Abstract
In order to investigate the spontaneous combustion characteristics of weathered coal, gas generation and thermal behavior of weathered and fresh coal were analyzed. A self-made high-temperature-programmed experimental system was applied to simulate the spontaneous combustion of weathered and fresh coal. The characteristic parameters during oxidation were between 30 and 650 °C. The growth rate obtained through the analysis of an indicator gas was adopted to calculate the characteristic temperatures of high-temperature spontaneous combustion of coal. A C80 Calvet calorimeter was used to capture the thermal behavior during oxidation. At a high temperature and low oxygen concentration, weathered coal continued oxidizing and releasing thermal energy to sustain oxidation. The concentration of gases produced through high-temperature oxidation was lower for weathered coal than for fresh coal. The exothermic onset temperature of weathered coal was 43 °C, which was lower than the exothermic onset temperature of fresh coal. A salient difference was observed in the thermal energy release between weathered coal and fresh coal at different oxidation stages. From the critical temperature to crack temperature, the percentage of thermal energy release of weathered coal was much lower than that of fresh coal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal is one of the most essential fuels on earth with a vast global reserve [1]. Substantial amounts of weathered coal are exposed at outcrops and shallow seams. Weathering effects alter the spontaneous combustion characteristics of coal. To understand the hazards of weathered coal, we analyzed the gaseous products of oxidation of fresh coal and weathered coal. The thermal energy of weathered coal was also clarified through a series of experiments. The occurrence and development of the spontaneous combustion of coal are a result of intricate dynamic physical and chemical processes [2].
Numerous scholars have conducted research on coal oxidation and studied the rates of oxygen consumption, gas generation, and gas composition. Kinetics have also been investigated [3,4,5,6,7]. Guo et al. [8] used a digital rock-released scope measurement and optical borehole survey system to evaluate seam loosening and scope breakage under weathering and extreme climate conditions. Cox [9] concluded that coal weathering can alter product composition and thus influence gasification yields. Zhang et al. [10] analyzed the causes and characteristics of spontaneous combustion of weathered coal in an open pit. They also discussed the temperature changes and CO emission during the oxidation of weathered coal. Marchioni [11] tested the degree of coal weathering and elucidated the reasons for color changes. Davis et al. [12] found that the subtle oxidation of weathered coal can be understood by comparing fresh and weathered coal in blue light. Kruszewska et al. [13] studied the elasticity index of vitrinite in weathered coal and found that it increased with weathering time. Misz et al. [14], Wagner [15], Jolanta et al. [16], and Magdalena et al. [17] have shown that weathered coal is shiny because of structural accumulation and cracks in the surface molecules that develop during high-temperature oxidation. Wang et al. [18] adopted thermogravimetry–Fourier transform infrared–gas chromatography–mass spectrometry to investigate the change in the properties of Chinese long-flame coal during low-temperature pyrolysis; in this study, the researchers found that oxygen in the gas mainly existed in the form of CO2 and CO, whereas oxygen in tar was primarily in the form of phenolic compounds.
Rotaru et al. [19] studied the thermal effect of humic acid in low-temperature coal, and all the steps in the decomposition process were revealed using nonisothermal linear temperature ranges. Cui et al. [20] used Fourier transform infrared spectroscopy to quantitatively analyze the functional groups in Datong coal from Shanxi Province, People’s Republic of China, and found that a higher content of oxygen in the functional group led to higher levels of aromatic structure and lower levels of fat structures. Numerous researchers have contributed toward the study of weathered coal; however, the release characteristics of all types of gases and the thermal behavior during high-temperature spontaneous combustion of weathered coal have scarcely been investigated.
The study of spontaneous combustion of coal examines the stage of low-temperature oxidation (< 200 °C), and the study of more than 200 °C focuses on the combustion part. However, the study of high-temperature oxidation of coal at the low-temperature stage and the high-temperature stage during the period of ignition point is relatively scant. Therefore, we used a self-developed high-temperature-programmed experimental system to test the characteristics of weathered and fresh coal. A C80 Calvet calorimeter was used to study thermal behavior during the oxidation of weathered and fresh coal. The results of investigating the gaseous products and thermal behavior of weathered coal provide a favorable basis for predicting spontaneous combustion on the surface of open-pit and shallow coal seams.
