Abstract
Ionic liquid 1-ethylpyridinium bis(trifluoromethylsulfonyl)imide ([C2py][NTf2]) was synthesized and characterized by 1H NMR spectroscopy, 13C NMR spectroscopy and thermal gravity analysis. The molar heat capacities of [C2py][NTf2] were measured using a heat-flow calorimeter with “3D Calvet” calorimetric sensor from (293 to 312) K. The experiment value of molar heat capacity 502.15 J K−1 mol−1 at 298.15 K was obtained. Moreover, the estimation values of molar heat capacity were calculated by using 4 methods at 298.15 K, and the result showed the Paulechka et al.’s method was more appropriate for predicting the molar heat capacity of IL [C2py][NTf2], and the error was less than 2%. In addition, the freezing point T * was calculated by freezing point depression, which was approximately equal to experimental value 305.08 K. The molar enthalpy of fusion Δf H m = 26.77 kJ mol−1, molar melting entropy Δde S m = 90.60 J mol−1 K−1 and the freezing constant K f were also calculated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are known as “green solvents” due to their great capacity and their “environmentally friendly” properties in comparison with common organic solvents [1,2,3]. The capability of ILs to be used as solvents for the desulfurization of fuels has been tested [4,5,6,7,8] and considered promising agents for gas separation as they show a great variety in the ability to absorb gases during last years [9, 10]. Ionic liquid with anion of bis(trifluoromethylsulfonyl)imide also has an application prospect as solvent to extract benzene from alkanes [11]. Moreover, during the practical application of ionic liquids, thermal management of industrial processes is very important for safety production and reducing energy consumption, so heat capacity of ILs is indispensable basic data.
The low-temperature heat-flow calorimeter BT2.15 from Setaram with “3D Calvet” calorimetric sensor can be used to determine the heat capacity [12,13,14], which compared with traditional differential scanning calorimetry (DSC) with 2D flat sensors, the sensor has a novel structure, and the sample was surrounded by multiple layers of sensors; it means all the heat flow was captured for both sample and reference pool, rather than just detected the heat changes in bottom of the crucible, in which the premise was the sample plate contacted with the sensor closely. In addition, Zhang et al. [15]. reported the uncertainty of the heat capacity measurements did not exceed 0.01 by using BT2.15, and the average relative deviation was found to be 0.33%, and the maximum relative deviation was 0.79% in the accuracy of the measurement. However, the calculated standard uncertainties were ± 1 K for the temperature in the thermal analysis and ± 5% for the determination of the molar heat capacity when using a differential scanning calorimeter according to Gómez et al.’s report [16]. That is BT2.15 has a higher accuracy in determination of the heat capacity than DSC.
As a continuation of our investigation [17,18,19,20], in this paper, the IL [C2py][NTf2] was synthesized and the molar heat capacities in temperature range of (293–312) K were measured using a heat-flow calorimeter with “3D Calvet” calorimetric sensor. The value of molar heat capacity at 298.15 K was calculated by polynomial fitting equation. The molar enthalpy of fusion Δf H m and molar melting entropy Δde S m were also calculated.
Experimental
Preparation of IL [C2py][NTf2]
[C2py][Br] (N-Alkylpyridinium bromide) was synthesized according to Tong et al.’s report [21]. IL [C2py][NTf2] (1-Ethylpyridinium bis(trifluoromethylsulfonyl)imide) was synthesized by using the ion exchange reaction from [C2py][Br] and HN(SO2CF3)2 (bis(trifluoromethanesulfonyl)imide) in distilled water and stirred for 3 h at room temperature. The product was washed several times with distilled water until no Br− was indicated by the solution of AgNO3/HNO3. The final product was dried in vacuum for 2 days at 353 K. Structure of the resulting [C2py][NTf2] was confirmed by 1H NMR, 13C NMR spectroscopy and thermal gravity analysis (TGA) (see Figures S1, S2 and S3 in Supporting Information). The 1H NMR (600 MHz, CDCl3, δ): 9.77 (d, 1H, N–CH), 8.49 (t, 1H, CH–CH–CH), 8.01 (t, 2H, CH–CH–N), 4.61 (m, 2H, CH2–CH3), 1.63 (t, 3H, N–CH3). From 1H NMR and 13C NMR spectroscopy, impurity peaks were not found. TGA spectroscopy showed a mass loss peak of IL [C2py][NTf2]. In addition, water content was measured using a Karl Fischer moisture titrator (ZSD-2 type) before measurement for three times (81, 85 and 82 ppm) which are all less than 85 ppm.
