complex formation have been calculated by the program HEAT developed to simultaneously calculate the enthalpies of reaction and the equilibrium constants of complex formation for systems with any stoichiometry [30]. The HEAT use and application were described in detail in previous publications for the treatment of calorimetric data of the molecular complex formation of amino acids and peptides with crown ethers and cryptand [2.2.2] mixed solvents [25, 31]. The algorithm for the calculation of lgK and ΔrH used by HEAT consists in the numerical minimization of function F:
where N is the number of experimental points; ωi is the mass of the single measurement; and ΔcomplH and ΔcalcH are the experimental and calculated molar enthalpies of the process, respectively. In this work, all ΔcomplH experimental values have been considered to be determined with the same precision, so wi = 1.
UV–Vis spectroscopy
The UV–Vis spectral data were processed using FTMT program [30]. The FTMT program applies for equilibrium modeling in solutions and data processing of spectral measurements with the purpose of determining equilibrium constants. For this, an approach based on the statistical maximum likelihood principle was used. The mathematical model of the system sets the number and stoichiometry of the reactions, the values of the equilibrium constants, the partial molar properties of the particles or reactions and the total concentrations of the components.
The calculations were performed basing the experimental dependencies of absorbance at one wavelength on the initial concentration ratio of the reagents. The molar extinction coefficients of QCT and HPβCD required for calculations in FTMT, at each pH and wavelength value, were preliminarily determined using calibration plots. The sum of mean square deviations for calculated and experimental values of optical density was in the range from 0.0001 to 0.1.
Differential scanning calorimetry
Aiming to verify the possible different interactions of ethanol and water with cyclodextrins, thermoanalytical tests have been run on HPβCD using a TA Q20 differential scanning calorimeter (DSC; TA Instruments, USA). In particular, DSC spectra have been obtained on HPβCD as received, and on the dry residue of HPβCD solubilized in water, ethanol and hydroalcoholic solutions (water/ethanol 9:1 and 8:2 volumetric ratios). All DSC tests have been carried out in the solid state, on the samples preliminarily dried in the hood overnight. Samples were accurately weighted (~ 5 mg) and placed in hermetic aluminum pans. Then, the samples were heated from 20 to 240 °C at 10 °C min−1 under an inert nitrogen atmosphere at a constant flow rate (50 mL min−1). An empty aluminum pan was used as a reference. Triplicate scans have been performed.
Results and discussion
Previous studies focused on the complex formation between d-metal ions and amine or carboxylate complexes in aqueous–organic solvents have revealed, on the basis of the solvation-thermodynamic approach, the possibility of predicting the thermodynamic parameters of the ionic complex formation reactions in different media according to a change in the solvation state of ligands [32,33,34]. In particular, the complexation in water is considered as a set of reactions of stepwise replacement of water molecules in the first solvate shell of the central ion by a ligand molecule [32]. In a binary solvent, the set of reactions is more complicated, since there is a preferential solvation of reagents with one of the solvent components [33, 34]. In the complexation of inorganic cations with crown ethers, cryptands and other macrocyclic structures, the central ion is completely or almost completely isolated from the solvent. For less stable complexes between macrocycle (host) and organic molecule (guest), the guest molecule and the solvent most probably compete for the formation of the complex with the HPβCD.
In this work, the thermodynamic parameters of the complex formation between QCT and HPβCD in water and in ethanol/water mixed solvent, at pH = 7.0 and 8.1, compared with the relevant literature data in water are presented in Table 1. The results of calorimetric titrations showed that the complexation occurs in ethanol/water mixtures of composition X(EtOH) = 0.00, 0.05 and 0.10 molar fraction. Conversely, no complex formation was found out when ethanol molar fraction was ≥ 0.20 at pH = 7.0 or when pH was lowered to 3.6 with a 0.10 EtOH molar fraction, according to the calorimetric titration and UV–Vis data.
The formation of the QCT/HPβCD complex can be envisaged by the total heat of complexation Σ(Qcompl) dependence on the concentration of HPβCD in the cell. More in detail, when the complex formed, Σ(Qcompl) becomes HPβCD concentration independent as observed in Fig. 2a–c. Differently, as shown in Fig. 2d, e, in the presence of weak molecular interactions, the total heat of complexation Σ(Qcompl) dependence on the concentration of HPβCD in the cell is linear. Binding constants at pH = 7.0 were also calculated by UV–Vis titration spectra (Fig. 3a, b), and the results are shown in Table 1.
