Abstract
Preparation of nanofluid is of prime importance to obtain better thermal and physical properties. Different preparation parameters used in nanofluid preparation sometimes perform contrarily even if prepared with same nanoparticles and base fluid. Stability, thermal conductivity, and viscosity of the nanofluid are significantly affected by the cluster (agglomerate) size of nanoparticles in the base fluid which deteriorate thermal performance. In order to break the agglomerates and improve the dispersion of nanoparticles, ultrasonication is a more prevalent method. Nanofluids react differently for different sonication time and the reaction of the nanofluid with the change in sonication time varies for different nanofluids, which is dependent on various factors. In this regard, research works pertinent to the effect of ultrasonication on different properties of nanofluids are confined. In this paper, review of investigations carried out on experimentally evaluated ultrasonication effects on thermal properties and various physical properties of nanofluid. It is found that with an increased sonication time/energy, reduces the particle size and thus aids in obtaining a better dispersion leading to enhancement of stability, thermal conductivity and reducing viscosity. However, the longer ultrasonication duration was not found to be better in all cases where best performance was obtained for an optimum duration of ultrasonication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanofluids consist of particles that are of nanometer size known as nanoparticles dispersed in the fluid. These nanoparticles are generally metals, carbides, oxides, carbon nanotubes etc. [1,2,3,4]. Water, glycerol, oil, and ethylene glycol (EG) have been used as base fluids to suspend these nanoparticles [5,6,7,8,9]. Nanofluids have potential applications in electronics cooling system, fuel cells, heat exchangers, refrigeration systems, pharmaceutical processes, solar collectors, chillers, machines for temperature management and many others [10,11,12,13]. Uniform distribution of nanoparticles in the base fluid is a critical factor in deciding the behavior of nanofluid. Nanoparticles tend to agglomerate and form clusters in the base fluid, which is a setback in the performance of the nanofluids. This is observed due to high surface energy of nanoparticles that leads to sedimentation causing a reduction in performance and further affecting its thermophysical properties like viscosity, thermal conductivity, and pressure drop. [14,15,16,17].
Therefore, to reduce the agglomeration and sedimentation of nanoparticles, they must be properly dispersed and stabilized. Stability of a nanofluid is the main concern before preparing it. The stability of the nanofluid is the ability of nanoparticle to stay dispersed in the base fluid without forming any clusters. Stability of a nanofluid depends on various parameters such as particle size, surfactant used, sonication time, volume concentration, and type of sonication (pulse or nonplused). [18,19,20,21,22,23]. Zeta potential gives a measure of effective electric charge on the surface of the nanoparticle suspended in any fluid. The magnitude of the zeta potential provides information about particle stability. Particles with higher magnitude zeta potentials exhibit increased stability due to a larger electrostatic repulsion between particles [24,25,26,27,28]. The higher agglomeration size leads to change in density and they tend to settle down hence reducing stability. Agglomeration also effects thermal conductivity of the nanofluid. Therefore, the agglomeration is to be subdued as much as possible [29,30,31,32,33,34,35].
Sonication of nanofluid is achieved by providing sound energy to agitate the nanoparticles in the suspension. Sonication breaks the nanoparticles in the base fluid to obtain more uniform finer-sized particles. More than 20 kHz of ultrasonic frequencies are applied to the solution leading to the commonly known process as ultrasonication [36,37,38]. Bath-type and probe-type ultrasonicators are employed nowadays. Probe type is found to be more efficient than bath-type sonicator due to its high intensity of sonication [39,40,41,42]. The stability of a nanofluid can be improved by using surfactants. Again, stability is also dependent on surfactant type and its concentration. It is always a challenge to obtain a low viscous fluid with better dispersion. As the dispersion is better the more the viscous the fluid becomes. In most cases, use of surfactant increases the viscosity of nanofluid. However, the effect of surfactant is out of the scope of this study to make the review focused on ultrasonication effect only. Nevertheless, the effects of surfactants in conjunction with ultrasonication time/energy are considered here.
Various scientific instruments and machines are used by the researchers to study the stability, particle distribution, cluster size of the nanoparticles in the base fluid after the preparation of nanofluid. Some of the largely used devices include X-ray powder diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, field emission scanning electron microscopy (FESEM), thermogravimetry analysis (TGA), Brunauer–Emmett–Teller (BET) surface area analysis, chemical analysis (elemental analysis), Raman spectrum, laser granulometry, transmission electron microscope (TEM), UV–Vis spectrometer, etc. [31, 42,43,44,45,46,47,48,49,50,51].
There is no concrete solution on how much ultrasonication time/energy is required to homogenize a suspension to get better and prolong stability. One of the approaches now being recently practiced to enhance stability is by ultrasonicating the nanofluid for longer durations. However, some researchers obtained better stability after a certain duration of ultrasonication and after which stability parameters were found to be reduced. Many studies are reported in literature in which effect of ultrasonication time on the thermal, physical and chemical properties of nanoparticles are experimented [22, 41, 43, 44, 52,53,54,55,56,57,58,59,60,61]. Hence this article is intended to review the same. Various ultrasonication techniques are used to stabilize the nanoparticles dispersed in the nanofluids. Among the nanoparticles studied to enhance the stability and other properties include Al2O3 (alumina), TiO2 (titania), carbon nanotubes (CNTs) and few others. TiO2 and Al2O3 are most widely investigated nanoparticles as they have a huge application ranging from solar heaters to food industry [15, 16, 62]. The following sections include information of the nanofluid preparation methods, experimental works about ultrasonication effect on some common nanofluids. Each section contains information about the effect of ultrasonication on colloidal dispersion and thermophysical properties of that type of nanofluids. The outline of this review is expressed in Fig. 1.
Preparation of nanofluids
Stable nanofluid preparation is the one of the major step and key issue in assessing the thermophysical properties of nanofluids for any application. Wu et al. [63] described the nanofluids preparation aspects by three different methods: kinetic stability, dispersion stability, and chemical stability. The determination of nanoparticles and right base fluid will help to get rid of chemical stability for the working environment. Henceforth, these all parameter should be considered for the preparation of nanofluids to obtain good stability by reducing the expansion of nanoparticles inside the base fluid and avoid the sedimentation for longer durations.
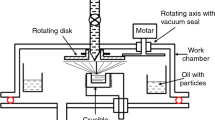
Preparation of nanofluids is not as straightforward as adding nanoparticles into base liquid. There are two distinct systems primarily utilized for combining nanoparticles into a base fluid: single-step process and two-step method. In single-step method, the nanoparticles are directly dispersed and condensed in the based fluid solution at a single/one time [20]. Normally, physical vapor deposition (PVD) procedure/fluid compound technique/VEROS (vacuum evaporation to a running oil substrate) is utilized for single-step processing technique. This technique has favorable circumstances, for example, stability increment and limited agglomeration.
Figure 2 illustrates the two-step method which is generally adopted for the preparation of graphite nanofluids. In a two-step method, nanoparticles are at first arranged and then dispersed into the fluid medium by ultrasonication or other processes. Nowadays, accessibility of nanoparticles from various sources makes two-step method genuinely appealing to the researchers. Two-step method functions efficiently for different oxide nanoparticles. A large number of investigators used two-step dispersion technique to get proper and homogeneous mixture and stability of nanofluids [63,64,65,66,67,68,69,70].

