Abstract
In this paper, the thermodynamic (energy and exergy) analysis and water analysis of a modified solar still augmented with copper tube heat exchanger in coarse aggregate have been carried out and compared with conventional still performance under the same climatic conditions. Basin water temperature, solar intensity, wind velocity, cumulative yield, water conductivity, total hardness, pH value and fluoride concentration are obtained from experimental results for saline, basin and distilled water. Energy efficiency, evaporation and convective heat transfer coefficient, exergy evaporation rate and exergy efficiency are determined from energy and exergy analysis. The results show that the modified still has an efficiency of 28% and 17% greater than the conventional still. The productivity of modified and conventional still is 6.23 kg m−2 and 2.41 kg m−2, respectively. The exergy efficiency depends on the time of the test day and reaches a maximum value of 5.5% and 1.1%, respectively, for the modified and conventional still. From the water analysis, it is observed that the maximum distilled water pH, water conductivity, hardness and fluoride content are 7.5, 0.8 × 10−4 S m−1 (0.8 µS cm−1), 0.5 × 10−3 kg m−1 (0.5 mg L−1) and 0.7 × 10−3 kg m−3 (0.7 mg L−1), respectively, with the still salinity removal efficiency of 99%. The results indicate that the modified still has higher energy and exergy efficiencies and better water quality with cumulative yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solar desalination is one of the widely followed and effective methods to produce fresh water from saline water by evaporation and condensation process in solar still. The incident solar radiation penetrates through the glass plate and heats up the saline water in the basin. The water evaporates due to rise in temperature and condenses on the inside of the glass plate. The condensed water is collected in the collection tank by gravitational force. At last, the salt contaminants are settled down in the still basin. Fabrication of a solar still is very simple, and the still requires zero fuel cost since solar energy is available abundantly to produce drinking water. Solar desalination techniques are a low-cost method, eco-friendly and do not need skilled labors to handle the process. In addition to the corrosion of the basin plate, the limitation of the conventional solar desalination system is lesser efficiency because the process is mainly based on daily solar radiation.
Many studies have been carried out by various researchers in order to increase the productivity of pure water from saline water. A variety of modifications in solar desalination technique have been devised by them to accelerate the evaporation rate of the process which leads to improve the daily productivity of the system. A heat pump-assisted solar desalination system tested by Belyayev et al. [1] showed that the productivity improves by 80%. The energy efficiency of solar still coupled with heat pump was enhanced to 62% with the daily productivity of 12.5 kg m−2 during summer times.
Badran [2] obtained a yield increase up to 51% in a single slope solar still by varying operational parameters. Also the results revealed that the daily efficiency of the still was improved by minimizing the depth of the saline water level and employing sprinklers to accelerate the condensation rate of the system.
Al-Hayek and Badran [3] have conducted experiments on simple solar still with black-coated absorber plate to obtain high solar energy into the basin to enhance the evaporation rate. The overall efficiency of the system increased by 20% with the daily distilled water collection of around 5.5 kg m−2. Charcoal was utilized as a heat absorber medium, for enhancing evaporation rate in the solar still by Naimet et al. [4]. They achieved 15% enhanced efficiency along with average daily distillate water rate of 2.5 kg m−2.
The experimental study of Aboabboud et al. [5] shows that the single slope solar still which used thermal energy recycle unit has produced 12 kg m−2 of distillate water. Sun tracking system was employed by Abdallah et al. [6] to absorb the most amount of solar energy. The efficiency of the system was 30% with the daily distilled water output of 5.6 kg m−2 day−1. George Ayoub and Malaed [7] used rotating cylinder in the solar still to improve the condensation rate to enhance the yield of the system. The economic analysis carried by them indicates that the cost of resultant distilled water was between $4.7 and 5.7 m−3. El-Sebaii et al. [8] used phase change material (PCM) as energy storing material in solar still. Stearic acid with the thickness of 3.3 cm has been used as PCM and showed still efficiency of 85.3% with a yield of 9.005 kg m−2.
Akash et al. [9] conducted tests with still area of 3 m2 by using different types of energy absorbing materials like black rubber, black ink and black dye. The results showed that the efficiency of the system was increased from 38 to 60%. Panchal [10] investigated the double basin solar still by coupling vacuum tube with black granite gravel and proved that the efficiency of the system was increased by 65%. Voropoulos et al. [11] showed that solar still coupled with solar collector gives the maximum efficiency of 66.6%. They proposed a modification on the solar still to enhance the heat transfer rate by connecting solar collector with a solar still. Murugavel et al. [12] conducted a simulation experiment on a double slope solar still by using several wick materials. The results showed that light cotton cloth with a solar still was more effective than other materials like light jute cloth and sponge sheets.
