Abstract
The sufficient energy to transfer the crude oil to the surficial wellbore facilities would be reduced dramatically which was done by CO2, especially for heavy and super-heavy oil reservoirs. The objective of this comprehensive study is to measure the considerable influence of CO2 solubility on the recovery factor, density, viscosity at different pressures and temperatures. According to the results of this study, recovery factor at the pressure of 0.2 MPa and 0.5 MPa experienced the lowest recovery factor among other pressures. They were about 38.37% and 43.81% after the injection of about 15 pore volumes of CO2. For the pressures of 1 MPa, 2 MPa, 3 MPa, and 4 MPa, at the first stages of CO2 injection (up to 3 pore volumes of CO2 injection), the recovery factor experienced the same value. Since then, by the increase in pressure from 1 to 4 MPa, the recovery factor was increased slightly. Moreover, recovery factor at the temperature of 333 K was measured about 34.5%. The measured recovery factor for other temperatures was 40.17%, 47.68%, 51.97%, 58.42%, and 63.54% at the temperature of 363 K, 393 K, 423 K, 453 K, and 483 K, respectively. On the other hand, the density of heavy oil which was saturated with CO2 was decreased with the increase in pressure and temperature and the higher temperatures caused the lower effect on the viscosity and density of heavy oil. Consequently, the dissolution of CO2 had decreased the heavy oil viscosity in the higher temperatures and pressures, and due to the increase in pressure and temperature, the heavy oil recovery factor was increased. Furthermore, the recovery factor for the 70 mL min−1 of CO2 injection was lower than the 700 mL min−1 of CO2 injection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
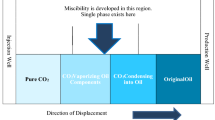
Enhanced oil recovery methods have been divided mainly into two different stages, which are known as primary and secondary recovery methods [2, 5, 11, 20, 39]. These methods contain waterflooding injection or reinjection of natural gas which was produced on the surface, relatively thirty percent of the total original oil-in-place (henceforth, OOIP) to itself [12, 16, 19, 24, 43]. However, by utilizing the tertiary recovery methods, production rate could reach 40–60% of total oil-in-place in the reservoir. EOR methods are generally divided into two principal categories: nonthermal recovery and thermal recovery methods [9, 14, 32, 36, 46]. In the recent decades, chemical flooding and gas injectivity processes have widely reported in the literature in the case of operational and laboratory performances to improve the oil recovery [10, 17, 28, 37, 48]. Thermal recovery performances were considered as one of the efficient techniques in the oil recovery enhancement of heavy and super-heavy oil reservoirs [13, 15, 26, 42, 44]. SAGD procedures by the utilization of steam and CO2 utterly depended on the segregationally gravity to produce more oil volume [4, 21, 24, 33, 35]. SAGD processes are usually considered as the optimum methods of recovery performances by the drilling of a new horizontal well near the main reservoir to inject the steam continuously. One of the important things about CO2 which is profoundly impacted the viscosity and density is that CO2 regarding its mass transfer and the compositional exchange between heavy oil and CO2 makes an efficient displacement in the reservoir [1, 6, 30, 31, 34]. Another efficient mechanism of improving displacement efficiency was related to the interfacial tension in the miscible flooding of CO2. Therefore, the lower interfacial tension of oil–CO2 rather than water–oil interfacial tension helps to more mobilization of oil phase in the reservoir. On the contrary, the macroscopic sweep efficiency of the CO2 is poor enough regarding the relatively low density and viscosity of the CO2, which considered as one of the main consequences of CO2 in some of the reservoirs [3, 18, 22, 23, 27, 45].
The high potential of CO2 regarding its inexpensive and availability was considered as one of the optimum techniques in enhanced recovery of heavy and super-heavy oil reservoirs. The reason for this CO2 efficiency is the higher solubility of the gas phase in oil, which caused to decrease the viscosity dramatically and subsequently increase the volume of produced oil. According to the Jadhawar and Sarma’s [27] study, they concluded that the CO2 injection at the pressure ranges of 1–6 MPa caused to the reduction of viscosity about 80% and the total volume of produced oil was increased about 10–20% [27]. The swelling of oil caused to rise the elastic energy and improve the mobility of residual oil which was trapped in the reservoir. The dissolution of CO2 regarding the interfacial tension reduction between oil and CO2 and rise in the relative permeability of oil phase which had decreased the heavy oil viscosity in the higher temperatures and pressures were studied by Cui et al. [8]. As CO2 has filled those pore volumes which were occupied by the oil phase, some parts of CO2 could dissolve in the water and residual oil and the others would be remained in the formation. Therefore, CO2 would be an optimum choice for the recovery enhancement of heavy oil reservoirs [7, 8].
