Abstract
Prochlorperazine (PCP) and promethazine (PMT) are dopamine (D2) receptor antagonists with a phenothiazinic structure that used nowadays mainly for their antiemetic properties, including for the pre- and postoperative setting. In this paper, the thermal behavior of PCP and PMT was studied, including a complete kinetic analysis for the evaluation of the degradation process in heterogenous media. Samples were analyzed using Fourier transform infrared spectroscopy, thermogravimetric analysis (TG/DTG/HF) and a preliminary kinetic analysis using the ASTM E698 kinetic method, the obtained data being later completed with an in-depth kinetic analysis according to ICTAC 2000 protocol, using the isoconversional methods of Friedman, Ozawa–Flynn–Wall, Kissinger–Akahira–Sunose, respectively, the modified nonparametric kinetics method, the latter allowing the determination of the kinetic triplet without using any approximation, but as well the separation of parallel reaction that occurs at decomposition. The results confirmed that PCP dimaleate has a better stability in comparison with PMT hydrochloride, in terms of apparent energy of activation, probably due to solid-state structure of solid and the contribution of intermolecular bondings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prochlorperazine ((2-chloro-10-[3-(4-methylpiperazin-1-yl)propyl]phenothiazine), abbreviated PCP) is a phenothiazine derivative that has antihistaminic, antiemetic, antipsychotic and anticholinergic properties, being a dopamine D2 receptor antagonist [1, 2].
The compound is used in various psychotic affections, including bipolar disorder [1], nonpsychotic anxiety [2], the management of nausea and vomiting, especially chemotherapy-induced emesis [3] or migraine headaches [4].
The severity of the possible side effects, such as sedation and extrapyramidal reactions, is directly linked to prochlorperazine’s action mechanism, the intensity of the reactions increasing with the dose of the active substance [5, 6]. Because of these considerations, pharmaceutical research focuses on obtaining a drug formulation that can boost bioavailability without increasing the concentration of the active ingredient.
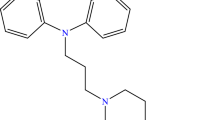
Prochlorperazine (Fig. 1a) is a white or light yellow crystalline powder that melts at about 228 °C [7]. It is slightly soluble in water (15 mg L−1, at 24 °C) and alcohol. This property can be modified by using the substance as a salt, namely dimaleate, whose water solubility reaches 0.3 mg mL−1 [8, 9].
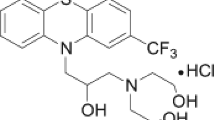
Promethazine ((2-dimethylamino-2-methyl) ethyl-N-dibenzoparathiazine, abbreviated PMT, Fig. 1b), a phenothiazine-related compound historically known for its antipsychotic and sedative effects [10] is more currently used for its antiallergic, sedative and antiemetic properties in the treatment of motion sickness [11, 12]. This application is due to its biological activity as a histamine H1 and muscarinic M1 receptor inhibitor [13]. PMT is involved in studies regarding its effectiveness in the management of chronic pain [14].
Although its absorption after oral administration is over 80%, due to its first pass clearance, promethazine’s bioavailability decreases at about 25% [15].
Regarding its physicochemical profile, it is known that PMT is a white or very light yellow crystalline powder that has the molecular weight equal to 284.421 g mol−1 and a water solubility of 15.6 mg L−1 (at 24 °C) [13, 16]. In order to improve PMT solubility and in turn its bioavailability, the compound is used as a salt, namely promethazine hydrochloride, chosen because of its increased stability, whose molecular mass and solubility reach 320.88 g mol−1 and 100 g L−1, respectively [17, 18]. Promethazine has a melting point of 230–232 °C [17].
Due to the rigid hydrophobic ring hydrophilic amine side chain, a few solubilization methods such as micelle formation, complexation with cyclodextrins or microemulsions have been developed. Because of the various compounds involved in these processes, a complete thermal and kinetic study of the active substance is necessary in order to assure the proper selection of the solubilization method [19, 20].