(Reprinted from [20], copyright (2017) with permission from Elsevier)
Two-step method of nanofluids preparation.
An ultrasonicator is used to prepare nanofluid by ultrasonication to disperse nanoparticles in the base fluid. For nanofluids prepared by any method are initially calculated for volume fraction (φ) of nanoparticles by,
where wn is the weight of nanoparticle, ρn is the density of nanoparticles, wb and ρb are weight and density of base fluid, respectively.
Effect of ultrasonication on oxide nanofluids
Effect of ultrasonication on Al2O3 nanofluids
The effect of ultrasonication duration on the stability (as zeta potential) of Al2O3–water nanofluid was studied in [50] and found zeta potential to increase with ultrasonication time and reached a maximum at 5 h of duration and later reduced. Nguyen et al. [71] investigated the effect of dispersion stability and ultrasonication on the cluster size of alumina nanofluid. The increased ultrasonication time on suspended nanoparticles in aqueous solution could lead to nanoparticle re-agglomeration. In work [52], the ultrasonication effect on Al2O3–W nanofluid was analyzed. Their results showed that the dispersion was dependent on ultrasonication time. Sufficient sonication time aided in obtaining better stability and less agglomeration, insufficient sonication time lead to lower stability and agglomeration. The results also show that sedimentation % of nanoparticles was more for nanofluid prepared by longer storage duration. The particle size diameter reduced with the increase in ultrasonication duration.
Another research study [72] carried out on properties of Al2O3–W nanofluids for ultrasonication with two types of pulses and found that continuous pulses developed more stable solution than discontinuous pulses for similar sonication time. The effect of ultrasonication duration on colloidal structure and viscosity of alumina–water nanofluid was investigated by [39]. The effect of sonication time on colloidal structure, stability and temperature-dependent viscosity of nanofluids were the main focus of the study [39]. Cluster size or agglomeration size of the nanoparticles in the nanofluids is high before sonication and is dispersed after ultrasonication for specific time as shown in Fig. 3. Colloidal dispersion was not uniform till 150 min of sonication but was more uniform for 180 min of ultrasonication as shown in Fig. 4 [39]. In another study focused to find the effective ultrasonication process for better colloidal dispersion of nanofluid. The main aim of this research is to get a better stable nanofluid. The samples were sonicated for 1–5 h. The particle size distribution analyses showed that the agglomerated size was decreased with increase in sonication duration, the sample sonicated at 50% amplitude had a better zeta potential and the same are shown in Fig. 5 [40]. However, erosion of the sonicator tip was observed when the sonication was carried out 5 h with 50% amplitude, which can be seen in Fig. 6.

(Reprinted from [39])
Al2O3 nanoparticles as observed in FESEM i 10 μm scale and ii 1 μm.

(Reprinted from [39])
Al2O3–W nanofluid microstructure captured by TEM after sonicating for a 30 min, b 60 min, c 90 min, d 120 min, e 150 min and f 180 min.

(Reprinted from [40], copyright (2015) with permission from Elsevier)
Variation in properties of Al2O3–W nanofluid for different ultrasonication duration a average cluster size and b zeta potential.

(Reprinted from [40], copyright (2015) with permission from Elsevier)
TEM images of Al2O3–water nanofluid samples after 5 h of ultrasonication.
Adio et al. [51] noted the influence of ultrasonication energy on the dispersion consistency of aluminum oxide–glycerol nanofluid based on viscosity data, and model development for the required ultrasonication energy density in this experiment. Using viscometer the optimum energy density necessary for preparing homogeneous nanofluids was found for all particle sizes. And the volume fraction by energy density model was derived using dimensionless analysis considering the interaction/binding energy in the base fluid. The stability of alumina nanofluid was studied by UV–vis spectrum in [73]. The results showed that increase in sonication time caused cluster size to reduce, whereas further sonication leads to constant cluster size. Effective thermal conductivity reduced as cluster size decreased and then improved. At higher temperature enhancement of effective thermal conductivity was higher as shown in Fig. 7 [73]. The study [39] showed that the higher relationship exists between the sonication and viscosity of the fluid. It was found that the maximum viscosity was found at 60 min of sonication and then it was found to lean toward the base fluid viscosity. Another study [70], investigated the effects of ultrasonication duration on colloidal dispersion and thermophysical properties of alumina–water nanofluids. The particle size distribution analysis showed that the decrease in cluster size was due to the increase in sonication time. Initially, the decrease was rapid but the absolute zeta potential was maximum at the sonication duration of 3 h. Viscosity and density also changed with the change in sonication time and temperature as shown in Fig. 8 [70]. The relation between yield stress and ultrasonication period of nanofluids are studied in [74] and found that non-Newtonian flow characteristics were noticed and also the yield stress was found to be decreased with the increase of the fluid temperature. The flow characteristics as flow behavior index and the shear stress at various shear rates were studied for different temperatures from 10 to 50 °C which are shown in Figs. 9 and 10, respectively. The consistency index, the yield stress and the flow behavior index were studied using the Herschel–Bulkley rheological model. It was noticed that the decrease of yield stress was rapid at the beginning of ultrasonication time and further the decrease was retarded [74]. Many more details related to alumina nanofluids are provided in Table 1.