According to the numerical simulation of waterflooding, CO2 flooding, and alternative injection of CO2 and water in heavy oil reservoir by Sohrabi et al. [40], it was witnessed that displacement efficiency by the CO2 had provided better results rather than waterflooding. Moreover, alternative injection of steam and CO2 was recommended as the efficient technique on the recovery enhancement of heavy oil reservoirs [40]. Zheng et al. [47] proposed a three-dimensional model to consider the influence of CO2 injection under the pressure maintenance in a heavy oil reservoir. They concluded that CO2 injection under the pressure maintenance would be a beneficial choice in the improvement of oil recovery. Kavousi et al. [29] proposed that thermal processes such as CO2 injection caused to CO2 initial pressure, and subsequently, the molecular diffusion and solubility of CO2 were increased. Thereby, the recovery factor of the heavy oil would be improved rather than conventional techniques such as waterflooding [29]. Seyyedsar and Sohrabi [38] investigated the profound impact of intermittent CO2 and its viscosity-reducing gas parameter which help to improve the recovery during the CO2 injection [38]. Ebadati et al. [20] had investigated different injectivity scenarios of water and CO2 for an heavy oil, and they concluded that how alternative injection of water and gas would be a good choice to improve the oil recovery factor. They concluded that hot WAG injection regarding the feasible mobility of gas in the pores would improve the recovery factor more than waterflooding.
Although there are numerous research and experimental investigations that have been widely reported in the literature to espouse the importance of CO2 flooding on the heavy oil reservoirs, in this experimental study, the degasified heavy oil and the heavy oil that were saturated with CO2 were investigated. In this study, the crucial parameters of density, solubility of CO2, viscosity and the recovery factor were taken into the consideration at different pressures and temperatures. As a result, the dissolution of CO2 had decreased the heavy oil viscosity in the higher temperatures and pressures. Moreover, due to the increase in pressure, the heavy oil recovery factor was increased and the recovery factor of heavy oil was increased due to the increase in temperature.
Materials and methods
Materials
Sand pack: the provided sand pack which is used in this experimental investigation is extracted from one of the Iranian sandstone reservoirs in the Pazanan oilfield with the approximate length of 8.25 cm and 3 cm of outer diameter. The sand packs were consisted of the 120–160 range of meshes with the approximate particle diameter of 315–500 μm.
Performed gas: carbon dioxide (CO2) which was used in this experiment was purified to the percent of 99% which is to be administered in the flooding procedures.
Crude oil: the administered oil in this study was provided from Pazanan oilfield with the initial viscosity of 1.158 × 106 mPa s−1 at the atmospheric temperature and pressure.
To measure the solubility, density, and viscosity of the provided heavy crude oil which was saturated with CO2, the following steps were done. As can be seen in Fig. 1, a high resistant mixer (at the temperature of 573 K) was capable of mixing the heavy oil with CO2 at the predetermined temperature and pressure. A mixing hammer was added to the mixer to reduce the adverse impact of high temperature. The main purpose of adding an intermediate container in the system was to check the volume of heavy oil that was mixed in the sample mixer with the maximum value of 70 mL. To ensure the maximum pressure and the equipment’s security during the experiment performance, backpressure valves and gate valves were used. To measure the viscosity, a thermo-scientific rheometer was used in this investigation, which is operated as an automation system by the utilization of a close loop to calculate the rheology property. To measure the oil mass, a scientific scale was utilized in the system in which subsequently the value of density was calculated. Finally, to ensure the solution of CO2 in the heavy oil, a gas controller was set in the system.
After providing the necessary materials for performing the experiment, the apparatus is designed as Fig. 2 to measure the displacement efficiency of heavy oil which is saturated with CO2. The designed apparatus was consisted of a sand pack which was taken in holder, injection system (gas controller and CO2 bottle with high pressure), and data measurement system by the utilization of a computer. Gas controller was used by the adjustment of injected CO2 rate during the CO2 injectivity procedures. Pressure sensors were put at the outlet and inlet sides of the sand pack to record the pressure on each time. Oil production from the sand pack was transferred by a backpressure valve to a scale container. After that, the recovery factor was calculated by the mass of oil which was stored in the container.