Velmurugan et al. [13] obtained an optimum salinity of the solar stills integrated with a mini solar pond as 80 g kg−1 of water through their experimentation and achieved an efficiency of 27.60%. Sponge cubes were introduced in solar still coupled with mini solar pond, which had an efficiency of 57.8%. Nafey et al. [14] conducted experiments on a single slope solar still by using black rubber and gravel as an energy storage medium. The experimental results showed that black rubber with 10-mm thickness has solar still efficiency of 20% with glass angle of 15°. Sahoo et al. [15] investigated the performance parameters of solar still using blackened surface and thermocol insulation. The efficiency of this modification solar still was raised about 6% with 20 L of basin capacity.
Sakthivel et al. [16] conducted experiments on a single slope solar still with the black granite gravel size of 6 mm. The results showed that the daily yield of the system is 3.9 kg m−2 with efficiency increased from 44 to 52%. Khalifa et al. [17] carried a work on the effect of inclination of the external reflector on simple solar still for summer conditions. The optimum angle of the reflector and cover obtained was 20° which is 2.45 times doubled the productivity. Sakthivel et al. [18] established that using jute cloth as an energy storage medium in the solar still, the efficiency was raised about 20%, and deviation of this result to theoretical observation was 9%. El-Sebaii et al [19] conducted a study on the effect of fin configuration parameters in a single basin solar still performance. The results indicated that the efficiency of still is 7% increased by using 0.04 m (height) and 0.001 m (thickness) size of fins. Rajaseenivasan et al. [20] investigated the energy and exergy performance of a double basin solar still with different materials in the basin. The results showed that the maximum exergy efficiency of single and double basin solar still was 2.072% and 1.412%. Dhivagar et al. [21] reviewed the various solar desalination systems and recommended that the daily productivity of the solar still is increased with respect to the temperature of inlet water.
From the reports of the researchers, the main operational parameter of the solar still was found to be inlet saline water temperature and yield of the solar still depends on it. Also in order to improve the efficiency of the solar still, many researchers used a heat recovery system to increase the temperature of inlet saline water. The sensible heat storage medium can also be utilized to preheat the inlet of saline water from the solar energy in the daytime for better evaporation rate. The heat capacity of sensible heat storage medium should be higher to enhance the temperature of saline water through the preheating process [16]. The thermal conductivity of an energy storage medium should be moderately high to improve the heat transfer. The sensible heat storage medium should be of low cost and easily available.
Researchers have studied the performance of the solar still by changing its orientation, integrating with heat pump, solar tracking, black coating of absorber plate, applying heat absorbing medium like charcoal, black granite gravel, jute cloth and PCM, employing different fin configurations and employing double basin system. Each study reported certain improvement in the performance of the solar still. However, none of the researchers have conducted thermodynamic and water analysis on solar still augmented with copper tube heat exchanger in coarse aggregate. In the present work, the energy, exergy and water analyses have been carried out on a modified solar still augmented with copper tube heat exchanger in coarse aggregate at a climatic condition and compared with conventional still performance for the same conditions with saline, basin and distilled water as feed.
Proposed modification
The main focus of the present research work is to develop an efficient, low-cost, solar distillatory system suitable for a small unit of production. In this study, a conventional solar still is modified by augmenting copper tube heat exchanger in coarse aggregate to enhance the evaporative heat transfer rate through the preheated water. The copper tube heat exchanger is placed horizontally beneath the coarse aggregate. The coarse aggregate with thermal conductivity of 2.25 W m−1 K−1 and copper tubes with thermal conductivity of 369 W m−1 K−1 are used to preheat the inlet saline water to enhance the performance of a solar still. Coarse aggregate is selected based on its heat storing capacity in the daytime and used as heat energy storage medium. Coarse aggregate is capable of absorbing the solar thermal energy by conduction, convection and radiation during higher sunshine periods with heat capacity of 43.66 kJ kg−1 [22].