Both discussed compounds are used currently as therapy options, their physicochemical characteristics being the foundation of the pre-formulation stages of drug design. As to properly choose the best formulation and the appropriate additives, it is important that the stability profile of the compounds is analyzed and fully understood. The modifications regarding the properties of the active substances that occur with temperature variation can be easily observed and understood using thermal analysis, while kinetic techniques are useful in determining reaction mechanisms and required conditions.
Since thermal stability of active pharmaceutical ingredients (API) is crucial for the shelf life of the final formulation [21, 22], but also for a correct industrial approach of the necessary steps for obtaining said formulation, we set our goal in this study to realize a kinetic study over the decomposition of PCP and PMT, using the isoconversional methods of Friedman, Ozawa–Flynn–Wall, Kissinger–Akahira–Sunose, respectively, the modified nonparametric kinetics (NPK) method.
Materials and methods
Prochlorperazine dimaleate (PCP) and promethazine hydrochloride (PMT) were obtained from Sigma-Aldrich (purity > 98%) used as received without further purification. The samples were kept in closed containers, in the conditions mentioned by the supplier.
Thermoanalytical TG/DTG/HF data were collected using a PerkinElmer DIAMOND TG/DTA instrument. The data were collected on samples containing around 6 mg of each API, which were weighted into an open aluminum crucible. The thermal treatment was carried out in the 35–510 °C temperature range in a synthetic air atmosphere at a flow rate of 100 mL min−1, in non-isothermal conditions at heating rates β = 5, 7, 10, 12 and 15 °C min−1. The kinetic study was carried out for the decomposition step that took place in 160–350 °C temperature range.
The kinetic study (ASTM E698 method) was carried out using the AKTS—Thermokinetics software (AKTS AG TechnoArk, Siders, Switzerland). Friedman, Kissinger–Akahira–Sunose and Flynn–Wall–Ozawa methods were applied using a template developed by our group of study.
Fourier transform infrared spectroscopy (FTIR) spectra were recorded on PerkinElmer SPECTRUM 100 device. The data were collected in 4000–650 cm−1 spectral range, on an UATR device. Spectra were built up after a number of 32 co-added scans.
Results and discussion
FTIR analysis
Both compounds were investigated by FTIR analysis (Fig. 2), and in order to prove their identity and purity, a well-documented technique is used in pre-formulation stages [23], the obtained data being in agreement with published literature [24].
The FTIR spectra of PCP (Fig. 2a.) reveal the presence of functional moieties corresponding to the chemical structure by the characteristic vibrations from the spectra. According to this, in the spectral range 3500–2300 cm−1, two broad bands are observed, namely in the spectral range 3200–2800 cm−1, with peaks at 3010 and 2947 cm−1, and 2600–2300 cm−1, with a peak at 2315 cm−1. These bands appear due to the presence of OH groups from maleic acid, but as well to the presence of C–H bonds from alkyl chain from PCP structure. Prominent bands were observed in the spectral range 1700–700 cm−1, with two intense peaks corresponding to C=O stretching vibrations from maleate moieties at 1690 cm−1 (from protonated COOH group) and 1619 cm−1 (the asymmetric stretch of the carboxylate ion, COO−). The bands at 1535 and 1561 cm−1 can be attributed to the presence of the aromatic rings. Other intense bands are represented by the angular deformation of CH2 at 1477 and 1441 cm−1. Due to the 2,10-disubstituted phenothiazine skeleton, a characteristic and unique pattern of the absorption bands appears in the 1000–700 cm−1 range showing peaks at 943, 864, 799 and 778 cm −1. At lower wavenumbers, with a split peak at 746 cm−1 the rocking vibration of the C–H bond from the methylene groups appears.