CO2 solubility impact on the heavy oil
To consider the impact of CO2 solubility, degasified heavy oil and a large volume of CO2 were mixed in the sample mixer under different pressures (0.2 MPa, 0.5 MPa, 1 MPa, 2 MPa, 3 MPa, 4 MPa,) and temperatures (333 K, 363 K, 393 K, 423 K, 453 K, 483 K) within 2 days before commencing the experimental evaluation. The mixer was put vertically into the system by setting a pressure pump at a constant value less than the real pressure. Seventy milliliters of oil was injected into the container when it was full. Since then, the CO2 was separated by the flash evaporator and measured accurately by gas flow meter. Finally, the CO2 solubility was calculated at the determined pressure and temperature by the gas flow meter accurately.
CO2 impact on the heavy oil density
After the injection of oil volume into the container, it was saturated with the CO2 and then the masses of this mixture were measured by beaker. Finally, the density was calculated with crude oil density formula. In the second stage, degasified heavy oil was injected to the sample mixer under the definite pressure and temperature condition within 2 days and the density was calculated as same as the mixture of heavy oil and CO2.
CO2 impact on the heavy oil viscosity
The first sections of this technique were followed as the measurements of density and the solubility of gas phase. After the injection of required oil volume in the container, the saturated heavy oil with CO2 was put in the beaker and the viscosity was measured by the scientific rheometer at the determined pressure and temperature. To measure the viscosity of the degasified heavy oil, the heavy oil was injected into the high-pressure and high-temperature resistance mixer and its viscosity was measured as same as saturated heavy oil with CO2.
Oil recovery factor experimental evaluation with the injection of CO2
At the first stage of the experiment, the permeability, porosity, and oil saturation were estimated for each set of experiment in the average value to eliminate the experiment discrepancies. The backpressure valves which included nitrogen to designate the pressure value in a constant pressure and the temperature were set to 473 K to stimulate the steam circumstances in the system. (This pressure was more than the temperature of saturation condition.) The gate valve was opened to inject the constant volume of CO2 (600 mL min−1) in the system. Finally, the liquid volume that was extracted from the sand pack was accumulated and weighted sequentially until the water content had reached 99.9%.
Results and discussion
CO2 solubility effect at the different pressures and temperatures
The effect of CO2 solubility at different pressures and temperatures is schematically depicted in Figs. 3–8. As can be seen in Fig. 3, the CO2 solubility effect versus temperature for different pressures is plotted. According to Fig. 3, the pressure ranges were investigated from 3 to 13 MPa. In the pressure of 13 MPa, there is a dramatic decline in the solubility of CO2 when the temperature had increased from 135 to 185 °C. This reduction in the heavy oil was about 18 m3 m−3 (from 51.26 to 33.89 m3 m−3). Therefore, this CO2 solubility reduction in heavy oil is 35.13%, 41.12%, 46.27%, 48.92%, 52.61%, and 44.48 at 3 MPa, 5 MPa, 7 MPa, 9 MPa, 11 MPa, and 13 MPa, respectively, regarding the increase in temperature from 358 to 458 K. As it is evident from the results of this study, the increase in pressure had caused the increase in CO2 solubility in heavy oil.
As can be seen in Fig. 4, the CO2 solubility effect versus pressure for different temperatures is plotted. According to Fig. 4, the temperature was investigated in this study ranging from 358 K to 458 K. In the temperature of 458 K, there is a sharp decrease in the solubility of CO2 when the pressure had decreased from 13 to 3 MPa. This reduction in the heavy oil was about 10 m3 m−3 (from 18.34 to 8.67 m3 m−3). Therefore, this CO2 solubility reduction in heavy oil is 63.12%, 66.68%, 68.27%, 61.92%, and 55.61% at 358 K, 388 K, 408 K, 428 K, and 458 K, respectively, regarding the increase in pressure from 3 to 13 MPa. As it is evident from the results of this study, the increase in temperature had caused the decrease in CO2 solubility in heavy oil.
As a result, the CO2 solubility in heavy oil was decreased regarding the increase in temperature when the pressure has a constant alteration in the system. However, the CO2 solubility in heavy oil at high temperatures has its lower value; CO2 solubility in heavy oil was more impacted by the pressure. Due to the increase in pressure, the compressibility of the gas molecules has risen and subsequently caused to the CO2 solubility increase in the heavy oil. Furthermore, at higher pressures, regarding the more compressibility of heavy oil phase and the reduction in mutual distance which was occurred between the heavy oil molecules, the gas dissolution was not large enough. This phenomenon was discussed by He et al. [25].