Experimental
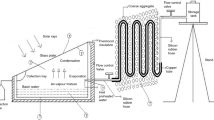
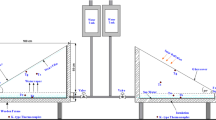
Figure 1 shows the schematic diagram of a modified solar still associated with copper tube heat exchanger and coarse aggregate. The dimensional details of solar still used for experimentation are given in Fig. 2a. The solar still contains a storage tank, coarse aggregate, copper tube and collection bottle. The solar still is fabricated from galvanized iron (G.I) sheet of thickness 2 mm which has a thermal conductivity of 31 W m−1 K−1. The basin area of solar still used is 1 m2. Fifty kilograms of coarse aggregate with 5-mm size is placed around the copper tube heat exchanger.
Figure 2b shows the dimension details of copper tube heat exchanger. This copper tube is used to hold a flow rate of 1 L h−1 of saline water and preheat it to more than 70 °C during peak sunshine hours. The basin of solar still and copper tube are coated with black paint to absorb the maximum amount of solar energy. The plain glass plate cover of 3-mm thickness is situated on the top of the conventional still with the angle of 27°. Transmissivity, emissivity and absorptivity of glass cover plate used are 0.88, 0.08 and 0.98, respectively. The solar still is kept inside the thermocol box to minimize the heat dissipation to the atmosphere. Silicon rubber hose is used to transfer the saline water from storage tank to still through copper tube. M sealant gel is used to block leakage from glass plate cover to conventional solar still. The coarse aggregate is heated by sun rays during daytime. The copper tube heat exchanger integrated with a solar still is buried into coarse aggregate for preheating the inlet saline water. The entire tube surface is heated by heat transfer from coarse aggregate and solar radiation.
The saline water is circulated through the copper tube heat exchanger every 1 h, and preheated water is sent to the solar still. The pictorial view of modified still is shown in Fig. 3.
A flow control valve is used between the copper tube and solar still to provide a batch mode of hot water and maintain a required depth of water level in the system. For every 1 h, the flow control valve is opened to allow the preheated water to enter the solar still. The temperature of saline water and glass plate cover is measured by using a K type thermocouple connected with temperature indicator. A calibrated Kipp–Zonen pyranometer is used to measure the solar intensity ranges, and the wind velocity of the air is measured by using a vane-type anemometer. The temperature of coarse aggregate is measured using a thermometer, and a measuring jar is used to measure the desalinated water from the collection tank. Experiments are carried out from 9 a.m. to 7 p.m. during January–July 2016. The entire system is cleaned periodically to remove the salt content in the basin as the saline water is used as feed water.
Energy analysis
The heat transfer is an important quantity used for the analysis of the performance of the solar still. Free convection, evaporation (diffusion) and radiation modes of heat transfer take place inside the still. The convection and evaporation heat transfer rate depends on radiation heat transfer.
From the assumption of small inclination of the glass cover, the water surface and glass plate are considered as an infinite parallel plane. The radiation heat transfer between glass plate cover and the water surface for radiation and convection is given by Eqs. (1) and (2) [23],
By combining Eqs. (1) and (2), the radiative heat transfer coefficient is
Convective heat transfer occurs from the water surface to glass plate cover due to buoyancy effect and density variation of the humid fluid with temperature gradients. The convective heat transfer rate and heat transfer coefficient are given by Dunkle’s relation [24],
The evaporation heat transfer takes place from the water surface to glass cover, and it is a function of convection heat transfer. In order to derive the evaporative heat transfer, the relation between convective heat transfer and the mass transfer coefficient is presented by Baum et al. [25],
Cooper [26] also derived the same relations as given in Eqs. (8) and (9),
By combining Eqs. (8) and (9), the evaporative heat transfer coefficient is given in Eq. (10),
The values of Pws and Pgl can be obtained from Eq. (11),
The insulated solar still system will help to minimize the side and bottom convective heat losses. Here, the bottom loss coefficient or overall heat transfer coefficient is considered from basin to ambient air through side and base of the solar still. It occurs due to the combined effect of convective and conductive heat transfers. The overall heat transfer coefficient is given in Eq. (12),
Top loss coefficient occurs due to radiation and convection heat transfers from glass plate cover to ambient, and it is expressed in Eq. (13),
Convective heat transfer from coarse aggregate to water in the copper tube is given in Eq. (14),
Equation (15) represents the hourly yield of the solar still presented by Tiwari et al. [27],
The thermal energy of the solar still can be defined as the amount of energy used for distilling the water to the amount of solar intensity observed by the system, and the energy efficiency of the system is given in Eq. (16) as,
Exergy analysis
Energetic and exergetic thermodynamic analyses have been widely applied to analyze the performance of thermal systems. Exergetic thermodynamic analysis is derived from the second law of thermodynamics, and it signifies that the maximum portion of energy can be converted into useful work [28]. The general exergy balance Eq. (17) for the solar still is presented by Helpbalsi [29],
or
where the exergy input energy is equal to the solar radiation and can be written by Petela [30]
The exergy of work rate is given by Eq. (20),
The exergy output of a solar still can be written as Eq. (21),
The monthly exergy input and output can be determined by multiplying Eqs. (20) and (22). The exergy efficiency of a solar still is defined as Eqs. (22) and (23),
Experimental uncertainty analysis
Uncertainty associated with experimental measurements is shown in Table 1. The error percentage is calculated for the measuring instruments, viz. thermocouple, pyranometer, thermometer, anemometer and measuring jar. The minimum error occurred in any instrument is same as the ratio between its least count and least minimum value of the output measured [31].