In the case of FTIR spectra of PMT (Fig. 2b), in the spectral range 3200–2300 cm−1, two weak bands are observed, with peaks at 3055 and 2984 cm−1, due to the presence of aromatic C–H bonds, respectively, aliphatic C–H, as well the in-plane vibrations of aromatic C=C bonds at 1591 and 1569 cm−1. Other intense bands appear at 1454 cm−1 (probably due to angular deformation of CH2 groups), 1227 and 733 cm−1. In the case of PMT, a phenothiazine without a substituent at position 2, a very strong absorption band in the 720–770 cm−1 region exhibits, with a peak at 726 cm−1, which is attributed to the out-of-plane bonding vibrations of the four adjacent hydrogens of the phenothiazine ring system.
Thermal stability of PCP and PMT
The thermoanalytical curves of PCP and PMT obtained during heating at β = 5 °C min−1 in air atmosphere exhibit a multistadial decomposition route (Fig. 3.). PCP shows a good thermal stability (up to 177 °C), when a decomposition process occurs (temperature range 177–225 °C, with a Δm = 32.92%) and a DTGmax at 197 °C. This first process is accompanied by an endothermal effect, according to the HF curve, in the same temperature range, with a HFmax at 197 °C. This process is immediately followed by another one, as evidenced by the DTG curve, in the temperature range 225–315 °C and DTGmax = 282 °C. In this temperature range, the mass loss is also rapid (at 315 °C, Δm = 81.40%), the process being accompanied by an exothermal event on the HF curve, with HFmax at 298 °C. With the increase in temperature, in the range 315–510 °C, the mass loss is relatively insignificant (Δm = 11.96%).
The thermal profile of PMT reveals another pathway of decomposition, simpler than the one of PCP. This fact can be probably associated with the lack of organic salt co-former and the existence of a simpler salt, namely a hydrochloride. This salt is stable up to 192 °C, when a decomposition process begins. This process is evidenced by the TG/DTG curves as a continuous one, with a rapid and considerable mass loss (Δm = 94.84%) in the 192–309 °C temperature range and a DTGmax at 269 °C. However, HF curve indicates two consecutive endothermal processes, the first occurring in the 192–239 °C temperature range and HFmax = 230 °C, while the second take place between 239 and 293 °C and HFmax = 266 °C. These processes are related to the decomposition of hydrochloride salt, followed by destruction of organic skeleton. At temperatures higher than 309 °C, the mass loss is considerable smaller, so that at 510 °C, the total mass loss is Δm = 98.94%.
Kinetic study
The kinetic study was carried out for the main decomposition step of each compound, namely in the 160–250 °C temperature range for PCP and 150–350 °C for PMT. The preliminary kinetic study was realized using the ASTM E698 kinetic method (Fig. 4a, b), based on the Ozawa plot. This method was developed on the assumption that the degree of conversion is independent of the heating rate β when a DTG curve reaches its maximum, at Tmax. The mathematical form of the ASTM E698 is represented by Eq. (1):
The value of the Ea is estimated from the slope of the linear plotting for experiments carried out at the selected five different heating rates (Fig. 4a, b).
The activation energies obtained by using the ASTM E698 methods are 146.0 kJ mol−1 for PCP and 98.5 kJ mol−1 for PMT, respectively.
As to obtain a more realistic view over the decomposition process of the bioactive compounds, with an adequate estimation of the decomposition process by obtaining the kinetic triplet, more complex kinetic models should be used. This fact is also recommended by the ICTAC 2000 protocols, by using isoconversional methods, both integral and differential. Following this, the differential method of Friedman and the integral ones of Flynn–Wall–Ozawa and Kissinger–Akahira–Sunose were used for the estimation of the apparent Ea versus the conversion degree α. For this, the progress of transformation versus modification of temperature is presented in Fig. 5a, b, for both bioactive compounds, as well the reaction rate versus temperature, in Fig. 6a, b.
By the analysis of the reaction rate versus temperature (Fig. 6), it is confirmed that the decomposition mechanism of PCP is more complex (Fig. 6a), consisting in several overlapped processes, as shown by the variation of reaction rate versus temperature, while in the case of PMT (Fig. 6b) such behavior is not observed.