CO2 solubility effect on the density of heavy oil
As can be seen in Fig. 5, the degasified heavy oil density versus pressure for different temperatures is plotted. According to Fig. 5, the pressure ranges were investigated from 5 to 12 MPa. In the comparison of different temperature profiles for the fixed pressure alterations (from 5 to 12 MPa), at the temperature of 358 K, there is a sharp decline in the value of degasified heavy oil density. It had decreased from 0.895 to 0.812. In the temperature of 458 K, there is a slight increase in the degasified heavy oil density when the pressure was decreased from 12 to 5 MPa. Therefore, degasified heavy oil density was decreased with the increase in temperature. The reason for this decrease is that regarding the temperature increase, heavy oil was expanded and subsequently the molecular space was increased which subsequently caused to the decrease in degasified heavy oil density. On the other hand, due to the increase in pressure, heavy oil was compressed and subsequently the molecular space was reduced which subsequently caused to the increase in degasified heavy oil density.
As can be seen in Fig. 6, the density of heavy oil which was saturated with CO2 versus pressure for different temperatures was plotted. According to Fig. 6, the pressure ranges were investigated from 5 to 12 MPa. In the temperature of 458 K, there is a slight increase in the degasified heavy oil density (from 0.894 to 0.87) when the pressure was decreased from 12 to 5 MPa. Therefore, the density of heavy oil which was saturated with CO2 was decreased with the increase in temperature, although the density reduction of heavy oil which was saturated with CO2 regarding the pressure increase was opposite to the degasified heavy oil. In the temperature of 458 K, the density reduction of heavy oil which was saturated with CO2 was about 12.15% in comparison with the degasified heavy oil. This phenomenon witnessed the appropriateness of CO2 in the lower density reduction at the high temperatures.
CO2 solubility effect on the viscosity of heavy oil
As can be seen in Fig. 7, the degasified heavy oil viscosity versus temperature for different pressures was plotted. According to Fig. 7, the temperature ranges were investigated from 358 K to 458 K. In the comparison of different pressure profiles for the fixed temperature alterations, there is a dramatic decline in the value of degasified heavy oil viscosity when the temperature had fallen from 358 to 458 K. It had decreased from 7951.23 to 448.61 mPa s−1 at the pressure of 13 MPa. The other trends for other pressures were experienced similar to the pressure of 3 MPa, and they had a decrease decline. The reason of this reduction was related to the compression of degasified heavy oil and subsequently the reduction of spaces for the molecular mobilization. Therefore, as it is shown in Fig. 7, however, regarding the pressure increase the degasified heavy oil viscosity had increased, and its viscosity had decreased with the increase in temperature.
As can be seen in Fig. 8, the viscosity of heavy oil that was saturated with CO2 versus temperature for different pressures was plotted. According to Fig. 8, the temperature ranges were investigated from 358 to 458 K. In the comparison of different pressure profiles for the fixed temperature alterations, there is a dramatic drop in the value of degasified heavy oil viscosity when the temperature had fallen from 358 to 458 K. It had decreased from 816.37 to 27.91 mPa s−1 at the pressure of 13 MPa. The other trends for other pressures were experienced similar to the pressure of 13 MPa, and they had a decrease decline. At the temperature of 185 °C, the viscosity of heavy oil which was saturated with CO2 had decreased from 59.74 to 27.91 mPa s−1 when the pressure increased from 3 to 13 MPa. In the comparison with the degasified heavy oil, the viscosity of heavy oil which was saturated with CO2 had been decreased regarding the pressure increase. Thereby, the viscosity of heavy oil which was saturated with CO2 was lower than the degasified heavy oil viscosity, which was clearly depicted the efficiency of CO2 in the reduction of viscosity at high temperatures. As Stalgorova and Babadagli [41] were discussed this phenomenon, the reduction of viscosity was caused by CO2. The main reason for this issue was the considerable influence of CO2 in the expansion of heavy oil volume. Hence, the friction of oil molecules was reduced due to the oil expansion and subsequently the viscosity of heavy oil which was saturated with CO2 was decreased drastically regarding the dissolution of CO2.