(Reprinted with permission from [73]. Copyright © 2014, Springer-Verlag Berlin Heidelberg)
Thermal conductivity variation of alumina nanofluid for a vol% of 3 and b vol% of 2.

(Reprinted from [70], copyright (2015) with permission from Elsevier)
Effect on a viscosity and b density of Al2O3–W nanofluid for different ultrasonication duration and temperature.

(Reprinted from [74], copyright (2015) with permission from Elsevier)
Variation in shear stress of Al2O3–W nanofluid for different ultrasonication duration a 0 h, b 1 h, c 2 h and d 3 h.
Effect of ultrasonication on TiO2 nanofluids
Chen et al. [64] studied the rheological behavior of EG-based TiO2 nanofluid for different temperature and ultrasonication time. The results indicated that the nanofluids were Newtonian and the shear viscosity was found to be the strong function of the temperature and particle concentration. Average nanoparticle size reduced with the increase in ultrasonication time as can be seen in Fig. 11 [64]. An experimental investigation was done [75] on addition of Ag nanoparticles to TiO2 nanofluid to achieve recyclability of nanofluid and retract the nanoparticles from the used waste base fluid. Segregation and recycling of waste fluid by rapid settling of TiO2 using increasing Ag nanoparticles and sonication was achieved. It was found that the settling time reduced very much for a particular concentration of Ag nanoparticles and for a particular value of sonication time as shown in Fig. 12 [75]. In [76], the researchers prepared TiO2–W nanofluid by stirred bead milling and ultrasonication, and checked viscosity and thermal properties of the nanofluid. Dispersions of sub-micron agglomerations of lower viscosity and increased thermal conductivity were obtained with more ultrasonication duration. Particle size and viscosity reduced significantly for increased ultrasonication time, which are shown in Figs. 13 and 14, respectively.

(Reproduced from [64] by permission of IOP Publishing. CC BY-NC-SA)
Colloidal particle size decreasing with increasing ultrasonication time for TiO2–W nanofluid. © Deutsche Physikalische Gesellschaft.

(Reprinted from [75], copyright (2012) with permission from Elsevier)
Effect of ultrasonication duration on settling time of nanoparticles in the base fluid.

(Reprinted from [76], copyright (2012) with permission from Elsevier)
Effect of probe sonication time on particle size of TiO2–W nanofluid.

(Reprinted from [76], copyright (2012) with permission from Elsevier)
Reduction in viscosity due to increase in probe sonicating time of TiO2–W nanofluid.
The effect of adding TiO2 nanoparticles into distilled water with continuous sonication and without sonication was investigated in [77]. The experiment was conducted for laminar flow region up to 0.1, 0.15 and 0.25 of vol%. It was noticed that the heat transfer improved significantly for the vol% considered due to continuous sonication. Heat transfer improvement in terms of Nusselt number (Nu) for different ultrasonication duration is shown in Fig. 15 [77]. In another study [72] on ultrasonic properties of TiO2–W found similar effects of obtaining more stable nanofluid by using continuous pulses sonication [72]. An experimental study on the significance of ultrasonic processing and surfactant on thermal conductivity, viscosity, and stability of Titania nanofluid (TiO2 nanoparticles of 25 nm diameter) is reported in [78]. They found that the addition of surfactant is beneficial to obtain highly stable nanofluid along with improved thermal conductivity [78]. Box–Behnken model was utilized in [79] to investigate thermal conductivity and stability of TiO2 (25 nm) nanofluid. Their result revealed that the nanofluids became stable and by low power and with lower sonication period for low concentrations with no change in thermal conductivity. Whereas, high thermal conductivity (Fig. 16) and stability of nanofluids coincided at 1 vol%, i.e., at higher concentrations [79].

(Reprinted from [77], copyright (2013) with permission from Elsevier)
Ultrasonication effect on local Nusselt number (Nu) for different vol% of nanoparticles at a, b different axial distance and c, d different Reynolds number (Re).

(Reprinted with permission from [79]. Copyright © 2012, Springer Science + Business Media Dordrecht)
Contour plots of TiO2–W nanofluid thermal conductivity at different sonication time and energy for a 0.1 vol%, b 0.55 vol% and c 1 vol%.
Leena and Srinivasan synthesized TiO2–W nanofluid using sol–gel method and carried out ultrasonic investigation on the same. They observed an increase in ultrasonic velocity of the nanoparticles during ultrasonication due to surface effect. This surface effect is nothing but the hydrogen bonding between the base fluid molecules and TiO2 nanoparticles [41]. Ultrasonication effect on thermal conductivity of synthesized TiO2 nanofluid with different base fluids using sol–gel method was determined experimentally and theoretically in [54].
Ethylene glycol, paraffin oil and water were the base fluids used to disperse TiO2 nanoparticle. Effect of sonication on different concentrations (3–6%) of nanoparticle was also performed. They found a pronounced effect of sonication on thermal conductivity of the prepared nanofluid. Till 60 min of sonication time, the thermal conductivity of nanofluids increased and there on decreased. Intermolecular force and Brownian motion increased after 60 min of sonication which leads to clustering of nanoparticles and intern reducing the surface area. This caused a decrease in thermal conductivity of the nanofluids. However, water based nanofluid showed more improvement in thermal conductivity till 60 min of sonication. Thermal conductivity of all the nanofluids was also more with increasing concentration of nanoparticles from 3 to 6% [54]. The optimum ultrasonication time for stability and better dispersion of TiO2 nanofluid was studied by [80] as shown in Fig. 17. They learnt that the nanoparticles re-agglomerated after 150 min of ultrasonication and the ultrasonication time required to get optimum dispersion is a multivariate parameter dependent on ultrasonic treatment, type and characteristics of nanofluid. The stability of the nanofluid was checked through zeta potential factor and compared the results with [39, 73], as shown in Fig. 18 [80]. Table 2 gives the various details like vol%, sonication time, temperature range, parameters studied, etc., of research works carried out on TiO2 based nanofluids.

(Reprinted from [80], copyright (2013) with permission from Elsevier)
Microstructure variation of TiO2–W nanofluid as seen in TEM for different sonication time.