Experiment and methods
Coal samples
Selected long-flame coal was sampled from Liulinggou, Inner Mongolia, and the People’s Republic of China. The proximate analysis of coal samples is presented in Table 1. The coal samples were wrapped in plastic and nylon bags and shipped back to the laboratory quickly after extraction from the coal mine. The procedure involved pulverizing a piece of the coal sample in the air and screening 6 kg of various grain sizes—0–0.9, 0.9–3, 3–5, 5–7, and 7–10 mm (1 kg each)—and placing them in sealed bags within sealed containers for a high-temperature-programmed experiment. In the C80 experiments, 1600 mg of the coal sample was used, and the particle sizes (diameter) were 0.18–0.38 mm.
High-temperature-programmed experimental system
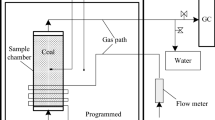
The XKGW-1 high-temperature-programmed experimental system was adopted, and the experimental setup (Fig. 1) consisted of four parts: an air supply system, an XKGW-1 high-temperature reaction furnace, coal sample tank, and a gas analysis system. The high-temperature reaction furnace [21] had dimensions of 65 cm × 45 cm × 40 cm. The inner layer was composed of ceramic fiber, and the outer layer was made of carbon steel. An S-type single thermocouple was used to measure temperature. The high-temperature-resistant silicon–carbon coal tank had a diameter of 10 cm and a height of 20 cm. The upper part of the tank was covered. The cover and the tank were connected by a flange, and graphite gaskets were clamped in the middle to ensure the airtightness of the tank. Artificial intelligence (AI)-based proportional integral differential temperature control was employed, and the temperature of the high-temperature reaction furnace was 30–1000 °C. Gas chromatography was used for the gas component analysis. The experiment was conducted in air with a temperature of 30–650 °C. The system used an air pump to supply air and a rotor flowmeter to control the flow.
XKGW-1 High-temperature-programmed experimental system [21]
C80 Calvet calorimeter
An ordinary differential scanning calorimeter can measure the physical and chemical thermal effects of a single phase transition: crystallization or melting. However, the C80 Calvet calorimeter can accurately measure the physical and chemical thermal effects of mixing, adsorption, and the reaction processes of mixtures. Moreover, its measurement accuracy reduces uncertainty during the initial stage of oxidation. Therefore, the thermal behavior of weathered and fresh coals was measured using the C80 Calvet calorimeter (Setaram, France).
To ensure that the coal sample oxidized sufficiently during the reaction, the air flow was set at 100 mL min−1. Considering the time of the experiment and the influence of the heating rate on the oxidation of coal, the heating rate was set at 0.1 °C min−1, and the heating range was 30–200 °C.
Experimental results and discussion
Gaseous concentration analysis
During the oxidation of coal, physical and chemical adsorption occurs, which consumes a substantial amount of oxygen. The release of gases is one of the macroscopic features of spontaneous combustion. Spontaneous reaction during coal oxidation released CO, CO2, C2H4, C2H6, and olefins. Oxygen consumption was measured during the oxidation of weathered and fresh coals release of CO, CO2, CH4, C2H4, and C2H6 gases.
Oxygen consumption
Oxygen supply, which is one of the three elements of the spontaneous combustion of coal, plays an influential role in the combustion process. The characteristics of oxygen consumption and the release characteristics of gases reflect the severity of spontaneous combustion. The experimental oxygen consumption of fresh and weathered coals is illustrated in Fig. 2. Oxygen consumption in fresh and weathered coals was similar. Because of the physical adsorption of oxygen, the oxygen concentration was stable below 70 °C and consumption was slow. Between 70 and 200 °C, the oxygen concentration decreased rapidly in both weathered and fresh coals. At this time, the active functional groups in the coal molecules were gradually activated, the reaction rate of the coal samples was accelerated, and the active functional groups participated in the reaction and consumed a large quantity of oxygen. The oxygen concentration dropped to 4% over time and generally stabilized below 2%. Because the reaction mechanisms between weathered and fresh coals differ at various stages of oxidation at low temperatures, the oxygen concentration of weathered coal was less than the oxygen concentration of fresh coal between the fission and ignition temperatures. At 320–510 °C, the oxygen concentration of weathered coal was higher than the oxygen concentration of fresh coal, because a considerable number of organic microstructures on the coal macromolecules were consumed during a long period of weathering, and the decrease in functional groups in weathered coal macromolecules resulted in a decrease in oxygen consumption during high-temperature oxidation. At the end of the reaction, the oxygen concentration of weathered coal was almost equal to that of fresh coal.