CO2 solubility effect on the recovery factor of heavy oil
Figures 9–11 depict the oil recovery factor at different pressures and temperatures with different rates of CO2 injection. As can be seen in Fig. 9, the recovery factor of heavy oil at different rates of CO2 injection under different temperatures was investigated. At the first steps of CO2 injection, the displacement of CO2 was unstable enough which was caused to CO2 breakthrough. This breakthrough was led to the lower recovery factor (lower oil displacement) and viscous fingering phenomenon. The recovery factor at the temperature of 333 K was measured about 34.5%. The measured recovery factor for other temperatures was 40.17%, 47.68%, 51.97%, 58.42%, and 63.54% at the temperature of 363 K, 393 K, 423 K, 453 K, and 483 K, respectively. Thereby, it was witnessed that the recovery factor of heavy oil was increased due to the increase in temperature.
As can be seen in Fig. 10, the recovery factor of heavy oil at different rates of CO2 injection under different pressures was investigated. The recovery factor at the pressure of 0.2 MPa and 0.5 MPa was experienced the lowest recovery factor among other pressures. They were about 38.37% and 43.81% after the injection of about 15 pore volumes of CO2. For the pressures of 1 MPa, 2 MPa, 3 MPa, and 4 MPa, at the first stages of CO2 injection (up to 3 pore volumes of CO2 injection) the recovery factor experienced the same value. Since then, by the increase in pressure from 1 to 4 MPa, the recovery factor was increased slightly. Therefore, it was indicated that, due to the increase in pressure, the recovery factor was increased.
As can be seen in Fig. 11, the heavy oil recovery factor during the injection of CO2 with the concentration of 70 mL min−1 and 700 mL min−1 was investigated. As it is evident, the recovery factor for the 70 mL min−1 of CO2 injection was lower than the 700 mL min−1 of CO2 injection. However, the CO2 concentration had a profound impact on the increase in recovery factor; its effect does not enough rather than its effect on the viscosity.
Conclusions
Due to the increase in pressure, the heavy oil recovery factor was increased. The recovery factor of heavy oil was increased due to the increase in temperature. The recovery factor for the 70 mL min−1 of CO2 injection was lower than the 700 mL min−1 of CO2 injection. Moreover, according to the results of this study, recovery factor at the pressure of 0.2 MPa and 0.5 MPa experienced the lowest recovery factor among other pressures. They were about 38.37% and 43.81% after the injection of about 15 pore volumes of CO2. For the pressures of 1 MPa, 2 MPa, 3 MPa, and 4 MPa, at the first stages of CO2 injection (up to 3 pore volumes of CO2 injection), the recovery factor experienced the same value. Since then, by the increase in pressure from 1 to 4 MPa, the recovery factor was increased slightly. Thereby, recovery factor at the temperature of 333 K was measured about 34.5%. Although degasified heavy oil density was increased regarding the increase in pressure, its density was decreased with the increase in temperature, the density of heavy oil which was saturated with CO2 was decreased with the increase in pressure and temperature. Thereby, the dissolution of CO2 had decreased the heavy oil density in the higher temperatures and pressures. At the higher temperatures, the rate of solubility was more slowly due to the increase in pressure. On the other hand, the density of heavy oil which was saturated with CO2 was decreased with the increase in pressure and temperature. Therefore, the dissolution of CO2 had decreased the heavy oil density in the higher temperatures and pressures.
References
Al-Bayati D, Saeedi A, Myers M, et al. Insight investigation of miscible SCCO2 water alternating gas (WAG) injection performance in heterogeneous sandstone reservoirs. J CO2 Util. 2018;28:255–63.
Ampomah W, Balch R, Cather M, et al. Optimum design of CO2 storage and oil recovery under geological uncertainty. Appl Energy. 2017;195:80–92.
Bayat Ali E, Junin R, Hejri S, et al. Application of CO2-based vapor extraction process for high pressure and temperature heavy oil reservoirs. J Pet Sci Eng. 2015;135:280–90.
Bayestehparvin B, Abedi J, Ali S. Non-equilibrium reservoir simulation of solvent-steam processes, based on mass and heat transfer inside a pore. In: SPE reservoir simulation conference. Society of Petroleum Engineers; 2017.
Bera A, Mandal A. Microemulsions: a novel approach to enhanced oil recovery: a review. J Pet Explor Prod Technol. 2015;5:255–68.
Chen S, Li H, Yang D. Optimization of production performance in a CO2 flooding reservoir under uncertainty. J Can Pet Technol. 2010;49:71–8.