(Reprinted from [80], copyright (2013) with permission from Elsevier)
Zeta potential of TiO2–W nanofluid for different sonication time.
Effect of ultrasonication on ZnO nanofluids
Chung et al. [81] synthesized ZnO–W nanofluid of varying purity by different preparation methods and checked the effectiveness of dispersion. Their result showed that ultrasonic horn was effective in reducing particle size, sedimentation rate and in obtaining minimum achievable size than other types of sonication method [81]. Effect of ultrasonication on thermal conductivity of ZnO–EG nanofluids was studied in [82]. They observed that excess sonication broke the particles into finer segments and better dispersion of ZnO, as observed by light scattering intensity % and TEM images of the nanofluid, which are shown in Figs. 19 and 20, respectively. Variation in thermal conductivity at different sonication hours obtained is shown in Fig. 21 [82]. More details of investigations carried out related to ZnO nanofluids are presented in Table 3.

(Reprinted from [82], copyright (2013) with permission from Elsevier)
Dynamic light scattering intensity % of ZnO nanofluid for different ultrasonication durations.

(Reprinted from [82], copyright (2012) with permission from Elsevier)
1 vol% of ZnO–EG nanofluid in TEM images after ultrasonicating for a 4 h, b 12 h, c 60 h and d 100 h.

(Reprinted from [82], copyright (2012) with permission from Elsevier)
Variation in thermal conductivity of 3.75% ZnO–EG nanofluid for different sonication time at 30 °C temperature.
Effect of ultrasonication on other oxide based nanofluids
A work on copper oxide nanoparticles (10–30 nm) dispersed in ethylene glycol and experimented for viscosity and thermal conductivity is reported in [83]. TEM images showed prolate spheroid shaped particles having an aspect ratio of 3, and despite of sonicating for a long time particles were still in aggregated state. Thermal conductivity increased only when the particle concentration was under dilute limit [83]. A study on agglomeration and stability of Silica–W nanofluid prepared using ultrasonic probe is found in [56]. Necessary ultrasonication time required to completely disperse the nanoparticles in the base fluid was 5 min at a pH of 10. Asadi et al. [84] experimented the effect of sonication time and surfactant on stability and thermal conductivity of Mg(OH)2 nanoparticle for varying solid concentration, sonication time and for different surfactant. They realized that cetyl trimenthyl ammonium bromide (CTAB) surfactant improved stability compared with other surfactants. The zeta potential of the nanofluid with surfactant after a week is shown in Fig. 22 and relative thermal conductivity for different sonication time is shown in Fig. 23 [84]. Table 4 gives studies carried out on different nanofluids and details are mentioned in different columns.

(Reprinted from [84], copyright (2016) with permission from Elsevier)
Variation in zeta potential for different sonication time and at different days of Mg(OH)2–W nanofluids.

(Reprinted from [84], copyright (2016) with permission from Elsevier)
Variation in relative thermal conductivity for different sonication time and at different solid concentrations of Mg(OH)2–W nanofluids.
Effect of ultrasonication on CNT nanofluids
Yang et al. [85] investigated the rheological and thermal properties of MWCNTs dispersed in olefin oil by varying sonication energy and time. They found that the nanoparticle agglomeration and its colloidal suspension has major effect on heat transfer characteristics of the nanofluid. If the agglomeration of particle is high, it leads to increased thermal conductivity and more viscosity [85]. Effect of sonication time on the dispersion of MWCNT in water and factors which optimize the efficiency were studied using TEM and UV–vis in [86]. MWCNTs dispersed to its maximum when stabilized by SDS for a certain amount of sonication energy. The adsorbed SDS molecules on the surface of MWCNTs prevented agglomeration maintaining the colloidal stability for few months which were seen through TEM images as shown in Fig. 24 [86]. In [87], the investigation was done on influence of ultrasonication time, temperature and volume fraction on thermo-physical properties of carbon nanofluid using thin layer technique. They identified that the clustering in CNT is lesser than any other suspensions for same vol% and higher sonication time thus, increasing thermal conductivity and setting time of nanoparticles [87].

(Reprinted from [86], copyright (2006) with permission from Elsevier)
TEM images of MWCNT nanoparticles treated with SDS surfactant for a 5 min of sonication and b 90 min of sonication.
Garg et al. [88] experimentally analyzed the effect of ultrasonication on various thermal properties of 1.0 vol% of Multi-Walled Carbon Nanotubes (MWCNT). Power law viscosity model was used to discuss the shear thinning effect. They varied the time period of ultrasonication, the maximum improvement in thermal conductivity and convective heat transfer was found to be 20 and 32%, respectively. Thermal conductivity increased considerably above 24 °C up to some optimum time and deprived on increasing ultrasonication time further. Figure 25 shows the microstructure of MWCNT suspension in which 80 min of ultrasonication breaks the CNTs finely. The viscosity of nanofluid prepared by various sonication time was studied at different temperature and highest viscosity was observed for the nanofluid prepared by 40 min of ultrasonication, whereas flow behavior index remains almost constant as shown in Fig. 26 [88]. Dispersion behavior of SWCNTs using different dispersants (o-DCB and DMF) and due to the variation of sonication time from 20 to 220 s is reported in [89]. Sonication time improved the dispersion of SWCNT in the presence of both solvents. They found that the debundling and dispersion of SWCNT in these solvents was critically dependent on the ultrasonication process and was highly dependent on many of the parameters of the base fluid, including the vapor pressure, viscosity, surface tension, density and molecular weight. This process is mainly guided by minimization of sonication requirements. Effect of ultrasonication on aggregation factors was also studied in [89] as shown in Fig. 27.

(Reprinted from [88], copyright (2009) with permission from Elsevier)
MWCNT nanoparticles TEM images when ultrasonicated for a 20 min, b 40 min, c 60 min and d 80 min.

(Reprinted from [88], copyright (2009) with permission from Elsevier)
Effect of different parameters on properties of MWCNT–W nanofluid when ultrasonicated for different time, at different temperature, GA surfactant and DI water. a Viscosity vs shear rate at 15 °C, b viscosity vs shear rate at 30 °C. At different ultrasonication time c flow consistency index and d flow behavior index.