Mathematical uncertainty analysis
The result R is the given function of the independent variables \(x_{1} ,x_{2} ,x_{3} \ldots x_{\text{n}}\).
Let \(\omega_{\text{R}}\) be the uncertainty in the result and \(\omega_{1} ,\omega_{2} ,\omega_{3} \ldots \omega_{\text{n}}\) be the uncertainties in the independent variables. If the uncertainties in the independent variables are all given with the same odds, then the uncertainty in the result having these odds is given by equation as [32],
If this equation is applied to the energy and exergy efficiency, we can get Eqs. (26) and (27),
Results and discussion
Experiments are conducted in conventional still (without copper pipe) and modified solar still to obtain the energetic, exergetic performance and water analysis under the same climatic conditions. An observation of both the stills has been noted on daily basis to obtain the concurrent value. In this work, the sample observation is taken for revealing the results when the solar energy is fairly the same. The solar intensity and wind velocity profile are shown in Fig. 4. It is observed that the wind velocity has a higher fluctuation from the beginning to the end of the experiments.
The variation of basin water temperature of both stills and its difference with respect to time are shown in Fig. 5. It is observed that the rise in basin temperature of modified still is faster than the temperature of conventional still. The effect of wind velocity over the glass plate cover is playing a major role in the pure water production. Since the wind velocity is a meteorological property, it cannot be controlled and cannot be stable with respect to time. After 10 a.m., the saline water inside the conventional solar still heats up with higher solar radiation. Then, the saline water temperature is reduced slowly during the late afternoon hours because of reduction in the solar intensity. But in a modified solar still, the temperature of saline water increases more due to preheated water sent as feed water. The saline temperature difference between conventional and modified still is observed to be 18 °C. This is due to the radiation loss from the water surface in the solar still [18]. It is known that a large amount of heat (~ 2553 kJ (Cp = 0.74 kJ kg−1 K−1)) is released from heat storage medium to copper tube for enhancing the latent heat of vaporization in the solar still. Even though the same mass flow rate is maintained in both stills, the latent heat of vaporization is more for the modified still due to heat storage medium.
Figure 6a, b shows the variation of evaporative and convective heat transfer coefficient of both stills. It is observed that the evaporative heat transfer coefficient is higher than the convective heat transfer coefficient. The maximum evaporative heat transfer coefficient of 112.65 W m−2 K−1 is achieved in the modified solar still. It is observed that the preheated water is heated up again with higher solar intensity periods in the modified still. During late noon periods, the water is heated up only through the preheating process and not much heating up from the solar still due to lower solar intensity. During this charging period, the water temperature is raised by heat storage medium [33]. The same observation is seen in convective heat transfer coefficient as given in Fig. 6b. Convective heat transfer occurs among different layers of water inside the still. From the water surface to the condensing glass cover, heat is accompanied by transport of water formed above the water surface through the air–vapor mixture. This process is accelerated due to preheated water. So, both the convective and evaporative heat transfers rise simultaneously in a solar still and are independent of radiative heat transfer. A significant increase in both convective and evaporative heat transfer coefficients of modified still is experiential when compared to the conventional still.