CO concentration
The relationship between CO concentration and temperature in the oxidation of fresh and weathered coals is shown in Fig. 3. The CO released from the fresh and weathered coals increased with the increase in temperature during oxidation. For slow coal–oxygen reactions at low temperatures, the CO released by fresh and weathered coals increased gradually below 140 °C. After reaching 140 °C, the CO concentration of the fresh and weathered coals commenced to increase rapidly. Between 165 and 290 °C, the CO released by weathered coal was slightly greater than the CO released by fresh coal. During low-temperature oxidation, the side chains and bridge bonds of nonaromatic structures in the coal molecules, including hydroxyl, carbonyl, methyl, and methylene structures, were vulnerable to oxygen attack, thus producing CO and CO2 gases [22]. The porosity of weathered coal was slack, and the active functional group participated easily in coal-oxygen recombination. The CO concentration of fresh coal gradually exceeded the CO concentration of weathered coal at 300–420 °C because of the substantial reduction in carbonyl ester functional groups, oxygen-containing functional groups, and carbonyl groups in the weathered coal macromolecules.
The concentration of CO released from the fresh and weathered coals reached the first peak point at 400 and 425 °C, respectively. Moreover, when the fresh and weathered coal samples were oxidized between the ignition temperature and the burned-out temperature, the CO concentration fluctuated within a certain range. At this stage, the concentration of CO released from fresh coal samples exceeded the concentration of CO released from weathered samples, but weathered coal remained longer in this stage because of the significant reduction in functional groups, such as carbonyl and aliphatic hydrocarbons. From 475 °C to the burned-out temperature, the CO concentration of fresh coal decreased and then increased again until the end of oxidation. The CO concentration of weathered coal increased rapidly after the fluctuation stage because weathering resulted in incomplete oxidation and low-temperature pyrolysis of carbon. The hydroxyl and ether bonds, and some stable oxygen heterocycles in the coal molecule, were cleaved to form CO [23].
Characteristic temperatures
Characteristic temperature is a macroindicator that influences the external environment during coal oxidation. CO was selected as the indicator gas [24] to determine the characteristic temperature of weathered coal. As shown in Fig. 4, the critical temperature (T1), crack temperature (T2), fission temperature (T3), ignition temperature (T4), and burned-out temperature (T5) [25] were obtained at 70, 120, 230, 375, and 580 °C, respectively. Except for the fission temperature, the characteristic temperature values of weathered coal were lower than the temperature values of fresh coal because weathering destroyed the internal structure of the coal, making it looser, and the active groups stored in the pores of the coal were exposed to a considerable amount of fluidity. The oxidation process can be further accelerated by combining oxygen, leading to a characteristic temperature that appeared in advance. The fission temperature was the turning point in this process. Moreover, weathered coal had fewer aromatic groups than fresh coal, leading to a hysteretic point in the fission temperature.
CO2 concentration
Figure 5 shows that the CO2 concentration increased parabolically for both fresh and weathered coals and was higher for fresh coal than for weathered coal throughout the oxidation. Because of CO2’s high molecular mass, weathering does not completely release CO2 gas, which is adsorbed by van der Waals force in the coal molecules. During coal oxidation, gradual desorption of CO2 occurred in coal pores. During weathering, the coal surface was oxidized, and part of the active functional group was consumed, which resulted in the CO2 concentration of weathered coal being less than the CO2 concentration of fresh coal. The CO2 concentration reached the peak point at the ignition temperature, and subsequently decreased because coal that would raise incompletely an oxidation reaction and low-temperature pyrolysis reaction. The hydroxyl group, ether bond, and some stable oxygen-containing groups in coal cracked to form CO. The concentration of CO2 released from the oxidation of weathered coal decreased from the ignition temperature to 500 °C and then fluctuated thereafter because of the decomposition of carboxyl groups on humic acid macromolecules in weathered coal produced CO2.