The differential isoconversional method of Friedman [25] is presented in the linearized form as Eq. 2, where α is the conversion degree, T is temperature, f(α) is the differential conversion function, R is the universal gas constant, β is the heating rate β = dT/dt (where t is time), A is the pre-exponential factor and Ea is the activation energy given by the Arrhenius equation.
The linear correlations between \(\ln \left( {\beta \frac{{{\text{d}}\alpha }}{{{\text{d}}T}}} \right)\) versus (1/T) for the thermogravimetric data obtained at five heating rates are presented in Fig. 7 for both PCP and PMT, but with a clear indication of multistep degradation of the compounds, since the slopes of these lines are drastically modified at high conversions, for both compounds, especially for PCP.
The integral method of Ozawa [26, 27] and Flynn–Wall [28] is generally presented in the linearized form from Eq. (3), where g(α) is the integral conversion function.
The linear dependencies were obtained after plotting ln β versus 1/T, as shown in Fig. 8, while the estimated apparent activation energy for each α is presented in Table 1.
The linearized form of isoconversional method proposed by Kissinger [29], Akahira and Sunose [30] is presented in Eq. (4), where g(α) is the integral conversion function.
The plotting of \(\ln \frac{\beta }{{T^{2} }}\) versus 1/T (Fig. 9) leads to good linear correlations, from which the apparent activation energy value was estimated for each conversion degree.
The results suggested by the KAS method (Table 1) are similar with those obtained by the FWO method, since the two methods differ solely by the approximation used for the integral form of the conversion function.
Another important observation by comparison of the results suggested by the differential method versus integral ones is the variation of Ea with the advance of the reaction: Solely the Friedman method suggests a modification of the decomposition mechanism by a considerable modification of the slopes of the linear dependencies, while the integral methods are less precise, due to the integral processing of the data. However, these inconsistencies lead to uncertain kinetic results and for an objective processing of kinetic data, the modified NPK method was used.
The nonparametric kinetics method (NPK) was firstly reported by Sempere et al. [31,32,33] and later modified by Vlase et al. [34,35,36,37]. Several advantages reside in using the modified NPK method, such as the evaluation of the kinetic triplet, which is not revealed by classical isoconversional methods.
The nonparametric kinetic method (NPK) is using a single assumption, namely that the reaction rate is a product of two independent functions, one dependent of the degree of conversion (f(α)) and another one of temperature (k(T)), which is the fundamental relation of heterogenous kinetics.
The obtained values of reaction rates are inserted in a matrix, expressed as a product of two vectors containing information over both dependencies, i.e., k(T) and f(α). From this matrix, two functions related to the temperature and conversion are obtained, without any a priori approximation, being based solely on the veracity of the Arrhenius equation. The experimental points obtained at five heating rates form by interpolation a three-dimensional continuous reaction rate surface, with the following coordinates: T, α and transformation rate, as presented in Fig. 10. By discretization, from this surface, it obtained an n × m matrix M, which is decomposed, using the singular value decomposition algorithm [38], into the product of matrixes, as shown by Eq. (5):
The results obtained using the modified NPK method are presented in Table 2. These data were obtained by analyzing the vector u (the first column of U) in respect of a kinetic model suggested by Šesták and Berggren [39]:
respectively, the vector v (the first column of V) for an Arrhenius-type dependence for the temperature.
NPK method indicates that both compounds are degraded in two processes. For PCP, the main process is characterized by an explained variance λ = 84.1%, consisting in both chemical degradation (with a reaction order n = 1), accompanied by a physical transformation (m = 1/3). The secondary process has a smaller contribution to the mean apparent activation energy, both as explained variance and energetic contribution. This secondary process is a different decomposition mechanism, since n = 1 and m = 1/3.
For PMT, the kinetic model for the main decomposition step with λ = 74.8% is the same as in the case of PCP, consisting in both chemical degradation (with a reaction order n = 1), accompanied by a physical transformation (m = 1/3). As for the secondary process, it consists solely in a chemical degradation (with a reaction order n = 1).