While the preheated water at 75 °C is sent to the solar still, the rise in temperature of the saline water is high for the modified still. The yield of the modified still increases due to exchange of sensible heat by coarse aggregate to copper tube. Figure 7 reveals that the yield of the modified still is more than the conventional still. The productivity of the modified still is about 6.23 kg m−2 day−1, whereas the conventional still is 2.14 kg m−2 day−1. The modified still yield of the present work is higher than that of the still with sand heat energy storage (5 kg m−2 day−1) reported by Sathyamurthy et al. [34].
Figure 8 shows the energy efficiency of conventional and modified stills for the trial days. The experimental results are compared with existing double active solar still which has 24.1% of enhanced efficiency [35]. The thermal efficiency of modified still varies from 14.3 to 28%, which proved that coarse aggregate heat storage medium has better efficiency. The maximum energy efficiency attained for the modified still is 28%, whereas the conventional still energy efficiency is 11%.
Figures 9 and 10 show the exergy evaporation rate and exergy efficiency of the stills obtained for the trial days. It is found that the exergy efficiency of modified still ranges from 1.6 to 5.5%. The experimental results are compared with existing double active solar still which has 1.34% of enhanced efficiency [35]. This variation occurs due to the usage of coarse aggregate along with copper tube heat exchanger. The maximum exergy efficiency of conventional still is found to be 1.1%.
Water analysis
The output water from the stills has been analyzed for its quality in the chemistry laboratory. Water conductivity, hardness, pH and fluoride parameters are found to identify the quality of distilled water. Saline, basin and distilled water samples were taken for the treatment processes. Figure 11 shows the accomplishment of water conductivity with trial days. The conductivity of basin water is more than saline water due to the settling of salts in the basin plate. However, it does not affect distilled water conductivity. The maximum conductivity of distilled water was found to be around 0.8 × 10−4 S m−1 (10 µS cm−1).
Total hardness is a combination of calcium and magnesium ions present in water. Plumbing and scaling effects occurred in a solar still due to the settling of Ca+ and Mg+ minerals. Figure 12 shows the attainment of total hardness for trial days. The results reveal that the basin water hardness does not increase as conductivity. Instead it decreases due to precipitation process while the basin water gets evaporated in the still. The distilled water hardness was about 0.5 × 10−3 kg m−1.
The pH of saline water in the range of 6.5–8.5 leads to the formation of corrosion and plumping effect. Figure 13 shows the pH value attained for trial days. From the results of experimentation, the pH of the distilled water was found to be 6.5–7.5 which is acceptable range as drinking quality level. The variation in pH value of distilled water may be due to evaporation and condensation process.
The fluoride content is very harmful to human health and has a high soluble level than calcium and magnesium. Figure 14 reports that the range of fluoride concentration of basin water increases in the solar still due to less precipitation during the evaporation process. The concentration of fluoride increases from 12 × 10−3 to 21 × 10−3 kg m−3 in basin water with the maximum of 0.7 × 10−3 kg m−3 (0.7 mg L−1) in distilled water.
Conclusions
In this work, integration of coarse aggregate with copper tube heat exchanger in a solar still has been made. The results show that preheated feed to still enhances the daily productivity of the solar still. The following results are drawn from the energetic, exergetic and water analyses.
-
(a)
The daily productivity of conventional still is increased from 2.41 to 6.23 kg m−2 by modifying the still with augmenting coarse aggregate with copper tube heat exchanger.
-
(b)
The energy efficiency of modified still is 28%, whereas conventional still is found to be 11%. The improvement in efficiency of 17% is achieved. The maximum exergy efficiency of a modified still and conventional still is 5.5% and 1.1%, respectively. Energy efficiency has been always higher than that of exergy efficiency owing to not taking into consideration losses [36].
-
(c)
Sensible heat energy storage materials are having a major role to enhance the productivity of the solar still. The saline water is preheated to a temperature of 75 °C which is appreciably enhancing the evaporation rate. Continuous heat transfer to the solar still through a copper tube could accelerate the evaporation process to attain the maximum efficiency. The effective temperature of coarse aggregate, glass and saline water increases the yield of the solar still.
-
(d)
Chemical tests to determine pH, hardness, conductivity and fluoride concentration are done to know the quality of distillate water. The maximum value of distilled water pH, water conductivity, hardness and fluoride contaminants was found to be 7.5, 0.8 × 10−4 S m−1 (0.8 µS cm−1), 0.5 × 10−3 kg m−1 (0.5 mg L−1) and 0.7 × 10−3 kg m−3 (0.7 mg L−1) with the still salinity removal efficiency of 99%.