Alkane and olefin gases
The alkane and olefin gases released included CH4, C2H4, and C2H6. Figures 6–8, show that the changes in the concentrations of CH4, C2H4, and C2H6 in fresh coal were similar to changes in concentrations in weathered coal during oxidation. The CH4, C2H4, and C2H6 gases released by weathered coal appeared at 50, 120, and 120 °C, respectively. At the beginning of oxidation, the concentrations of the three gases were low, and increased gradually as the temperature increased. Fresh and weathered coals generated CH4 during oxidation and reached the peak point at 480 °C. Therefore, weathering had little effect on the CH4 concentration produced by the coal samples. The CH4, C2H4, and C2H6 gases generated by the oxidation of fresh and weathered coals increased from 300, 280, and 330 °C, respectively. For the intensification of oxidation, the temperatures of the peak values of C2H4 and C2H6 concentration were 440 and 480 °C, respectively. Low-temperature pyrolysis reactions occurred in the coal samples, and the aliphatic structure in the coal became aromatic to form biphenyl and release CH4. The aliphatic side chain of the aromatic ring in coal was dissociated from the free radical of the aliphatic hydrocarbon in the free phase to form C2H4 and C2H6. Additionally, the thermal decomposition reaction of humic acid in weathered coal [26, 27] yielded CH4, C2H4, and C2H6. The prolonged chemical weathering resulted in a substantial depletion of the active functional groups in weathered coal; the microstructure of the coal molecules changed, explaining why the peak values of C2H4 and C2H6 concentration were much higher in fresh coal than they were in weathered coal. C2H4 and C2H6 remained in low concentrations above 525 and 550 °C, respectively, and the gas concentration of fresh coal was similar to that of weathered coal. The active groups in the coal molecules were cleaved step by step, and humic acid decomposition proceeded gradually in weathered coal, which caused CH4 to abate above 480 °C.
Particle size influence
The porosity of coal varies with size. The smaller the particles are, the greater the porosity is. The results revealed that the contact area between coal and oxygen is different for weathered and fresh coal. Different numbers of active functional groups of coal reacted with oxygen with different particle sizes, resulting in different gas concentrations. In the low-temperature oxidation process, the coal particle size was small and could readily react with oxygen. High sensitivity and high stability are essential characteristics of indicator gases. The influence of coal granularity on the spontaneous combustion of weathered coal was analyzed by selecting CO concentration as the indicator gas.
Different particle sizes (0–0.9, 0.9–3, 3–5, 5–7, and 7–10 mm) of coal led to different reaction surface areas, thereby resulting in different concentrations of various gases. The influence of particle size on the spontaneous combustion of weathered coal was different than the influence of particle size on the spontaneous combustion of fresh coal [28,29,30]. Figure 9a shows that at different particle sizes, the CO concentration increased with temperature. With the increase in temperature, the CO concentration increased drastically. During low-temperature oxidation (Fig. 9b) of weathered coal, the CO concentration for particles 0–0.9 mm increased primarily. The CO concentration reached the first peak value at the ignition temperature and then fluctuated within a certain range before increasing sharply at the burned-out temperature among different particle sizes. When the oxidation temperature for particles 0–0.9 mm was below the ignition temperature and over 520 °C, the CO concentration was higher than the CO concentration for other particle sizes.
