Abstract
Heat capacity for 1-hexyl-3-methyl imidazolium perrhenate ionic liquid [C6MIM][ReO4] in the temperature range from 79 to 396 K has been measured by a fully automated adiabatic calorimeter. For [C6MIM][ReO4], glass transition temperature, the melting temperature, standard molar heat capacity, enthalpy and entropy of solid–liquid phase transition were determined to be (202.164 ± 0.405) K, (226.198 ± 0.265) K, (480.702 ± 0.013) J K−1 mol−1, (15.665 ± 0.195) kJ mol−1 and (69.250 ± 0.780) J K−1 mol−1, respectively. In addition, the thermodynamic characteristics and solid–liquid phase change behavior of [C6MIM][ReO4] were compared with the ones of [C7MIM][ReO4] reported in the literature. The thermodynamic functions (HT− H298.15), (ST− S298.15) and (GT− G298.15), for the compound in the experimental temperature range were calculated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Usually, the melting temperatures of ionic compounds are higher than room temperature due to their strong and long-range interionic interactions. However, it is found that melting temperatures of a series of organic ionic compounds are lower than room temperature, and they are called room-temperature ionic liquids (ILs) [1, 2]. ILs are made up of a bulky organic cation and a counterion and possess special physical and chemical properties, making them attractive for both pure scientific and applied studies [3,4,5,6,7]. In recent years, all kinds of applications, extraction and separation processes, synthetic chemistry, catalysis, materials science and so on have been proposed for ILs; for example, rhenium ionic liquids can be used for epoxidation of olefins due to their excellent activity and selectivity [8,9,10,11]. The parameters of fusion of ILs are crucial for their applicability. Many results, such as melting temperature, glass transition temperature, standard molar heat capacity, enthalpy and entropy of solid–liquid phase transition, and other important information about the structure and energetics of the ILs, can be obtained from the experimental heat capacity data. All in all, the low-temperature heat capacity has very significant role in the theoretical research and application development of ILs [12,13,14].
A report about the preparation and thermodynamic properties of ionic liquid [C7MIM][ReO4] has been published in our laboratory [1]; as a continuation of our previous investigation, this paper reports the following: (1) 1-hexyl-3-methyl imidazolium perrhenate ionic liquid [C6MIM][ReO4] were prepared and characterized by 1H NMR spectroscopy and Raman spectrum; (2) low-temperature heat capacities of [C6MIM][ReO4] were measured by a high-precision automated adiabatic calorimeter over the temperature range from 79 to 396 K; (3) the thermodynamic functions (HT− H298.15), (ST− S298.15) and (GT− G298.15) were also calculated based on the experimental results.
Experimental
Reagents
The purities and sources of the reagents are listed in Table 1.
Preparation of IL [C6MIM][ReO4]
In this work, [C6MIM][ReO4] was synthesized and the scheme 1 shows the synthetic route. The N-methylimidazole (1 mol) and the bromohexane (1.2 mol) were placed in a round-bottomed flask and stirred under reflux at 80 °C for 48 h to obtain the [C6MIM] Br [15, 16]. Then, [C6MIM] Br and 1.2 equiv. of NH4ReO4 were reacted in acetone under argon and stirred at room temperature for 48 h to obtain the target product. In addition, ethyl acetate and acetonitrile were used as an extractant in this experiment. The content of Br− was determined by dripping the silver nitrate solution; the results reveal that any yellow deposition did not appear. The [C6MIM][ReO4] was characterized by 1H NMR spectroscopy and Raman spectrum (see Figure S1 and S2); its purity is more than 99.8%. And the water content was determined by a Karl Fischer moisture titrator (ZSD-2 type), and it is less than 0.3 mass%.
Measurement of molar heat capacities of IL [C6MIM][ReO4] by adiabatic calorimeter
To verify the dependability of the adiabatic calorimeter, the molar heat capacities for reference standard material, α-Al2O3 was measured. The deviations of our experimental results from the recommended values by NIST are within ± 0.5%, while the uncertainty is within ± 0.37%, as compared with the values given by the former National Bureau of Standards [17] in the temperature range of (78–400) K.
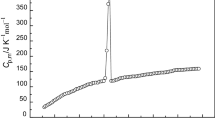
The molar heat capacities of [C6MIM][ReO4] (1.74589 g) were repeatedly measured by the adiabatic calorimeter, established by Dalian Institute of Chemical Physics [18,19,20]. Over the temperature range between 79 and 396 K, the first experimental data are listed in Table 2 and plotted in Fig. 1, and the second experimental data are listed in Table S1 and plotted in Figure S3 in Supporting Information, respectively.