Calorimeter calibration
The molar heat capacity, C p, was determined using a heat-flow calorimeter BT2.15 from Setaram, which adopts “3D Calvet” calorimetric sensor with resolution 0.1 µW, the accuracy of temperature measurement is ± 0.01 K, and the mass is with an uncertainty of ± 0.00001 g. The calorimeter was performed calibration with the standard molar heat capacity of KCl (purity > 99.95%) at 298.15 K and repeated for seven times. The results were 0.687333, 0.688046, 0.687966, 0.687269, 0.688009, 0.687719 and 0.687580 J K−1 g−1, respectively. The relative standard deviation RSD was 0.066%, which compared with 0.6879 J K−1 g−1 [22] are within the limit.
The heat capacities measurement of the IL
In this experiment, the test temperature was firstly set to 293 K and remain for 5 h, then heat to 312 K with a heating rate of 0.02 K min−1 under nitrogen environment. In this condition, the heat flow signals (experimental before-after) are at a same height.
Results and discussion
Measurement of molar heat capacity
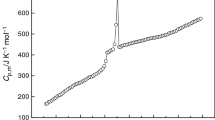
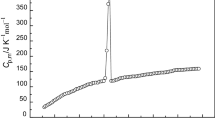
The experimental isobaric heat capacities of [C2py][NTf2] with a temperature step of 0.1 K are given in Table 1; by plotting molar heat capacity C p/J mol−1 K−1 against temperature T/K, the curve with r 2 exceeding 0.999 was obtained (see Fig. 1). Figure 1 shows the melting process with a sharp endothermic peak from 299 to 308 K; the melting point was determined to be 305.08 ± 0.05 K, which compared with 303.65 K [23] was approximately equal. The experimental values of the molar heat capacity were fitted before and after the melting process according to the following polynomial equations:
where X is the reduced temperature, X = [T − (T max + T min)/2]/[(T max − T min)/2]; T is the experimental temperature; T max and T min are the maximum and minimum of the temperature in the experimental temperature range. The correlation coefficient r-square of the four fittings is all above 0.999. Then, the value of molar heat capacity, C p = 502.15 ± 0.33 J K−1 mol−1, at 298.15 K was calculated by the polynomial Eq. (1).
Estimation of molar heat capacity
The molar heat capacity of IL [C2py][NTf2] was estimated by using 4 methods. According to Paulechka et al.’s report, [24] molar heat capacity can be estimated using the molar volume (V/cm3 mol−1) through the following empirical equation:
where V = 252.55 cm3 mol−1 (298.15 K), which was calculated by using the equation, V = M/ρ, ρ = 1.5375 g cm−3 [23], the estimated value of C p(cal.1) = 492.23 J K−1 mol−1 for [C2py][NTf2] obtained from Eq. (5). Besides, according to the gene expression programming (GEP) model [25], the prediction of experimental heat capacity data can be estimated by using the operators of −, + and × . The mathematical formula is as follows:
where T is temperature, C C is number of carbon atoms in cation part of IL, number of atoms of anion part (N-a), M W is molecular weight of IL, (N-c) is number of atoms of cation part and (CH3 R-c) is number of methyl groups in cation counter parts, and C p(cal.2) = 293.50 J K−1 mol−1 (298.15 K) was calculated. Farahani et al. [26] also reported that it can be calculated by following formula:
where N C = number of atoms of cation, N a = number of atoms of anion, CH3 R C = number of methyl groups in cation counter parts, nH a = number of hydrogen atoms in anion, T = temperature; then, C p(cal.3) = 477.50 J K−1 mol−1 (298.15 K) was obtained. Ahmadi et al. [27] also put forward a formula, by which the molar heat capacity can be estimated through the numbers of atoms including C, N, S, O, F, Br, Cl and B:
where T = temperature; M W = molecular weight of IL; C A = number of carbon atoms of anion; C C = number of carbon atoms of cation; N, S, O, F, Br, Cl and B = number of nitrogen, sulfur, oxygen, fluorine, bromine, chlorine and boron atoms of IL, C p(cal.4) = 433.69 J K−1 mol−1 (298.15 K) was also calculated. The values of molar heat capacity for 4 estimating methods and experiment are all listed in Table 2.
As shown in Table 2, the estimated value calculated from Paulechka et al.’s method is the nearest when compared with the experiment, and the error is less than 2%. According to Benito et al.’s report [28], the heat capacity determined by DSC was 523 J K−1 mol−1, which is higher than our experiment and the error was more than 4% when compared with the estimated value 492.23 J K−1 mol−1 and the experimental value 502.15 J K−1 mol−1. The deviation of experimental data may be caused by the different efficiencies of the instrument sensors. That is, the Paulechka et al.’s method which is based on the molar volume is more appropriate for predicting the molar heat capacity of IL [C2py][NTf2].