Between the ignition temperature and burned-out temperature, the CO concentration of coal with particles 0–0.9 and 0.9–3 mm was higher than the CO concentration for other particle sizes. From the ignition temperature to 500 °C, 0.9–3 mm particles produced more CO than particles 0–0.9 mm. From the ignition temperature to the burned-out temperature, the CO concentration of 7–10 mm particles was the lowest among all particle sizes. Physical weathering caused transformations in the coal particles, such as displacement and wrinkle, which changed from the surface to the interior structure, resulting in the weathered coal having a loose structure. When the particle size was sufficiently small, the concentration of gas produced by the weathering of coal increased.
Thermal behavior analysis
Thermal behavior is concomitant in physical and chemical reactions. The generation and accumulation of thermal energy cause the spontaneous combustion of coal. In the oxidation of coal at low temperatures, the main thermal reaction is thermal energy release.
Exothermic onset temperature
Thermal energy release is the main reason for the spontaneous combustion of coal. Therefore, the exothermic onset temperature of coal should be determined to restrict the substantial thermal energy release. Through analysis of the heat flow curve of low-temperature oxidation, we obtained the exothermic onset temperature of fresh and weathered coals. As shown in Fig. 10, the exothermic onset temperature of fresh and weathered coals was 50 and 43 °C, respectively, which was lower than the critical temperatures. Water evaporation in the coal during oxidation caused the exothermic onset temperature of weathered coal to be 7 °C less than that of fresh coal. Table 1 shows that the moisture content of fresh coal was 13.42%, which was higher than the moisture content of weathered coal, owing to the release of more thermal energy through water evaporation. Thermal energy accumulation was slow in weathered coal. The change in grain structure caused by weathering led to the loss of water in the coal.
Thermal energy release analysis
The thermal energy release was obtained from the C80 experiment. As shown in Fig. 11, the exothermic processes of both fresh and weathered coals followed a similar pattern. At the initial stage of oxidation, the thermal energy release was small. As the oxidation proceeded, with an increase in temperature, the thermal energy release rate increased. Before the critical temperature was reached, fresh coal was in an inert state. After the critical temperature was reached, the thermal energy release rate of fresh coal gradually increased with the increase in temperature, whereas weathered coal remained inert. When the crack temperature was reached, the thermal energy increased. Because of the decrease in hydroxyl and aliphatic hydrocarbon content in weathered coal molecules, the reaction heat and thermal energy release at low temperatures was lower macroscopically. After the critical temperature was attained, the thermal energy release of fresh coal was greater than the thermal energy release of weathered coal. Above 170 °C, various structures of the coal molecules fractured to various degrees, and the thermal energy release rates of fresh and weathered coals were similar.
As shown in Fig. 12, the total thermal energy release from the exothermic onset temperature of the coal sample to 200 °C was obtained through integration. The total thermal energy release of fresh coal was 2125.8 J g−1 and the total thermal release of weathered coal was 1234.7 J g−1. The thermal energy release of fresh coal was much larger than the thermal energy release of weathered coal because chemical weathering had consumed a large number of active functional groups, releasing some thermal energy. Additionally, the internal porosity of weathered coal was low, resulting in thermal energy produced through oxidation of the coal body system, which was difficult to release. Because weathering evaporated the water in coal, weathered coal exhibited a higher heat flow value at the initial stage than fresh coal.
To determine the thermal energy release characteristics of weathered coal at different stages, oxidation was divided into three stages: the exothermic onset-critical temperature stage, the critical-crack temperature stage, and the after crack temperature stage. The thermal energy release was determined by integrating the heat flow curve. Despite a large temperature range, fresh and weathered coals exhibited small thermal energy release in the two stages before the crack temperature, as shown in Table 2. Before the critical temperature, the amount of heat released from the oxidation of coal samples was extraordinarily small (< 2.5%). However, the exothermic characteristics of fresh and weathered coals at different stages of oxidation were different. The amount of thermal energy release from fresh coal was much larger than that from weathered coal, which was attributed to the broken weathered coal structure and weak absorption effect.