Total heat effect of the interaction between QCT and HPβCD in H2O–EtOH solvent, depending on the total molar concentration of HPβCD in the cell, a—in H2O at T = 298.15 K, pH = 7.0; b—at X(EtOH) = 0.10 molar fraction, at T = 298.15 K, pH = 7.0; c—at X(EtOH) = 0.10 molar fraction, at T = 298.15 K, pH = 8.1; d—at X(EtOH) = 0.10 molar fraction, at T = 298.15 K, pH = 3.6; e—at X(EtOH) = 0.20 molar fraction, at T = 298.15 K, pH = 7.0
An inspection of Table 1 shows that the thermodynamic parameters of QCT/HPβCD complex formation have been obtained in water by various methods, under different conditions [17, 23, 35,36,37]. In most of them, the values of the association constants are in satisfactory agreement with each other and with our values obtained by both calorimetric and UV–Vis methods. In our previous paper, we found the association constant in water at pH = 8.0 [23], and in this work we were able to obtain calorimetric data in water at pH = 7.0. At this pH, the association constant was one order of magnitude higher and the values of ΔrH and ΔrS showed an increase in both the exothermicity of complexation and the entropic contribution to the Gibbs energy change for the complex formation.
The different ΔrH and ΔrS values obtained by Liu et al. can be reasonably explained, considering that they were indirectly extracted by the phase solubility analysis at a different pH of 7.4 [17]. After the addition of ethanol to water, the stability of the complex was unchanged for X(EtOH) = 0.00, 0.05 and 0.10 molar fraction. However, along with this, an increase in the exothermicity of complexation and a decrease in the entropic contribution to the Gibbs energy change of the formation reaction were detected.
In a previous publication, an insignificant effect of added DMSO on the stability of molecular complexes was found in the study of the complexation of triglycine with cryptand [2.2.2] [31]. This finding was discussed based on the compensation effect of the entropy/enthalpy contributions to the Gibbs energy of complexation. Furthermore, in the case of molecular complexes of crown ethers with amino acids and peptides, the addition of ethanol, DMSO and acetone resulted in an increased stability of molecular complexes and in an increase in the exothermicity of their formation reactions [25]. These results were explained in terms of changes in solvation of guest molecules with the replacement of waters by organic solvents.
Considering previous studies, we could hypothesize that moving from water to hydroalcoholic solvent, the thermodynamics of QCT/HPβCD complex formation is influenced by the change in solvation of QCT. The solubility of QCT is higher in ethanol/water mixtures than in water [41], and this indicates that ethanol molecules displace water molecules in QCT hydration shell, affecting both the enthalpic and entropic contributions to Gibbs energy of complexation, so that the lgK for low X(EtOH) remains the same as in water. Increasing ethanol molar fraction, EtOH is able to effectively solvate QCT molecules, by subtracting them from the complexation, and competes for occupation of HPβCD cavities or fills the residual empty space of the cavity of HPβCD, as already found in another study [42]. The overall result is that, for X(EtOH) greater than 0.10 molar fraction, no complexation occurs.
DSC scans have been run to provide further information on the physical and chemical processes occurring during heating. Commercial HPβCD displays a broad endothermic peak, which is indicative of the release of superficial and strongly retained water from the hydrophobic core at 106 °C (Fig. 4) [38, 39]. After HPβCD dissolution in water, the DSC peak appears at a very similar temperature (about 103.5 °C), therefore indicating that the same interaction occurs after immersion of HPβCD in water. The DSC peak appears at slightly lower temperatures after dissolution in 9:1 and 8:2 water/ethanol mixtures, around 100°. The decrease in these peak temperatures can be explained by the formation of the host–guest molecular inclusion compound that allows the replacement of the strongly retained water molecules inside the cavity by the ethanol moieties. The obtained complex mainly most probably contains superficial water molecules that are more easily released, and hence, the peak temperature decreases [40]. This indicates that the total water content is lower and/or weakly bound to HPβCD after immersion in ethanolic or hydroalcoholic solvents, compared to the untreated, commercial CD. These results therefore corroborate the existence of the competition between ethanol and water for the complexation within the hydrophobic cavity of HPβCD which in turn contributes in decreasing the efficacy of QCT complexation in the presence of ethanol.