(Reprinted with permission from [89]. Copyright (2009) American Chemical Society)
At different ultrasonication time, effect of concentration on aggregation fractions (Xagg) of SWCNTs.
Meibodi et al. [47] studied the prominence of various parameters on thermal conductivity and stability of carbon nanotubes. They found that the more stable solution might not have higher thermal conductivity value and thermal conductivity of nanofluid is dependent on time soon after sonication, whereas independent of time at longer durations [47].
Nasiri et al. [90] researched on the outcome of ultrasonication dispersion method on thermal conductivity and stability of SWCNT, Double Wall CNT (DWCNT), Few Wall CNT (FWCNT) and two different MWCNT nanofluid. The experiment showed that the functionalized nanofluid has best stability and thermal conductivity compared to nanofluid prepared by ultrasonic bath and probe method (refer Fig. 28). After 50 h, the functionalized profile began to level due to stability, while other two profiles declined [90]. Colloidal dispersion of SWCNT in water using sodium deoxycholate as a surfactant for different sonicator parameters was studied by Yu et al. [91]. Sonication power was found to largely influence dispersion of SWCNT than sonication time on the resonance ratio. Ultrasonication was found to be more effective in removing bundles of SWCNT to obtain homogeneous solution. Figure 29 shows variation in count (%) for change in nanoparticle length and diameter for different ultrasonication time [91].

(Reprinted from [90], copyright (2009) with permission from Elsevier)
Variation of effective thermal conductivity (Keff) for change in temperature of functionalized nanofluid and nanofluid prepared by ultrasonic bath and probe a SWNTs, b DWNTs, c MWNTs1 and d MWNTs2.

(Reprinted from [91], copyright (2012) with permission from Elsevier)
a Diameter and b length statistics of SWCNTs analyzed using histogram for different ultrasonication duration in minutes.
Ruan and Jacobi [92] experimented the ultrasonication effects on rheological and thermal properties of MWCNT nanofluid. They investigated the effects macroscopically and microscopically. With sonication specific energy input, thermal conductivity increased non-linearly. Viscosity of nanofluid decreased with increase in shear rate. Using GA as a surfactant and sonicating for 120 min was found to be more stabilizing than without sonication [92]. Another study [48] was performed on the investigation of high energy probe sonication effect on thermoelectric power of MWCNT having larger diameter obtained by chemical vapor deposition with hydrogen/argon mixture carrier gas, liquid carbon precursor, and iron catalyst particles. The size of the nanotubes shortened, thus reducing electrical conductivity (Fig. 30) due to over sonication time [48]. In [59], the effect of sonication time on MWCNT–Epoxy resin was studied. It was observed that increasing the sonication time from 20 to 30 min showed improved electrical conductivity and strength of the nanocomposite [59]. Montazeri and Chitsazzadeh [93] studied the effect of sonication parameter on mechanical properties of MWCNT–Epoxy resin nanocomposite. Dynamic mechanical thermal analysis (DMTA) and tensile test were conducted under different dispersion state. The result showed that the increase in the sonication time increased the Young’s modulus of MWCNT [93]. Investigations pertinent to CNT nanofluids mentioned in this section are also mentioned in Table 5, which provides minor details of the studies.