Colonna S, Bastianini M, Sisani M, et al. CO2 adsorption and desorption properties of calcined layered double hydroxides. J Therm Anal Calorim. 2018;133:869–79.
Cui M, Wang R, Lv C, et al. Research on microscopic oil displacement mechanism of CO2 EOR in extra-high water cut reservoirs. J Pet Sci Eng. 2017;154:315–21.
Davarpanah A. Feasible analysis of reusing flowback produced water in the operational performances of oil reservoirs. Environ Sci Pollut Res. 2018;25:35387–95.
Davarpanah A. A feasible visual investigation for associative foam > polymer injectivity performances in the oil recovery enhancement. Eur Poly J. 2018;105:405–11.
Davarpanah A. The integrated feasibility analysis of water reuse management in the petroleum exploration performances of unconventional shale reservoirs. Appl Water Sci. 2018;8:75.
Davarpanah A, Akbari E, Doudman-Kushki M, et al. Simultaneous feasible injectivity of foam and hydrolyzed polyacrylamide to optimize the oil recovery enhancement. Energy Explor Exploit. 2019;37:44–59.
Davarpanah A, Mirshekari B. Experimental study and field application of appropriate selective calculation methods in gas lift design. Pet Res. 2018;3:239–47.
Davarpanah A, Mirshekari B. A simulation study to control the oil production rate of oil-rim reservoir under different injectivity scenarios. Energy Rep. 2018;4:664–70.
Davarpanah A, Mirshekari B, Behbahani TJ, Hemmati M. Integrated production logging tools approach for convenient experimental individual layer permeability measurements in a multi-layered fractured reservoir. J Pet Explor Prod Technol. 2018;8(3):743–51.
Davarpanah A, Mirshekari B. Mathematical modeling of injectivity damage with oil droplets in the waste produced water re-injection of the linear flow. Eur Phys J Plus. 2019;134(4):180. https://doi.org/10.1140/epjp/i2019-12546-9.
Davarpanah A, Shirmohammadi R, Mirshekari B. Experimental evaluation of polymer-enhanced foam transportation on the foam stabilization in the porous media. Int J Environ Sci Technol. 2019. https://doi.org/10.1007/s13762-019-02280-z.
Deng J, Song J-J, Zhao J-Y, et al. Gases and thermal behavior during high-temperature oxidation of weathered coal. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08103-0.
Ding M, Yuan F, Wang Y, et al. Oil recovery from a CO2 injection in heterogeneous reservoirs: the influence of permeability heterogeneity, CO2–oil miscibility and injection pattern. J Nat Gas Sci Eng. 2017;44:140–9.
Ebadati A, Akbari E, Davarpanah A. An experimental study of alternative hot water alternating gas injection in a fractured model. Energy Explor Exploit. 2018;37(3):945–59. https://doi.org/10.1177/0144598718815247.
Ebadati A, Davarpanah A, Mirshekari B. Stimulated-based characterization recovery enhancement feedback of oil-rim reservoirs. Energy Sources Part A Recovery Util Environ Eff. 2018;40:2528–41.
Ganjkhanlou Y, Bulánek R, Kikhtyanin O, et al. Study on thermal stabilities and symmetries of chemisorbed species formed on K-zeolites upon CO2 adsorption by TPD and in situ IR spectroscopy. J Therm Anal Calorim. 2018;133:355–64.
Giraldo L, Moreno-Piraján JC. CO2 adsorption on activated carbon prepared from mangosteen peel. J Therm Anal Calorim. 2018;133:337–54.
Gu H, Cheng L, Huang S, et al. Steam injection for heavy oil recovery: modeling of wellbore heat efficiency and analysis of steam injection performance. Energy Convers Manag. 2015;97:166–77.
He L, Ping-ping S, Xin-wei L, Qi-Chao G, Cheng-sheng W, Fangfang L. Study on CO2 EOR and its geological sequestration potential in oil field around Yulin city. J Pet Sci Eng. 2015;134:199–204.
Huang S, Cao M, Cheng L. Experimental study on the mechanism of enhanced oil recovery by multi-thermal fluid in offshore heavy oil. Int J Heat Mass Transf. 2018;122:1074–84.
Jadhawar PS, Sarma HK. Effect of well pattern and injection well type on the CO2-assisted gravity drainage enhanced oil recovery. J Pet Sci Eng. 2012;98–99:83–94.