The molar enthalpy of fusion
The molar enthalpy of fusion of IL [C2py][NTf2], Δf H m, can be obtained from the following equation:
where n is the amount of substance, T de is the temperature of solid–liquid phase transition (peak melting temperature), T i is the temperature at which the solid–liquid phase transition started, T f is the temperature at which the solid–liquid phase transition ended, Q is total heat including sample and pool from T i to T f, C p(i) is molar heat capacity at T i, C p(f) is the molar heat capacity at T f, H 0 is molar heat capacity (T i − T f). The molar enthalpy of fusion Δf H m of IL was obtained using Eq. (9). The value of Δf H m = 26.77 kJ mol−1 is bigger than 22.44, 6.83 and 5.90 kJ mol−1 for [C2py][PF6], [C3py][PF6] and [C5py][PF6] [27], respectively. It may because the higher relative molecular mass and volume need more heat from solid to liquid.
Determination of melting point by freezing point depression
The trace impurities can also make a difference for the experimental result even the purity of 99 mass% is high. In that case, IL can be taken as a dilute solution which consists of solvent and impurity. Since the freezing point of dilute solution is certain to below the melting point of pure IL, then freezing point depression equation was obtained according to phase equilibrium principle as follows:
where x A is molar fraction, T * is melting point of pure IL, R is Avogadro constant. Since T de T * ≈ T 2 de , then Eq. (10) was rearranged:
From Eq. (11), the value of T * = 306.48 K was obtained, which compared with the average 305.08 K was a little bigger. The reason was caused by trace water in ionic liquid [C2py][NTf2].
The molar melting entropy Δde S m of IL can also be calculated according to melting point of pure IL:
From Eq. (12), Δde S m = 90.60 ± 0.06 J mol−1 K−1 was calculated, which compared with 60.04, 18.41 and 17.98 J mol−1 K−1 [27] for [C2py][PF6], [C3py][PF6] and [C5py][PF6], respectively, is bigger. Besides, the freezing constant K f was also calculated by the following equation:
where M A is molar mass, then the freezing constant K f = 1.09 × 104 g mol−1 K−1 was calculated.
Conclusions
In this paper, IL [C2py][NTf2] was synthesized and characterized. The molar heat capacities of ionic liquid [C2py][NTf2] were measured using a calorimeter with “3D Calvet” calorimetric sensor from (293 to 312) K. The value of molar heat capacity 502.15 J K−1 mol−1 was obtained at 298.15 K. Moreover, the estimation values of molar heat capacity were calculated by using 4 methods at 298.15 K, and the result showed the estimating formula which based on the molar volume was more appropriate for predicting the molar heat capacity of IL [C2py][NTf2] with the error less than 2%. The molar enthalpy of fusion Δf H m = 26.77 kJ mol−1, molar melting entropy Δde S m = 90.60 J mol−1 K−1 and the freezing constant K f were also calculated.
References
Calvar N, Gómez E, Macedo EA, Domínguez Á. Thermal analysis and heat capacities of pyridinium and imidazolium ionic liquids. Thermochim Acta. 2013;565:178–82.
Rocha MAA, Bastos M, Coutinho JAP. Santos LMNBF. Heat capacities at 298.15 K of the extended [CnC1im][Ntf2] ionic liquid series. J Chem Thermodyn. 2012;53:140–3.
Lin PY, Soriano AN, Leron RB, Li MH. Electrolytic conductivity and molar heat capacity of two aqueous solutions of ionic liquids at room-temperature: measurements and correlations. J Chem Thermodyn. 2010;42:994–8.
Bösmann A, Datsevich L, Jess A, Lauter A, Schmithz C, Wasserscheid P. Deep desulfurization of diesel fuel by extraction with ionic liquids. Chem Commun. 2001;23:2494–5.
Rodríguez-Cabo B, Francisco M, Soto A, Arce A. Hexyl dimethylpyridinium ionic liquids for desulfurization of fuels. Effect of the position of the alkyl side chains. Fluid Phase Equilib. 2012;314:107–12.
Arce A, Francisco M, Soto A. Evaluation of the polysubstituted pyridinium ionic liquid [hmmpy][Ntf2] as a suitable solvent for desulfurization: phase equilibria. J Chem Thermodyn. 2010;42:712–8.
Gao HS, Li YG, Wu Y, Luo MF, Li Q, Xing JM, Liu HZ. Extractive desulfurization of fuel using 3-methylpyridinium-based ionic liquids. Energy Fuels. 2009;23:2690–4.
Verdía P, González EJ, Rodríguez-Cabo B, Tojo E. Synthesis and characterization of new polysubstituted pyridinium-based ionic liquids: application as solvents on desulfurization of fuel oils. Green Chem. 2011;13:2768–76.
Han XX, Armstrong DW. Ionic liquids in separations. Acc Chem Res. 2007;40:1079–86.