Conclusions
Weathered coal was more easily oxidized than fresh coal, and spontaneous combustion also occurred more readily. Accordingly, its prevention in actual production processes should be closely considered. During high-temperature oxidation, fresh and weathered coals released a substantial amount of thermal energy and carbon-containing gases. For the oxidation of weathered coal, the selection of the index gas was the same as that for fresh coal. CO was selected as the index gas, which was produced by weathered coal, and it increased exponentially with an increase in temperature. The CO concentration obtained through the oxidation of weathered coal was greater than that obtained through the oxidation of fresh coal at the initial stage of reaction because of the loose pores and larger pore volume of weathered coal. At the final stage of the reaction, the CO concentration was greater than the CO concentration of fresh coal. The reaction of coal molecules with oxygen consumed a substantial number of active functional groups, such as hydroxyl groups, carbonyl groups, and ether bonds; thus, the CO concentration in weathered coal was less than that in fresh coal. The characteristic temperatures of fresh coal were higher than those of weathered coal. The weathering effect on the CH4 concentration was only negligibly different between the two coal types. The CO2 concentration reached the peak value at the ignition temperature. The amounts of C2H4 and C2H6 gases released by weathered coal were lower than the amounts of C2H4 and C2H6 gases released by fresh coal. Particle size had a substantial effect on the spontaneous combustion of weathered coal. Coal with particles 0–0.9 mm in size released the highest amounts of gas among the six tested sizes. The total thermal energy generated from the exothermic onset temperature to 200 °C by weathered coal was much less that generated by fresh coal. From the critical temperature to the crack temperature, the percentage of thermal energy generated by weathered coal was much smaller than that by fresh coal.
Abbreviations
- A ad :
-
Ash content on an air-dried basis (%)
- FCad :
-
Fixed carbon on an air-dried basis (%)
- M ad :
-
Moisture on an air-dried basis (%)
- T 1 :
-
First characteristic temperature, critical temperature (°C)
- T 2 :
-
Second characteristic temperature, active temperature (°C)
- T 3 :
-
Third characteristic temperature, pyrolysis temperature (°C)
- T 4 :
-
Fourth characteristic temperature, ignition temperature (°C)
- T 5 :
-
Fifth characteristic temperature, burned-out temperature (°C)
- V ad :
-
Volatile content on an air-dried basis (%)
References
Saini V, Gupta RP, Arora MK. Environmental impact studies in coalfields in India: a case study from Jharia coal-field. Renew Sustain Energy Rev. 2016;53:1222–39.
Lei CK, Deng J, Cao K, Ma L, Xiao Y, Ren LF. A random forest approach for predicting coal spontaneous combustion. Fuel. 2018;223:63–73.
Petit JC. Calorimetric evidence for a dual mechanism in the low temperature oxidation of coal. J Therm Anal Calorim. 1991;37(8):1719–26.
Ma SP, Hill JO, Heng S. A thermal analysis study of the oxidation of brown coal chars. J Therm Anal Calorim. 1989;35(5):1611–9.
Deng J, Xiao Y, Li QW, Lu JH, Wen H. Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel. 2015;157:261–9.
Deng J, Zhao JY, Xiao Y, Zhang YN, Huang AC, Shu CM. Thermal analysis of the pyrolysis and oxidation behaviour of 1/3 coking coal. J Therm Anal Calorim. 2017;129(3):1–8.
Xiao Y, Ren SJ, Deng J, Shu CM. Comparative analysis of thermokinetic behavior and gaseous products between first and second coal spontaneous combustion. Fuel. 2018;227:325–33.
Guo BC, Wang NB, Qi T, Lai XP. Detection and determination of seam releasing zone in slope of Zhundong surface mine. Coal Sci Technol. 2010;38(3):5–7.
Cox JL. Concern over coal samples. Fuel. 1984;63:1030–1.
Zhang RX, Xie HP, Xie ZK. Experimental study on spontaneous combustion of ground coal. J China Univ Min Technol. 2000;29(3):235–8.
Marchioni DL. The detection of weathering in coal by petrographic, rheologic and chemical methods. Int J Coal Geol. 1983;2:231–59.
Davis RC, Noon SW, Harrington J. The petroleum potential of tertiary coal from Western Indonesia: relationship to mire type and sequence stratigraphic setting. Int J Coal Geol. 2007;70:35–52.