Conclusions
The paper describes the comparative thermal stability of prochlorperazine dimaleate (PCP) and promethazine hydrochloride (PMT), which are dopamine (D2) receptor antagonists.
The thermoanalytical curves of PCP and PMT obtained during heating at β = 5 °C min−1 in air atmosphere exhibit a multistage decomposition route. PCP shows a good thermal stability (up to 177 °C), when a decomposition process occurs (temperature range 177–225 °C, while the thermal profile of PMT reveals another pathway of decomposition, simpler than the one of PCP. This fact can be probably associated with the lack of organic salt co-former and the existence of a simpler salt, namely a hydrochloride. This salt is stable up to 192 °C, when a decomposition process begins.
The results obtained by means of thermal analysis were also confirmed by the kinetic study which, by the employment of apparent activation energy values, suggests a better stability of PCP dimaleate in comparison with PMT hydrochloride. Using structural means, this can be explained by the fact that in solid state, the maleate anion assures not only a salt-like structure as is the case of PMT hydrochloride, but also the possibility of H-bonding in the crystalline lattice as supplementary attractive force.
References
Yan MA, Zhu YG, Li HD, Chen BM, Ma N, Lei YQ, Liu YP. Quantification of prochlorperazine maleate in human plasma by liquid chromatography-mass spectrometry: application to a bioequivalence study. J Chromatogr, B: Anal Technol Biomed Life Sci. 2009;877(27):3243–7.
https://www.drugbank.ca/drugs/DB00433. Accessed 11 Nov 2017.
Nishimura M, Matsuura K, Tsukioka T, Yamashita H, Inagaki N, Sugiyama T, Itoh Y. In vitro and in vivo characteristics of prochlorperazine oral disintegrating film. Int J Pharm. 2009;368(1–2):98–102.
Trottier ED, Bailey B, Lucas N, Lortie A. Prochlorperazine in children with migraine: a look at its effectiveness and rate of akathisia. Am J Emerg Med. 2012;30(3):456–63.
Fitzgerald TJ, Bishop-jodoin M, Laurie F, Sacher A, Aghababian RV, Dickson E. Oncologic emergency medicine. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-26387-8.
Morgan RJ Jr, Synold T, Carr BI, Doroshow JH, Womack EP, Shibata S, Somlo G, Raschko J, Leong L, McNamara M, Chow W, Teref M, Margolin K, Akman S, Longmate J. Continuous infusion prochlorperazine: pharmacokinetics, antiemetic efficacy, and feasibility of high-dose therapy. Cancer Chemother Pharmacol. 2001;47(4):327–32.
Council of Europe. European Pharmacopoeia 7.0. Strasbourg: Council of Europe; 2010.
https://pubchem.ncbi.nlm.nih.gov/compound/prochlorperazine. Accessed 11 Nov 2017.
https://pubchem.ncbi.nlm.nih.gov/compound/Prochlorperazine_dimaleate. Accessed 11 Nov 2017.
Kopera J, Armitage AK. Comparison of some pharmacological properties of chlorpromazine, promethazine, and pethidine. Br J Pharmacol. 1954;9:392–401.
Adam K, Oswald I. The hypnotic effects of an antihistamine: promethazine. Br J Clin Pharmacol. 1986;22(6):715–7.
Deitrick CL, Mick DJ, Lauffer V, Prostka E, Nowak D, Ingersoll G. A comparison of two differing doses of promethazine for the treatment of postoperative nausea and vomiting. J PeriAnesthesia Nurs. 2015;30(1):5–13.
https://www.drugbank.ca/drugs/DB01069. Accessed 22 Oct 2017.
Lynch KL, Shapiro BJ, Coffa D, Novak SP, Kral AH. Promethazine use among chronic pain patients. Drug Alcohol Depend. 2015;150:92–7.