Future work
In this work, ground water is used as feed water to the stills for experimentation and analysis purpose. By incorporating some more changes in the configuration of simple solar still with the usage of different sensible heat storage medium, works can be carried out to produce purified water from industrial effluents.
Abbreviations
- h :
-
Heat transfer coefficient (W m−2 K−1)
- h fg :
-
Latent heat of evaporation (J kg−1)
- k :
-
Thermal conductivity (W m−1 K−1)
- L :
-
Length (m)
- U o :
-
Overall heat transfer coefficient (W m−2 K−1)
- m :
-
Yield per unit area (kg m−2)
- P :
-
Pressure (Pa)
- C p :
-
Specific heat at constant pressure (J kg−1 K−1)
- q :
-
Rate of heat transfer (W m−2)
- T :
-
Temperature (K)
- W :
-
Work (W)
- A :
-
Basin area of the solar still (m2)
- I(t)s :
-
Hourly incident solar radiation (W m−2)
- \({\dot{\text{E}}\text{x}}\) :
-
Exergy (W)
- \({\dot{\text{E}}\text{x}}_{\text{dest}}\) :
-
Exergy destructed in the solar still water (W)
- \({\dot{\text{E}}\text{x}}_{\text{sun}}\) :
-
Exergy input from the sun to solar still (W)
- \(\varepsilon_{\text{eff}}\) :
-
Effective emissivity
- \(\sigma\) :
-
Stefan–Boltzmann constant (5.67 × 10−8 W m−2 K−4)
- \(\eta_{\text{E}}\) :
-
Energy efficiency of the system (%)
- \(\eta_{\text{Ex}}\) :
-
Exergy efficiency of the system (%)
- \(\omega\) :
-
Uncertainty
- a:
-
Ambient air
- ba:
-
Basin
- c:
-
Convection
- ca:
-
Coarse aggregate
- eva:
-
Evaporation
- gl:
-
Glass
- i:
-
Input to the solar still
- ins:
-
Insulation
- out:
-
Output from the solar still
- hx:
-
Heat exchanger
- r:
-
Radiation
- S:
-
Sun
- sd:
-
Side of still
- ws:
-
Water surface
- w:
-
Water
- work:
-
Work rate of the solar still
References
Belyayev Y, Mohanraj M, Jayaraj S, Kaltayev A. Thermal performance simulation of a heat pump assisted solar desalination system for Kazakhstan climatic conditions. Heat Transf Eng. 2018;134:1–13.
Badran OO. Experimental study of the enhancement parameters on a single slope solar still productivity. Desalination. 2007;209:136–43.
A-Hayek I, Badran OO. The effect of using different designs of solar stills on water distillation. Desalination. 2004;169:121–7.
Naima MM, El Kawi MAA. Non-conventional solar stills Part 1. Non-conventional solar stills with charcoal particles as absorber medium. Desalination. 2002;153:55–64.
Aboabboud MM, Horvath L, Szepvolgyi J, Mink G, Radhika E, Kudish AI. The use OD thermal energy cycle unit in conjunction with a basin type solar still for enhanced productivity. Energy. 1997;22:83–91.
Abdallah S, Badran O, Abu-Khader MM. Performance evaluation of a modified design of a single slope solar still. Desalination. 2008;219:222–30.
Ayoub GM, Malaeb L. Economic feasibility of a solar still desalination system with enhanced productivity. Desalination. 2014;335:27–32.
El-Sebaii AA, Al-Ghamdi AA, Al-Hazmi FS, Faidah AS. Thermal performance of a single basin solar still with PCM as a storage medium. Appl Energy. 2009;86:1187–95.
Akash BA, Mohsen MS, Osta O, Elayan Y. Experimental evaluation of a single basin solar still using different absorbing materials. Renew Energy. 1998;14:307–10.
Panchal HN. Enhancement of distillate output of double basin solar still with vacuum tubes. J King Saud Univ Eng Sci. 2015;27:170–5.
Voropoulos K, Mathioulakis E, Belessiotis V. Experimental investigation of a solar still coupled with solar collectors. Desalination. 2001;138:28–31.
Murugavel KK, Chokalingam KK, Sridhar K. An experimental study on single basin double slope simulation solar still with thin layer of water in the basin. Desalination. 2008;220:687–93.