Kruszewska KJ, Du Cann VM. Detection of the incipient oxidation of coal by petrographic techniques. Fuel. 1996;75:769–74.
Misz M, Fabiańska M, Ćmiel S. Organic components in thermally altered coal waste: preliminary petrographic and geochemical investigations. Int J Coal Geol. 2007;71:405–24.
Wagner NJ. The abnormal condition analysis used to characterize weathered discard coals. Int J Coal Geol. 2007;72:177–86.
Jolanta K, Uwe M, Jianwei M. Oxidation and carbonization of coals: a case study of coal fire affects coals from the Wuda coalfield, Inner Mongolia, China. Gen Assem Eur Geosci Union. 2010;12:EGU2010–EG11851.
Magdalena M, Monika F. Thermal transformation of organic matter in coal waste from Rymer Cones (Upper Silesian Coal Basin, Poland). Int J Coal Geol. 2010;81:343–58.
Wang ZB, Wang C, Kang RN, Bin F, Wei XL. Deoxygenation of Chinese long-flame coal in low-temperature pyrolysis. J Therm Anal Calorim. 2017;3:1–9.
Rotaru A, Nicolaescu I, Rotaru P, Neaga C. Thermal characterization of humic acids and other components of raw coal. J Therm Anal Calorim. 2008;92(1):297–300.
Cui X, Li XL, Li YM, Li S. Evolution mechanism of oxygen functional groups during pyrolysis of Datong coal. J Therm Anal Calorim. 2017;1:1–12.
Jin YF, Guo J, Wen H, Liu WY, Wang K, Ma XF. Experimental study on the high temperature lean oxygen oxidation combustion characteristic parameters of coal spontaneous combustion. J China Coal Soc. 2015;40(3):596–602.
Kus J. Impact of underground coal fire on coal petrographic properties of high volatile bituminous coals: a case study from coal fire zone No. 3.2 in the Wuda coalfield, inner Mongolia autonomous region North China. Int J Coal Geol. 2017;171:185–211.
Kus J. Oxidatively and thermally altered high-volatile bituminous coals in high-temperature coal fire zone No. 8 of the Wuda coalfield (North China). Int J Coal Geol. 2017;176:8–35.
Zhang YL, Wang JF, Wu JM, Chang LP. Modes and kinetics of CO2 and CO production from low-temperature oxidation of coal. Int J Coal Geol. 2015;140:1–8.
Deng J, Zhao JY, Zhang YN, Geng RL. Study on coal spontaneous combustion characteristic temperature of growth rate analysis. Procedia Eng. 2014;84:796–805.
Válková D, Kislinger J, Pekař M, Kučerík J. The kinetics of thermo-oxidative humic acids degradation studied by isoconversional methods. J Therm Anal Calorim. 2007;89(3):957–64.
Nagar BR, Waight ES, Meuzelaar HLC, Kistemaker PG. Studies on the structure and origin of soil humic acids by curie point pyrolysis in direct combination with low-voltage mass spectrometry. Plant Soil. 1975;43:681–5.
Saleh M, Nugroho YS. Thermogravimetric study of the effect of particle size on the spontaneous combustion of Indonesian low rank coal. Appl Mech Mater. 2013;330(330):101–5.
Küçük A, Kadıoğlu Y, Gülaboğlu MŞ. A study of spontaneous combustion characteristics of a Turkish lignite: particle size, moisture of coal, humidity of air. Combust Flame. 2003;133(3):255–61.
Qin YP, Song YM, Yang XB, Qin C. Experimental study on coal granularity influencing oxidation rate in goaf. J China Coal Soc. 2010;35:132–5.
Acknowledgements
This manuscript was edited by Wallace Academic Editing. This Project was supported by National Natural Science Foundation of China (Grant No. 5167-4191), National Natural Science Foundation of China of China (Grant No. 5180-4246), and Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2017JQ5047).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, J., Song, JJ., Zhao, JY. et al. Gases and thermal behavior during high-temperature oxidation of weathered coal. J Therm Anal Calorim 138, 1573–1582 (2019). https://doi.org/10.1007/s10973-019-08103-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08103-0