Conclusions
In this work, it has been established that the addition of ethanol to water leads to an insignificant decrease in the stability of the QCT/HPβCD host–guest complex. However, along with this, there are an increase in the exothermicity of complexation and a decrease in the entropic contribution to the change of the reaction Gibbs energy. Above X(EtOH) = 0.10 molar fraction, no complexation occurs. It seems unexpected because the decrease in the complex stability should be more significant at increasing ethanol content in the solvent. Probably, in H2O–EtOH solvent with 0.1 and more molar fraction of EtOH the replacement of QCT in the cavity of CD by molecules of EtOH is presented. In consequence, the complex QCT-CD does not form. This can be reasonably ascribed to the outcomes of DSC results, which sowed that ethanol actively competes for the inclusion within HPβCD cavity, therefore hampering the effective formation of QCT–HPβCD inclusion complex. Overall, the data showed that the ethanol affects QCT solvation, shifting the equilibrium far from QCT/HPβCD complex formation, and competes for occupation of HPβCD cavities. Taken altogether, these results highlight a counterintuitive conclusion, in that the expected solubility enhancement of the active molecule in the presence of ethanol did not match a higher affinity between QCT and HPβCD. However, further studies on different molecules in mixed solvents will be devoted to shed light on the thermodynamic interactions between pharmacologically active natural compounds and HPβCD.
References
D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–71.
Zahedi M, Ghiasvand R, Feizi A, Asgari G, Darvish L. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: a double-blind randomized controlled clinical trial. Int J Prev Med. 2013;4:777–85.
Shaik YB, Castellani ML, Perrella A, Conti F, Salini V, Tete S, Madhappan B, Vecchiet J, De Lutiis MA, Caraffa A, Cerulli G. Role of quercetin (a natural herbal compound) in allergy and inflammation. J Biol Regul Homeost Agents. 2006;20:47–52.
Kim HP, Mani I, Iversen L, Ziboh VA. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot Essent Fatty Acids. 1998;58:17–24.
Marsh DT, Das S, Ridell J, Smid SD. Structure-activity relationships for flavone interactions with amyloid β reveal a novel anti-aggregatory and neuroprotective effect of 2’,3’,4’-trihydroxyflavone (2-D08). Bioorg Med Chem. 2017;25:3827–34.
Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–80.
Ohnishi E, Bannai H. Quercetin potentiates TNF-induced antiviral activity. Antivir Res. 1993;22:327–31.
Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–7.
Khonkarn R, Mankhetkorn S, Hennink WE, Okonogi S. PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur J Pharm Biopharm. 2011;79:268–75.
Ghasemzadeh A, Jaafar HZ, Rahmat A, Ashkani S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) R.M.Sm grown in different locations of Malaysia. BMC Complement Altern Med. 2015;15:335–45.
Scambia G, Ranelletti FO, Panici PB, Piantelli M, Bonanno G, De Vincenzo R, Ferrandina G, Rumi C, Larocca LM, Mancuso S. Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumours and cultured cells. Br J Cancer. 1990;62:942–6.
Larocca LM, Piantelli M, Leone G, Sica S, Teofili L, Panici PB, Scambia G, Mancuso S, Capelli A, Ranelletti FO. Type II oestrogen binding sites in acute lymphoid and myeloid leukaemias: growth inhibitory effect of oestrogen and flavonoids. Br J Haematol. 1990;75:489–95.
Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20:2572–82.
Park SN, Lee MH, Kim SJ, Yu ER. Preparation of quercetin and rutin-loaded ceramide liposomes and drug-releasing effect in liposome-in-hydrogel complex system. Biochem Biophys Res Commun. 2013;435(3):361–6.
Bose S, Du Y, Takhistov P, Michniak-Kohn B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int J Pharm. 2013;441:56–66.
Gao X, Wang B, Wei X, Men K, Zheng F, Zhou Y, Zheng Y, Gou M, Huang M, Guo G, Huang N, Qian Z, Wei Y. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. 2012;4:7021–30.
Liu M, Dong L, Chen A, Zheng Y, Sun D, Wang X, Wang B. Inclusion complexes of quercetin with three β-cyclodextrins derivatives at physiological pH: spectroscopic study and antioxidant activity. Spectrochim Acta A Mol Biomol Spectrosc. 2013;115:854–60.
Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6:E329–57.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–25.
Szejtli J. Cylodextrin in drug formulations: part I. Pharm Technol Int. 1991;3:15–23.
Szente L, Szejtli J. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv Drug Deliv Rev. 1999;36:17–38.
Matsuda H, Arima H. Cyclodextrins in transdermal and rectal delivery. Adv Drug Deliv Rev. 1999;36:81–99.
D’Aria F, Serri C, Niccoli M, Mayol L, Quagliariello V, Iaffaioli RV, Biondi M, Giancola C. Host–guest inclusion complex of quercetin and hydroxypropyl-β-cyclodextrin. J Therm Anal Calorim. 2017;130:451–6.
Savic IM, Nikolic VD, Savic-Gajic I, Nikolic LB, Radovanovic BC, Mladenovic JD. Investigation of properties and structural characterization of the quercetin inclusion complex with (2-hydroxypropyl)-β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2015;82:383–94.