Velmurugan V, Srithar K. Solar stills integrated with a mini solar pond: analytical simulation and experimental validation. Desalination. 2007;216:232–41.
Nafey AS, Abdelkader M, Abdelmotalip A, Mabrouk AA. Solar still productivity enhancement. Energy Conversat Manag. 2001;42:1401–8.
Sahoo BB, Sahoo N, Mahanta P, Borbora L, Kalita P, Saha UK. Performance assessment of a solar still using blackened surface and thermocol insulation. Renew Energy. 2008;13:1703–8.
Sakthivel M, Shanmugasundaram S. Effect of energy storage medium (black granite gravel) on the performance of a solar still. Int J Energy Res. 2008;32:68–82.
Khalifa AJN, Ibrahim HA. Effect of inclination of the external reflector of simple solar still in winter: an experimental investigation for different cover angles. Desalination. 2010;264:129–33.
Sakthivel M, Shanmugasundaram S, Alwarsamy T. An experimental study on a regenerative solar still with energy storage medium: jute cloth. Desalination. 2010;264:24–31.
El-Sebaii AA, Ramadan MRI, Aboul-Enein S, El-Naggar M. Effect of fin configuration parameters on single basin solar still performance. Desalination. 2015;365:15–24.
Rajaseenivasan T, Murugavel KK, Elango T. Performance and exergy analysis of a double-basin solar still with different materials in basin. Desalination Water Treat. 2014;55:1786–94.
Dhivagar R, Sundararaj S. A review on methods of productivity improvement in solar desalination. Appl Mech Mater. 2018;877:414–29.
Kumar RK, Babu BG, Mohanraj M. Thermodynamic performance of forced convection solar air heater using pin-fin absorber plate packed with latent heat storage materials. J Therm Anal Calorim. 2016;126:1657.
Watmuff JH, Charters WWS, Proctor D. Solar and wind induced external coefficients for solar collectors. Rev Int. 1977;2:56.
Dunkle RV. Solar distillation-a roof type solar still and multi effect diffusion stills. Int Heat Transf Conf. 1961;1:895–902.
Baum VA, Bayaramov RB, Malevsky VM. Solar still in the deserts. In: Proceedings of international solar energy congress; 1976. p. 426.
Cooper PI. Maximum efficiency of single effect solar still. Sol Energy. 1973;15:205.
Tiwari GN, Gupta SP, Lawrence SN. Transient analysis of solar still in the presence of dye. Energy Conversat Manag. 1989;29:59–62.
Pandya B, Kumar V, Matawala V, Patel J. Thermal comparison and multi objective optimization od single stage aqua-ammonia absorption cooling system powered by different solar collectors. J Therm Anal Calorim. 2018;134:1–14.
Hepbasli A. A key review on exergetic analysis and assessment of renewable energy resources for a sustainable future. Renew Sustain Energy Rev. 2006;12:593–661.
Petela R. Exergy of undiluted thermal radiation. Sol Energy. 2003;74:469–88.
Kianifar A, Heris SZ, Mahian O. Exergy and economic analysis of a pyramid-shaped solar water purification system: active and passive cases. Energy. 2012;38:31–6.
Holman JP. Experimental methods for engineers. 8th ed. New York: McGraw Hill Publication; 2012. p. 62–5.
Sathyamurthy R, El-Agouz SA, Dharmaraj V. Experimental analysis of a portable solar still with evaporation and condensation chambers. Desalination. 2015;367:180–5.
Sathyamurthy R, Nagarajan PK, Edwin M, Madhu B, El-Agouz SA, Ahsan A, Mageshbabu D. Experimental investigations on conventional solar still with sand heat energy storage. Int J Heat Technol. 2016;34:597–603.
Sethi AK, Dwivedi VK. Exergy analysis of double slope active solar still under forced circulation mode. Desalination Water Treat. 2013;51:7394–400.
Pandey AK, Tyagi VV, Rahim NA, Kaushik SC, Tyagi SK. Thermal performance evaluation of direct flow solar water heating system using exergetic approach. J Therm Anal Calorim. 2015;121:1365.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhivagar, R., Sundararaj, S. Thermodynamic and water analysis on augmentation of a solar still with copper tube heat exchanger in coarse aggregate. J Therm Anal Calorim 136, 89–99 (2019). https://doi.org/10.1007/s10973-018-7746-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7746-1