Raeissi S, Peters CJ. A potential ionic liquid for CO2-separating gas membranes: selection and gas solubility studies. Green Chem. 2009;11:185–92.
Requejo PF, Calvar N, Domínguez Á, Gómez E. Comparative study of the LLE of the quaternary and ternary systems involving benzene, n-octane, n-decane and the ionic liquid [BMpyr][NTf2]. J Chem Thermodyn. 2016;98:56–61.
Casás LM, Plantier F, Pineiro MM, Legido JL, Bessières D. Calibration of a low temperature calorimeter and application in the determination of isobaric heat capacity of 2-propanol. Thermochim Acta. 2010;507–508:123–6.
Casás LM, Legido JL, Pozo M, Mourelle L, Plantier F, Bessières D. Specific heat of mixtures of bentonitic clay with sea water or distilled water for their use in thermotherapy. Thermochim Acta. 2011;524:68–73.
Coulier Y, Ballerat-Busserolles K, Mesones J, Lowe A, Coxam J. Excess molar enthalpies and heat capacities of 2-methylpiperidine − water and N–methylpiperidine − water systems of low to moderate amine compositions. J Chem Eng Data. 2015;60:1563–71.
Dong L, Zheng D, Nie N, Li Y. Performance prediction of absorption refrigeration cycle based on the measurements of vapor pressure and heat capacity of H2O + [DMIM]DMP system. Appl Energy. 2012;9:326–32.
Calvar N, Gómez E, Macedo EA, Domínguez Á. Thermal analysis and heat capacities of pyridinium and imidazolium ionic liquids. Thermochim Acta. 2013;565(565):178–82.
Wei J, Chang C, Zhang YY, Hou SY, Fang DW, Guan W. Prediction of thermophysical properties of novel ionic liquids based on serine [C n mim][Ser] (n = 3, 4) using semiempirical methods. J Chem Thermodyn. 2015;90:310–6.
Ma XX, Wei J, Zhang QB, Tian F, Feng YY, Guan W. Prediction of thermophysical properties of acetate-based ionic liquids using semiempirical methods. Ind Eng Chem Res. 2013;52:9490–6.
Xing NN, Dai B, Ma XX, Wei J, Pan Y, Guan W. The molar surface Gibbs energy and prediction of surface tension of [C n py][DCA] (n = 3, 4, 5). J Chem Thermodyn. 2016;95:21–5.
Wei J, Zhang QB, Tian F, Zheng L, Guan W, Yang JZ. Study on the thermodynamic properties for ionic liquid [C6mim][OAc](1-hexyl-3-methylimidazolium acetate). Fluid Phase Equilib. 2014;371:1–5.
Tong B, Liu QS, Tan ZC, Welz-Biermann U. Thermochemistry of alkyl pyridinium bromide ionic liquids: calorimetric measurements and calculations. J Phys Chem A. 2010;114:3782–7.
Speight JG. Lange’s handbook of chemistry. 16th ed. New York: McGraw-Hill; 2005.
Liu QS, Yang M, Yan PF, Liu XM, Tan ZC, Welz-Biermann U. Density and surface tension of ionic liquids [C n py][NTf2] (n = 2, 4, 5). J Chem Eng Data. 2010;55:4928–30.
Paulechka YU, Kabo AG, Blokhin AV, Kabo GJ, Shevelyova MP. Heat capacity of ionic liquids: experimental determination and correlations with molar volume. J Chem Eng Data. 2010;55:2719–24.
Barati-Harooni A, Najafi-Marghmaleki A, Arabloo M, Mohammadi AH. Chemical structural models for prediction of heat capacities of ionic liquids. J Mol Liq. 2017;232:113–22.
Farahani N, Gharagheizi F, Mirkhani SA, Tumba K. A simple correlation for prediction of heat capacities of ionic liquids. Fluid Phase Equilib. 2013;337:73–82.
Ahmadi A, Haghbakhsh R, Raeissi S, Hemmati V. A simple group contribution correlation for the prediction of ionic liquid heat capacities at different temperatures. Fluid Phase Equilib. 2015;403:95–103.
Benito J, Garcı´a-Mardones M, Pe´rez-Gregorio V, Gasco´n I, Lafuente C. Physicochemical study of n-ethylpyridinium bis(trifluoromethylsulfonyl)imide Ionic Liquid. J Solut Chem. 2014;43:696–710.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (21373005, 21673107) and LNET (LR2015025).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, L., Li, L., Guo, YF. et al. The isobaric heat capacities and thermodynamic properties of ionic liquid 1-ethylpyridinium bis(trifluoromethylsulfonyl)imide. J Therm Anal Calorim 131, 2943–2949 (2018). https://doi.org/10.1007/s10973-017-6807-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6807-1