(Reprinted from [48], copyright (2012) with permission from Elsevier)
Electrical conductivity (σ) of MWCNTs dispersed in Deionized water (DI H2O) for different ultrasonication time a MWCNT synthesized, b MWCNT raw, c Seebeck coefficient of MWCNT synthesized and d Seebeck coefficient of MWCNT raw.
Conclusions and future remarks
-
Ultrasonication time has twofold effect on the nanofluids. At the optimum processing time, the ultrasonication aids in forming better dispersions, however, once the optimum time has been reached further ultrasonication results in an re-agglomeration.
-
The optimum ultrasonication time is dependent on sonicator power, frequency used, volume concentration, nanomaterial type, base fluid, ultrasonicator type, etc.
-
It is also observed that the sonication process not only reduces the agglomerate sizes but also decreases the size of the nanoparticle.
-
Initially, at the beginning of ultrasonication, thermal conductivity of nanofluids were found to be decreased and further ultrasonication, the thermal conductivity of the nanofluids increases for a certain optimum time depending on nanofluid.
-
In the case of viscosity two types of trends were observed where one trend is that viscosity of nanofluid was decreased with increasing ultrasonication and other trend is the viscosity of nanofluids increases to the maximum for certain ultrasonication time then decreases, finally approaching the viscosity of the pure base fluid.
-
Density of nanofluids was found to be increased with increasing ultrasonication durations.
-
The stability of the nanofluid also depends on type of surfactant used. Surfactant used are usually nanomaterial specific. The concentration of surfactant used is also of concern. Using lesser concentration may lead to lower stability and over use may increase viscosity and thus increasing pressure drop. The stability of the nanofluid with surfactant is very much enhanced in comparison with the nanofluid without surfactants. With surfactant the nanofluids achieved stability for over months.
Following are some remarks for future work:
-
It is necessary to consider as many sonication parameters as possible like durations, amplitudes, pulses, probe tip diameter, and sonication types.
-
Effect of ultrasonication is needed to be considered with the change of the size, type, and concentration of particles in different base fluids.
-
Surfactants also play some role in preparation and properties of nanofluids. Therefore, the effect of surfactant and temperature could be another review topic.
-
Change of pH with and without using surfactants is another important research area.
Abbreviations
- Al2O3 :
-
Alumina
- CTAB:
-
Cetyl trimethyl ammonium bromide
- CNT:
-
Carbon nanotube
- DMF:
-
Dimethylformamide
- DW:
-
Double walled
- EG:
-
Ethylene glycol
- FW:
-
Few walled
- FE:
-
Field emission
- GNP:
-
Graphene nanopowder
- GA:
-
Gum arabic
- MW:
-
Multi-walled
- Mg(OH)2 :
-
Magnesium hydroxide
- o-DCB:
-
Ortho-dichlorobenzene
- PU:
-
Poly-urethane
- SDS:
-
Sodium dodecyl sulfate
- SW:
-
Single walled
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscope
- TiO2 :
-
Titania
- Vol%:
-
Volume concentration percentage
- W:
-
Water
- XRD:
-
X-ray powder diffraction
- ZnO:
-
Zinc oxide
References
Saidur R, Leong KY, Mohammad HA. A review on applications and challenges of nanofluids. Renew Sustain Energy Rev. 2011;15(3):1646–68.
Sohel MR, Saidur R, Khaleduzzaman SS, Ibrahim TA. Cooling performance investigation of electronics cooling system using Al2O3–H2O nanofluid. Int Commun Heat Mass Transf. 2015;65:89–93.
Sohel MR, Khaleduzzaman SS, Saidur R, Hepbasli A, Sabri MFM, Mahbubul IM. An experimental investigation of heat transfer enhancement of a minichannel heat sink using Al2O3–H2O nanofluid. Int J Heat Mass Transf. 2014;74:164–72.
Sohel MR, Saidur R, Hassan NH, Elias MM, Khaleduzzaman SS, Mahbubul IM. Analysis of entropy generation using nanofluid flow through the circular microchannel and minichannel heat sink. Int Commun Heat Mass Transf. 2013;46:85–91.
Sundar LS, Ramana EV, Singh MK, Sousa AC. Thermal conductivity and viscosity of stabilized ethylene glycol and water mixture Al2O3 nanofluids for heat transfer applications: an experimental study. Int Commun Heat Mass Transf. 2014;56:86–95.
Thakur R. Experimental & CFD investigation of cooling performance of mini-channel heat sink using nanofluid (Al2O3–H2O). Patiala: Thapar University; 2015.
Zakaria I, et al. Thermal analysis of heat transfer enhancement and fluid flow for low concentration of Al2O3 water–ethylene glycol mixture nanofluid in a single PEMFC cooling plate, vol. 79. Amsterdam: Elsevier B.V.; 2015.
Selvakumar P, Suresh S. Thermal performance of ethylene glycol based nanofluids in an electronic heat sink. J Nanosci Nanotechnol. 2014;14(3):2325–33.
Nazari M, Karami M, Ashouri M. Comparing the thermal performance of water, ethylene glycol, alumina and CNT nanofluids in CPU cooling: experimental study. Exp Therm Fluid Sci. 2014;57(September):371–7.
Bobbo S, Fedele L, Fabrizio M, Barison S, Battiston S, Pagura C. Influence of nanoparticles dispersion in POE oils on lubricity and R134a solubility. Int J Refrig. 2010;33(6):1180–6.
Guo D, Xie G, Luo J. Mechanical properties of nanoparticles: basics and applications. J Phys D Appl Phys. 2014;47(1):13001.
Ingole S, Charanpahari A, Kakade A, Umare SS, Bhatt DV, Menghani J. Tribological behavior of nano TiO2 as an additive in base oil. Wear. 2013;301(1–2):776–85.
Afzal A, Samee ADM, Razak RKA. Experimental thermal investigation of CuO–W nanofluid in circular minichannel. Model Meas Control B. 2017;86(2):335–44.
Ahmad SHA, Saidur R, Mahbubul IM, Al-Sulaiman FA. Optical properties of various nanofluids used in solar collector: a review. Renew Sustain Energy Rev. 2017;73:1014–30.
Gorji TB, Ranjbar AA. A review on optical properties and application of nanofluids in direct absorption solar collectors (DASCs). Renew Sustain Energy Rev. 2017;72:10–32.
Sundar LS, Sharma KV, Singh MK, Sousa ACM. Hybrid nanofluids preparation, thermal properties, heat transfer and friction factor—a review. Renew Sustain Energy Rev. 2017;68:185–98.
Kumar M, Afzal A, Ramis MK. Investigation of physicochemical and tribological properties of Tio2 nano-lubricant oil of different concentrations. Tribol Finnish J Tribol. 2017;35(3):6–15.
Wu D, Zhu H, Wang L, Liu L. Critical issues in nanofluids preparation, characterization and thermal conductivity. Curr Nanosci. 2009;5:103–12.