Usacheva TR, Sharnin VA. A thermodynamic study of reactions of amino acids with crown ethers in nonaqueous media as examples of guest-host molecular complex formation. Russ Chem Bulletin. 2015;64:2536–44.
Krestov GA, Novosyolov NP. Ionic solvation. New York: Ellis Horwood Edi; 1994.
Donze C, Coleman AW. Solvent effects in competition between guest molecules for β-cyclodextrin. J Incl Phenom Mol Recogn Chem. 1995;23:11–21.
Yoshii H, Kometani T, Furuta T, Watanabe Y, Linko YY, Linko P. Formation of inclusion complexes of cycldextrin with ethanol under anhydrous conditions. Biosci Biotechnol Biochem. 1998;62:2166–70.
Vasiliev VP, Borodin VA, Kozlovsky EV. The use of computers in chemical-analytical calculations, vol. 112. Moscow: Higher Education school; 1993 in Russian.
Borodin VA, Vasil’ev VP, Kozlovskii EV. Mathematical problems of chemical thermodynamics, vol. 219. Novosibirsk: Nauka; 1985 (in Russian).
Usacheva TR, Pham Thi L, Terekhova IV, Kumeev RS, Sharnin VA. Thermodynamics of molecular complexation of glycyl–glycyl–glycine with cryptand [2.2.2] in water–dimethylsulfoxide solvent at 298.15 K. J Therm Anal Calorim. 2016;126:307–14.
Sharnin VA. Thermochemistry of formation of copper (II)-ethylendiamine complexes and solvation of reagents in aqueous organic solvents. J Therm Anal Calorim. 1995;45:721–8.
Zevakin MA, Grazhdan KV, Dushina SV, Sharnin VA. Thermodynamic characteristics of reagents and reaction of Ag+—nicotinamide complex formation in water-ethanol media. J Mol Liq. 2007;131–132:163–7.
Gesse ZF, Repkin GI, Isaeva VA, Sharnin VA. The influence of reagents solvation on enthalpy change of glycine-ion protonation and silver(I) glycine-ion complexation in aqueous-dimethylsulfoxide solutions. J Therm Anal Calorim. 2013;110:1457–62.
Jullian C, Moyano L, Yanez C, Olea-Azar C. Complexation of quercetin with three kinds of cyclodextrins: an antioxidant study. Spectrochim Acta A Mol Biomol Spectrosc. 2007;67:230–4.
Pralhad T, Rajendrakumar K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J Pharm Biomed Anal. 2004;34:333–9.
Zheng Y, Haworth IS, Zuo Z, Chow MS, Chow AH. Physicochemical and structural characterization of quercetin-β-cyclodextrin complexes. J Pharm Sci. 2005;94:1079–89.
Oprean C, Mioc M, Csányi E, Ambrus R, Bojin F, Tatu C, Cristea M, Ivan A, Danciu C, Dehelean C, Paunescu V, Soica C. Improvement of ursolic and oleanolic acids’ antitumor activity by complexation with hydrophilic cyclodextrins. Biomed Pharmacother. 2016;83:1095–104.
Tang P, Tang B, Wang Q, Xu K, Xiong X, Li H. Effect of hydroxypropyl-β-cyclodextrin on the bounding of salazosulfapyridine to human serum albumin. Int J Biol Macromol. 2016;92:105–15.
Hădărugă NG, Hădărugă DI, Isengard HD. “Surface water” and “strong-bonded water” in cyclodextrins: a Karl Fischer titration approach. J Incl Phenom Macrocycl Chem. 2013;75:297–302.
Razmara RS, Daneshfar A, Sahraei R. Solubility of quercetin in water + methanol and water + ethanol from (292.8 to 333.8) K. J Chem. 2010;55:3934–6.
Kanokthip B, Helmut V, Peter W, Luckhana L. Influence of ethanol as a co-solvent in cyclodextrin inclusion complexation: a molecular dynamics study. Sci Pharm. 2015;83:387–99.
Acknowledgements
The calorimetric measurements presented in this work were carried out at the Institute of Thermodynamics and Kinetics of Chemical Processes of the Ivanovo State University of Chemistry and Technology (ISUCT) using the equipment of the Center for Collective Use of ISUCT. The study was carried out under grant of Council on grants of the President of the Russian Federation (Project 14.Z56.18.877-MK). The authors thank the University of Naples Federico II for the financial support of their collaboration contributed to the preparation of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usacheva, T., Kabirov, D., Beregova, D. et al. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J Therm Anal Calorim 138, 417–424 (2019). https://doi.org/10.1007/s10973-019-08136-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08136-5