Results and discussion
Heat capacity
From Fig. 1, an endothermic step corresponding to a glass transition occurred at glass transition temperature Tg = 201.760 K. From 210 to 230 K, a sharply endothermic peak was observed with the peak temperature 225.934 K; it corresponds to a melting process. And smooth heat capacity curves without endothermic and exothermic peaks were observed in other experimental temperature regions.
The molar heat capacities are fitted to two following polynomial in reduced temperature (x) by means of the least square fitting.
For the first temperature range (80–196) K:
The correlation coefficient of the fitting R2 = 0.9992.
For the second temperature range (205–217) K:
The correlation coefficient of the fitting R2 = 0.9958.
For the third temperature range (229–396) K:
The correlation coefficient of the fitting R2 = 0.9991.
Where x is the reduced temperature, x = [T − (Tmax + Tmin)/2]/[(Tmax − Tmin)/2], T is the experimental temperature.
Both series of heat capacity measurements of the sample in the fusion region are carried out and shown in Fig. 2; from the figure, not only is verified the almost perfect reversibility and repeatability of the fusion process, but also the repeated heat capacity measurements of the both series. And the melting temperature is obtained to be (226.198 ± 0.265) K, and it is listed in Table 4. Compared with [C7MIM][ReO4] [1], the melting temperature of [C6MIM][ReO4] is higher, which is possibly due to the more carbonyl group in the cation, the more the steric hindrance strengthened, which leads to the melting temperature decrease with increasing the number of methylene group in the alkyl chains of the ILs, and the change trend is agreement with Tan’s work, that is mainly reported that the heat capacities and melting points of ionic liquids 1-ethylpyridinium bromide (EPBr) and 1-propylpyridinium bromide (PPBr) [16].
Thermodynamic functions
The thermodynamic functions related to the reference temperature 298.15 K were calculated in the temperature range (80–400) K with an interval of 5 K, using the polynomial equation for heat capacity and thermodynamic relationships as follows:
The calculated values of thermodynamic functions (HT− H298.15), (ST − S298.15) and (GT − G298.15) are listed in Table 3.
From Table 3, standard molar heat capacity at T = 298.15 K is 480.715 J K−1 mol−1, which is lower than the value of [C7MIM][ReO4] [1], that is, it can be seen that the values of the standard molar heat capacity increase along with increasing the number of methylene group in the alkyl chains of the ILs, and it is also consistent with Tan’s [16].
The molar enthalpy ΔfusHm and entropy ΔfusSm of fusion of the compound were calculated from the following equations:
where Ti is the temperature at the initial melting temperature, Tf is the temperature at the final melting temperature, Q is the total energy introduced to the sample cell from Ti to Tf, Cp(S) is the heat capacity of the sample in the solid phase at Ti, Cp(L) is the heat capacity of the sample in liquid phase at Tf and C0 is the average heat capacity of the empty sample cell at temperature (Ti + Tf)/2.
The results of the melting point, molar enthalpy and entropy of two phase transitions obtained from every series of repeated experiments have been listed in Table 4.
Conclusions
In this paper, the heat capacities of [C6MIM][ReO4] were measured in the temperature range (79–396) K by adiabatic calorimeter. And according to heat capacity values, glass transition temperature, the melting temperature, standard molar heat capacity, enthalpy and entropy of solid–liquid phase transition were determined to be (202.164 ± 0.405) K, (226.198 ± 0.265) K, (480.702 ± 0.013) J K−1 mol−1, (15.665 ± 0.195) kJ mol−1 and (69.250 ± 0.780) J K−1 mol−1, respectively. It may due to the fact that the longer carbon chain in imidazolium cation, the more steric hindrance and lattice energy, which results in a decrease in melting temperature but an increase in standard molar heat capacity. And the variation trends of the melting temperature and standard molar heat capacity at 298.15 K are consistent with Tan’s.
In addition, the corresponding thermodynamic functions (HT − H298.15), (ST − S298.15) and (GT − G298.15) were calculated in the temperature range from 80 to 400 K.
References
Fang DW, Zuo JT, Xia MC, Tong J, Li J. Low-temperature heat capacities and the thermodynamic functions of ionic liquids 1-heptyl-3-methyl imidazolium perrhenate. J Therm Anal Calorim. 2018;132:2003–8.