Kamari A, Sattari M, Mohammadi AH, et al. Reliable method for the determination of surfactant retention in porous media during chemical flooding oil recovery. Fuel. 2015;158:122–8.
Kavousi A, Torabi F, Chan CW, et al. Experimental measurement and parametric study of CO2 solubility and molecular diffusivity in heavy crude oil systems. Fluid Phase Equilib. 2014;371:57–66.
Kristóf J, Inczédy J. Continuous determination of carbon dioxide evolved during thermal decomposition reactions. J Therm Anal. 1993;40:993–8.
Lei H, Yang S, Zu L, et al. Oil recovery performance and CO2 storage potential of CO2 water-alternating-gas injection after continuous CO2 injection in a multilayer formation. Energy Fuels. 2016;30:8922–31.
Liu Y, Han X, Zou J, et al. New progress of the offshore thermal recovery technologies in Bohai Bay, China. In: SPE/IATMI Asia Pacific oil & gas conference and exhibition. Society of Petroleum Engineers. 2017.
Mazarei M, Davarpanah A, Ebadati A, et al. The feasibility analysis of underground gas storage during an integration of improved condensate recovery processes. J Pet Explor Prod Technol. 2019;9:397–408.
Neves Junior A, Dweck J, Filho RDT, et al. Determination of CO2 capture during accelerated carbonation of engineered cementitious composite pastes by thermogravimetry. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08210-y.
Pang Z-x, Z-b Wu, Zhao M. A novel method to calculate consumption of non-condensate gas during steam assistant gravity drainage in heavy oil reservoirs. Energy. 2017;130:76–85.
Pang Z, Lyu X, Zhang F, et al. The macroscopic and microscopic analysis on the performance of steam foams during thermal recovery in heavy oil reservoirs. Fuel. 2018;233:166–76.
Park S, Lee ES, Sulaiman WRW. Adsorption behaviors of surfactants for chemical flooding in enhanced oil recovery. J Ind Eng Chem. 2015;21:1239–45.
Seyyedsar SM, Sohrabi M. Intermittent CO2 and viscosity-reducing gas (VRG) injection for enhanced heavy oil recovery. Fuel Process Technol. 2017;164:1–12.
Singh H, Cai J. Screening improved recovery methods in tight-oil formations by injecting and producing through fractures. Int J Heat Mass Transf. 2018;116:977–93.
Sohrabi M, Jamiolahmady M, Al Quraini A. Heavy oil recovery by liquid CO2/water injection. In: EUROPEC/EAGE conference and exhibition. Society of Petroleum Engineers. 2007.
Stalgorova E, Babadagli T. Modified Random Walk–Particle Tracking method to model early time behavior of EOR and sequestration of CO2 in naturally fractured oil reservoirs. J Pet Sci Eng. 2015;127:65–81.
Sun F, Yao Y, Chen M, et al. Performance analysis of superheated steam injection for heavy oil recovery and modeling of wellbore heat efficiency. Energy. 2017;125:795–804.
Tavakoli HM, Jamialahmadi M, Kord S, et al. Experimental investigation of the effect of silica nanoparticles on the kinetics of barium sulfate scaling during water injection process. J Pet Sci Eng. 2018;169:344–52.
Wang Y, Ren S, Zhang L, et al. Numerical study of air assisted cyclic steam stimulation process for heavy oil reservoirs: recovery performance and energy efficiency analysis. Fuel. 2018;211:471–83.
Wu Y, Liu X, Xing D. Case study of hot water foam flooding in deep heavy oil reservoirs. In: SPE Asia Pacific enhanced oil recovery conference. Society of Petroleum Engineers. 2015.
Zhao DW, Wang J, Gates ID. Thermal recovery strategies for thin heavy oil reservoirs. Fuel. 2014;117:431–41.
Zheng S, Li H, Yang D. Pressure maintenance and improving oil recovery with immiscible CO2 injection in thin heavy oil reservoirs. J Pet Sci Eng. 2013;112:139–52.
Zhu Y. Current developments and remaining challenges of chemical flooding EOR techniques in China. In: SPE Asia Pacific enhanced oil recovery conference. Society of Petroleum Engineers. 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davarpanah, A., Mirshekari, B. Experimental study of CO2 solubility on the oil recovery enhancement of heavy oil reservoirs. J Therm Anal Calorim 139, 1161–1169 (2020). https://doi.org/10.1007/s10973-019-08498-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08498-w