Page CB, Duffull SB, Whyte IM, Isbister GK. Promethazine overdose: clinical effects, predicting delirium and the effect of charcoal. QJM. 2009;102(2):123–31.
https://pubchem.ncbi.nlm.nih.gov/compound/promethazine#section=Top. Accessed 22 Oct 2017.
https://cameochemicals.noaa.gov/chemical/20957. Accessed 23 Oct 2017.
http://www.chemblink.com/products/58-33-3.htm. Accessed 23 Oct 2017.
Naqvi AZ, Khatoon S. Cloud point variation of amphiphilic drug promethazine hydrochloride with added surfactants. Colloids Surfaces B Biointerf. 2011;88(2):568–73.
Saleh TA, Abulkibash AM, Ibrahim AE. Portable system of programmable syringe pump with potentiometer for determination of promethazine in pharmaceutical applications. Saudi Pharm J. 2012;20(2):155–60.
Popovici AR, Vlase G, Vlase T, Suta LM, Popoiu C, Ledeti I, Iovanescu G, Fulias A. Local Anesthetic Agents: III. Study of solid dosage forms with pharmaceutical excipients. Rev Chim. 2015;66(7):1046–51.
Raica M, Cimpean AM, Popovici RA, Balica AR, Vladau M, Gaje PN. Mast cells stimulate lymphangiogenesis in the gingiva of patients with periodontal disease. Vivo (Brooklyn). 2015;29(1):29–34.
Trandafirescu C, Soica C, Ledeti A, Borcan F, Suta L-M, Murariu M, Dehelean C, Ionescu D, Ledeti I. Preformulation studies for Albendazole A DSC and FTIR analysis of binary mixtures with excipients. Rev Chim. 2016;67(3):463–7.
Karpinska J, Starczewska B, Puzanowska-Tarasiewicz H. Analytical properties of 2- and 10-disubstituted phenothiazine derivatives. Anal Sci. 1996;12(April):161–70.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci Part B Polym Lett. 1969;7(1):41–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2(3):301–24.
Flynn JH, Wall L. A quick direct method for determination of activation energy from thermogravimetric data. J Polym Sci, Part C: Polym Lett. 1966;4:323–8.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Akahira T, Sunose T. Research report, Trans joint convention of four electrical institutes. China Inst Technol (Sci Technol). 1971;16:22–31.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data. Thermochim Acta. 1998;316(1):37–45.
Sempere J, Nomen R, Serra R. Progress in non-parametric kinetics. J Therm Anal Calorim. 1999;56(2):843–9.
Sempere J, Nomen R, Serra R, Soravilla J. The NPK method. Thermochim Acta. 2002;388(1–2):407–14.
Vlase T, Vlase G, Doca N, Bolcu C. Processing of non-isothermal TG data: comparative kinetic analysis with NPK method. J Therm Anal Calorim. 2005;80:59–64.
Vlase T, Vlase G, Birta N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88(3):631–5.
Ledeti A, Vlase G, Vlase T, Circioban D, Dehelean C, Ledeti I. Kinetic study for solid-state degradation of mental disorder therapeutic agents. J Therm Anal Calorim. 2018;131(1):155–65.
Ledeti A, Olariu T, Caunii A, Vlase G, Circioban D, Baul B, Ledeti I, Vlase T, Murariu M. Evaluation of thermal stability and kinetic of degradation for levodopa in non-isothermal conditions. J Therm Anal Calorim. 2018;131(2):1881–8.
Wall ME, Rechtsteiner A, Rocha LM. Singular value decomposition and principal component analysis. In: Berrar DP, Dunitzky W, Granzow M, editors. A practical approach to microarray data analysis. Boston: Kluwer Academic Publishers; 2003. p. 91–109.
Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3(1):1–12.
Acknowledgements
This work was supported by a grant financed by the University of Medicine and Pharmacy “Victor Babeş” Timişoara (Grant PIII-C3-PCFI-2016/2017, acronym STONES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ledeti, I., Pusztai, A.M., Muresan, C.M. et al. Study of solid-state degradation of prochlorperazine and promethazine. J Therm Anal Calorim 134, 731–740 (2018). https://doi.org/10.1007/s10973-018-7566-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7566-3