Murshed SMS, Leong KC, Yang C. Enhanced thermal conductivity of TiO2–water based nanofluids. Int J Therm Sci. 2005;44(4):367–73.
Zhang Z, Cai J, Chen F, Li H, Zhang W, Qi W. Progress in enhancement of CO2 absorption by nanofluids: a mini review of mechanisms and current status. Renew Energy. 2018;118:527–35.
Nabeel Rashin M, Hemalatha J. Magnetic and ultrasonic studies on stable cobalt ferrite magnetic nanofluid. Ultrasonics. 2014;54(3):834–40.
Nabeel Rashin M, Hemalatha J. Magnetic and ultrasonic investigations on magnetite nanofluids. Ultrasonics. 2012;52(8):1024–9.
Nabeel Rashin M, Hemalatha J. A novel ultrasonic approach to determine thermal conductivity in CuO–ethylene glycol nanofluids. J Mol Liq. 2014;197:257–62.
Kamatchi R, Venkatachalapathy S. Parametric study of pool boiling heat transfer with nanofluids for the enhancement of critical heat flux: a review. Int J Therm Sci. 2015;87:228–40.
Ghadimi A, Saidur R, Metselaar HSC. A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf. 2011;54(17–18):4051–68.
Mehrali M, et al. Preparation, characterization, viscosity, and thermal conductivity of nitrogen-doped graphene aqueous nanofluids. J Mater Sci. 2014;49(20):7156–71.
Li Y, Zhou J, Tung S, Schneider E, Xi S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009;196(2):89–101.
Abareshi M, Goharshadi EK, Mojtaba Zebarjad S, Khandan Fadafan H, Youssefi A. Fabrication, characterization and measurement of thermal conductivity of Fe3O4 nanofluids. J Magn Magn Mater. 2010;322(24):3895–901.
Perez-Maqueda JP-RLA, Franco F. Comparative study of the sonication effect on the thermal behaviour of 1:1 and 2:1 aluminium phyllosilicate clays. J Eur Ceram Soc. 2005;25:1463–70.
Perez-Maqueda JLP-RLA, Blanes JM, Pascual Jose M. The influence of sonication on the thermal behavior of muscovite and biotite. J Eur Ceram Soc. 2004;24:2793–801.
Lam C, Lau K, Cheung H, Ling H. Effect of ultrasound sonication in nanoclay clusters of nanoclay/epoxy composites. Mater Lett. 2005;59:1369–72.
Özcan-tas NG, Padron G, Voelkel A, Square MS. Chemical engineering research and design effect of particle type on the mechanisms of break up of nanoscale particle clusters. Chem Eng Res Des. 2008;7:468–73.
Pandey DK, Yadawa PK, Yadav RR. Ultrasonic properties of hexagonal ZnS at nanoscale. Mater Lett. 2007;61(30):5194–8.
Poli AL, Batista T, Schmitt CC, Gessner F, Neumann MG. Effect of sonication on the particle size of montmorillonite clays. J Colloid Interface Sci. 2008;325:386–90.
Rossell MD, et al. Impact of sonication pretreatment on carbon nanotubes: a transmission electron microscopy study. Carbon. 2013;61:404–11.
Phuoc TX, Massoudi M, Chen RH. Viscosity and thermal conductivity of nanofluids containing multi-walled carbon nanotubes stabilized by chitosan. Int J Therm Sci. 2011;50(1):12–8.
Han ZH, Yang B, Kim SH, Zachariah MR. Application of hybrid sphere/carbon nanotube particles in nanofluids. Nanotechnology. 2007;18:4–7.
Sundar LS, Singh MK, Sousa ACM. Enhanced heat transfer and friction factor of MWCNT—Fe3O4/water hybrid nano fluids☆. Int Commun Heat Mass Transf. 2014;52:73–83.
Mahbubul IM, et al. Effect of ultrasonication duration on colloidal structure and viscosity of alumina–water nano fluid. Ind Eng Chem Res. 2014;53:6677–84.
Mahbubul IM, Saidur R, Amalina MA, Elcioglu EB, Okutucu-ozyurt T. Effective ultrasonication process for better colloidal dispersion of nanofluid. Ultrason Sonochem. 2015;26:361–9.
Leena M, Srinivasan S. Synthesis and ultrasonic investigations of titanium oxide nanofluids. J Mol Liq. 2015;206:103–9.
Lei B, Majumder K, Shen S, Wu J. Effect of sonication on thermolysin hydrolysis of ovotransferrin. Food Chem. 2011;124(3):808–15.
Ilyas SU, Pendyala R, Marneni N. Preparation, sedimentation, and agglomeration of nanofluids. Chem Eng Technol. 2014;37(12):2011–21.
Haitao ZHU, Changjiang LI, Daxiong WU, Canying Z, Yansheng YIN. Preparation, characterization, viscosity and thermal conductivity of CaCO3 aqueous nanofluids. Technol Sci. 2010;53(2):360–8.
Show K, Mao T, Lee D. Optimisation of sludge disruption by sonication. Water Res. 2007;41:4741–7.
Siddiqui SW, Unwin PJ, Xu Z, Kresta SM. The effect of stabilizer addition and sonication on nanoparticle agglomeration in a confined impinging jet reactor. Colloids Surf A Physicochem Eng Asp. 2009;350:38–50.
Emami M, Vafaie-sefti M, Morad A, Amrollahi A, Tabasi M, Sid H. The role of different parameters on the stability and thermal conductivity of carbon nanotube/water nano fluids. Int Commun Heat Mass Transf. 2010;37(3):319–23.
Hewitt CA, Craps M, Czerw R, Carroll DL. The effects of high energy probe sonication on the thermoelectric power of large diameter multiwalled carbon nanotubes synthesized by chemical vapor deposition. Synth Met. 2013;184:68–72.
Kabir E, Saha MC, Jeelani S. Effect of ultrasound sonication in carbon nanofibers/polyurethane foam composite. Mater Sci Eng A. 2007;459:111–6.
Lee J, et al. Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf. 2008;51:2651–6.
Adio SA, Sharifpur M, Meyer JP. Influence of ultrasonication energy on the dispersion consistency of Al2O3—glycerol nanofluid based on viscosity data, and model development for the required ultrasonication energy density. J Exp Nanosci. 2015;11(8):630–49.
Nine MJ, Rehman H, Chung H-S, Bae K, Jeong H-M. Effect of ultrasonic action on Al2O3/water dispersion and thermal characterization with convective heat transfer. Nanosci Nanotechnol Lett. 2012;4(8):827–34.
Sakthipandi K, Rajendran V, Jayakumar T, Raj B, Kulandivelu P. Synthesis and on-line ultrasonic characterisation of bulk and nanocrystalline La0.68Sr0.32MnO3 perovskite manganite. J Alloys Compd. 2011;509(8):3457–67.
Sonawane SS, Khedkar RS, Wasewar KL. Effect of sonication time on enhancement of effective thermal conductivity of nano TiO2–water, ethylene glycol, and paraffin oil nanofluids and models comparisons. J Exp Nanosci. 2015;10(4):310–22.
Chakraborty S, Saha SK, Pandey JC, Das S. Experimental characterization of concentration of nanofluid by ultrasonic technique. Powder Technol. 2011;210(3):304–7.
Mondragon R, Julia JE, Barba A, Jarque JC. Characterization of silica-water nanofluids dispersed with an ultrasound probe: a study of their physical properties and stability. Powder Technol. 2012;224:138–46.
Paul G, Philip J, Raj B, Das PK, Manna I. Synthesis, characterization, and thermal property measurement of nano-Al95Zn05 dispersed nanofluid prepared by a two-step process. Int J Heat Mass Transf. 2011;54(15–16):3783–8.