Yamamuro O, Minamimoto Y, Inamura Y, Hayashi S, Hamaguchi H. Heat capacity and glass transition of an ionic liquid 1-butyl-3-methylimidazolium chloride. Chem Phys Lett. 2006;423:371–5.
Strechan AA, Paulechka YU, Blokhin AV, Kabo GJ. Low-temperature heat capacity of hydrophilic ionic liquids [BMIM][CF3COO] and [BMIM][CH3COO] and a correlation scheme for estimation of heat capacity of ionic liquids. J Chem Thermodyn. 2008;40:632–9.
Shimizu Y, Ohte Y, Yamamura Y, Saito K, Atake T. Low-temperature heat capacity of room-temperature ionic liquid, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J Phys Chem B. 2006;110:13970–5.
Yauheni UP, Andrey GK, Andrey VB. Calorimetric determination of the enthalpy of 1-butyl-3-methylimidazolium bromide synthesis: a key quantity in thermodynamics of ionic liquids. J Phys Chem B. 2009;113:14742–6.
Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids. J Am Chem Soc. 2005;127:2398–9.
Fang DW, Tong J, Guan W, Wang H, Yang JZ. Prediction of the thermodynamic properties of 1-alkyl-3-methylimidazolium lactate ionic liquids [Cnmim][Lact] (n = 2, 3, 4, 5, and 6) by parachor. Sci China Chem. 2010;53:2564–70.
Paulechka YU, Kohut SV, Blokhin AV, Kabo GJ. Thermodynamic properties of 1-butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid in the condensed state. Thermochim Acta. 2010;511:119–23.
Wasserscheid P, Boemanna A, Bolm C. Synthesis and performance of ionic liquids determined from the “chiral pool”. Chem Commun. 2002;3:200–1.
Baudequin C, Bregeon D, Levillain J. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron Asymmetry. 2005;16:3921–45.
Ma CC, Shi Q, Woodfield BF, Navrotsky A. Low temperature heat capacity of bulk and nanophase ZnO and Zn1−xCoxO wurtzite phases. J Chem Thermodyn. 2013;60:191–6.
Paulechka E, Blokhin AV, Rodrigues ASMC, Rocha MAA, Santos LMNBF. Thermodynamics of long-chain 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ionic liquids. J Chem Thermodyn. 2016;97:331–40.
Paulechka YU, Kabo GJ, Blokhin AV, Shaplov AS, Lozinskaya EI, Vygodskii YS. Thermodynamic properties of 1-alkyl-3-methylimidazolium bromide ionic liquids. J Chem Thermodyn. 2007;39:158–66.
Paulechka YU, Blokhin AV, Kabo GJ. Evaluation of thermodynamic properties for non-crystallizable ionic liquids. Thermochim Acta. 2015;604:122–8.
Fang DW, Wang H, Yue S. Physicochemical properties of air and water stable rhenium ionic liquids. J Phys Chem B. 2012;116:2513–9.
Tong B, Liu QS, Tan ZC, Urs WB. Thermochemistry of alkyl pyridinium bromide ionic liquids: calorimetric measurements and calculations. J Phys Chem A. 2010;114:3782–7.
Tan ZC, Di YY. Review of modern low-temperature adiabatic calorimetry. Prog Chem. 2006;18:1234 (in Chinese).
Tan ZC, Shi Q, Liu BP, Zhang HT. A fully automated adiabatic calorimeter for heat capacity measurement between 80 to 400 K. J Therm Anal Calorim. 2008;92:367–74.
Tan ZC, Sun LX, Meng SH, Li L, Zhang JB. Heat capacities and thermodynamic functions of p-chlorobenzoic acid. Chem J Chin Univ. 2002;34:1417 (in Chinese).
Tan ZC, Sun GY, Song YJ, Wang L, Han JR, Wang M. An adiabatic calorimeter for heat capacity measurement of small samples-the heat capacity of nonlinear optical materials KTiOPO4 and RbTiOAsO4 crystals. Thermochim Acta. 2000;247:252–3.
Acknowledgements
This project was financially supported by National Nature Science Foundation of China NSFC (Nos. 21673107 and 21703090) and Liaoning BaiQianWan Talents Program (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, DW., Liang, KH., Hu, XH. et al. Low-temperature heat capacity and standard thermodynamic functions of 1-hexyl-3-methyl imidazolium perrhenate ionic liquid. J Therm Anal Calorim 138, 1641–1647 (2019). https://doi.org/10.1007/s10973-019-08312-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08312-7