Buonomo B, Manca O, Marinelli L, Nardini S. Effect of temperature and sonication time on nanofluid thermal conductivity measurements by nano-flash method. Appl Therm Eng. 2015;91:181–90.
Ghaleb ZA, Mariatti M, Ariff ZM. Properties of graphene nanopowder and multi-walled carbon nanotube-filled epoxy thin-film nanocomposites for electronic applications: the effect of sonication time and filler loading. Compos Part A Appl Sci Manuf. 2014;58:77–83.
Zhang G, Wan T. Sludge conditioning by sonication and sonication-chemical methods. Procedia Environ Sci. 2012;16:368–77.
Zhang G, Zhang P, Yang J, Liu H. Bioresource technology energy-efficient sludge sonication: power and sludge characteristics. Bioresour Technol. 2008;99:9029–31.
Khurana D, Choudhary R, Subudhi S. A critical review of forced convection heat transfer and pressure drop of Al2O3, TiO2 and CuO nanofluids. Heat Mass Transf. 2016;53(1):343–61.
Wu D, Zhu H, Wang L, Liu L. Critical issues in nanofluids preparation, characterization conductivity. Curr Nanosci. 2009;5:103–12.
Chen H, Ding Y, Tan C. Rheological behaviour of nanofluids. New J Phys. 2007;9:367.
Kole M, Dey TK. Viscosity of alumina nanoparticles dispersed in car engine coolant. Exp Therm Fluid Sci. 2010;34(6):677–83.
Hojjat M, Etemad SG, Bagheri R, Thibault J. Rheological characteristics of non-Newtonian nanofluids: experimental investigation. Int Commun Heat Mass Transf. 2011;38(2):144–8.
Duan F, Wong TF, Crivoi A. Dynamic viscosity measurement in non-Newtonian graphite nanofluids. Nanoscale Res Lett. 2012;7(1):360.
Sidik NAC, Mohammed HA, Alawi OA, Samion S. A review on preparation methods and challenges of nanofluids. Int Commun Heat Mass Transf. 2014;54:115–25.
Paramashivaiah BM, Rajashekhar CR. Studies on effect of various surfactants on stable dispersion of graphene nano particles in simarouba biodiesel. IOP Conf Ser Mater Sci Eng. 2016;149:12083.
Mahbubul IM, Shahrul IM, Khaleduzzaman SS, Saidur R, Amalina MA, Turgut A. Experimental investigation on effect of ultrasonication duration on colloidal dispersion and thermophysical properties of alumina-water nanofluid. Int J Heat Mass Transf. 2015;88:73–81.
Nguyen VS, Rouxel D, Hadji R, Vincent B, Fort Y. Effect of ultrasonication and dispersion stability on the cluster size of alumina nanoscale particles in aqueous solutions. Ultrason Sonochem. 2011;18(1):382–8.
Tajik B, Abbassi A, Saffar-Avval M, Najafabadi MA. Ultrasonic properties of suspensions of TiO2 and Al2O3 nanoparticles in water. Powder Technol. 2012;217:171–6.
Sadeghi MHR, Etemad SGh, Keshavarzi E. Investigation of alumina nanofluid stability by UV–vis spectrum. Microfluid Nanofluidics. 2015;18(5–6):1023–30.
Mahbubul IM, Saidur R, Hepbasli A, Amalina MA. Experimental investigation of the relation between yield stress and ultrasonication period of nanofluid. Int J Heat Mass Transf. 2016;93:1169–74.
Chakraborty S, Mukherjee J, Manna M, Ghosh P, Das S, Denys MB. Effect of Ag nanoparticle addition and ultrasonic treatment on a stable TiO2 nanofluid. Ultrason Sonochem. 2012;19(5):1044–50.
Silambarasan M, Manikandan S, Rajan KS. Viscosity and thermal conductivity of dispersions of sub-micron TiO2 particles in water prepared by stirred bead milling and ultrasonication. Int J Heat Mass Transf. 2012;55(25–26):7991–8002.
Rayatzadeh HR, Saffar-avval M, Mansourkiaei M, Abbassi A. Effects of continuous sonication on laminar convective heat transfer inside a tube using water–TiO2 nanofluid. Exp Therm Fluid Sci. 2013;48:8–14.
Ghadimi A, Metselaar IH. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp Therm Fluid Sci. 2013;51:1–9.
Lotfizadehdehkordi B, Ghadimi A, Metselaar HSC. Box–Behnken experimental design for investigation of stability and thermal conductivity of TiO2 nanofluids. J Nanopart Res. 2013;15:1369–78.
Mahbubul IM, Elcioglu EB, Saidur R, Amalina MA. Optimization of ultrasonication period for better dispersion and stability of TiO2–water nanofluid. Ultrason Sonochem. 2017;37:360–7.
Chung SJ, et al. Characterization of ZnO nanoparticle suspension in water: effectiveness of ultrasonic dispersion. Powder Technol. 2009;194(1–2):75–80.
Kole M, Dey TK. Effect of prolonged ultrasonication on the thermal conductivity of ZnO–ethylene glycol nanofluids. Thermochim Acta. 2012;535:58–65.
Kwak K, Kim C. Viscosity and thermal conductivity of copper oxide nanofluid dispersed in ethylene glycol. Korea-Australia Rheol J. 2005;17(2):35–40.
Asadi A, Asadi M, Siahmargoi M, Asadi T, Gholami Andarati M. The effect of surfactant and sonication time on the stability and thermal conductivity of water-based nanofluid containing Mg(OH)2 nanoparticles: an experimental investigation. Int J Heat Mass Transf. 2017;108:191–8.
Yang Y, Grulke EA, Zhang ZG, Wu G, Yang Y, Grulke EA. Thermal and rheological properties of carbon nanotube-in-oil dispersions. J Appl Phys. 2006;99:114307-1–8.
Yu J, Grossiord N, Koning CE, Loos J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon. 2007;45:618–23.
Amrollahi A, Hamidi AA, Rashidi AM. The effects of temperature, volume fraction and vibration time on the thermo-physical properties of a carbon nanotube suspension (carbon nanofluid). Nanotechnology. 2008;19(31):315701.
Garg P, Alvarado JL, Marsh C, Carlson TA, Kessler DA. An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int J Heat Mass Transf. 2009;52(21–22):5090–101.
Cheng Q, Gregan E, Byrne H. Ultrasound-assisted SWNTs dispersion: effects of sonication parameters and solvent properties ultrasound-assisted SWNTs dispersion: effects of sonication parameters and solvent properties. J Phys Chem C. 2010;114(19):8821–7.
Nasiri A, Shariaty-Niasar M, Rashidi A, Amrollahi A, Khodafarin R. Effect of dispersion method on thermal conductivity and stability of nanofluid. Exp Therm Fluid Sci. 2011;35(4):717–23.
Yu H, Hermann S, Schulz SE, Gessner T, Dong Z, Li WJ. Optimizing sonication parameters for dispersion of single-walled carbon nanotubes. Chem Phys. 2012;408:11–6.
Ruan B, Jacobi AM. Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Res Lett. 2012;7(1):1–14.
Montazeri A, Chitsazzadeh M. Effect of sonication parameters on the mechanical properties of multi-walled carbon nanotube/epoxy composites. Mater Des. 2014;56:500–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afzal, A., Nawfal, I., Mahbubul, I.M. et al. An overview on the effect of ultrasonication duration on different properties of nanofluids. J Therm Anal Calorim 135, 393–418 (2019). https://doi.org/10.1007/s10973-018-7144-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7144-8