Abstract
In this paper, UV–vis spectrum method and dynamic light scattering (DLS) methods were used to evaluate the stability of γ-Al2O3 nanofluid. The results of DLS method indicate that zeta potential increases by increasing ultrasonic mixing time. After 150 min, zeta potential was 50 mV, which under these conditions the nanofluid was stable. The results also show that the size of the nanoclusters decreases with ultrasonic mixing time. The results obtained by UV–vis spectrum indicate that by increasing the ultrasonic mixing time, the value of maximum absorbency increases and after 30 min, it decreases gradually. After 150 min of ultrasonic mixing time, the maximum absorbency becomes constant. This mixing time can be considered as optimum mixing ultrasonic time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the recent decade, many studies in the field of heat transfer are related to the nanofluids as a new heat transfer media (Yu and Xie 2012). Thermal conductivity is one of the most important parameters affecting thermal behavior of nanofluids. Comparison between the results of related investigations available in the literature, emphasizes the existence of a great discrepancy on the available experimental data (Masuda et al. 1993; Eastman et al. 1997; Pak and Cho 1998; Lee et al. 1999; Xie et al. 2002; Putra et al. 2003; Chon et al. 2005; Li and Peterson 2006).
The stabilities of nanofluids and nanocluster size are affecting parameters on the thermal conductivity (Prasher et al. 2006; Hong and Kim 2012); however, in many published studies, these key parameters are ignored (Masuda et al. 1993; Eastman et al. 1997; Pak and Cho 1998; Lee et al. 1999; Xie et al. 2002; Putra et al. 2003; Chon et al. 2005; Li and Peterson 2006). The stability of nanofluids is defined as the resistance of nanoparticles against aggregation. Forces such as Van der Waals attraction cause aggregation which results in nanoclusters formation (Jiang et al. 2003; Hwang et al. 2007; Rao 2010; Xian-Ju et al. 2011; Yu and Xie 2012). As nanoclusters are formed, depending on their size, clusters are settled down in the nanofluid.
Hong and Kim (2012) have investigated the effects of nanoparticles aggregation in alumina/water nanofluid. They showed that the nanoparticles can be well dispersed, and the thermal conductivity is consistent with the Maxwell lower band. According to their results, aggregation of nanoparticles causes the enhancement of nanofluids thermal conductivity.
Shalkevich et al. (2010) have studied the effect of nanoparticles aggregation on the thermal conductivity of silica and alumina/water nanofluids. Their results were similar to the results of Hong and Kim (2012).
Thermal conductivity of Fe/water nanofluids was measured by Hong et al. (2006). They showed that nanofluid thermal conductivity decreases by increasing the ultrasonic mixing time. Their result showed that the thermal conductivity is reduced by nanoparticles aggregation.
Wu et al. (2010) have shown that the aggregation of silica nanoparticles has no effect on the thermal conductivity of nanofluids.
One of the most important parameters on nanoparticles aggregation and thermal conductivity of nanofluids is Brownian motion (Jang and Choi 2004; Azizian et al. 2012; Prasher 2005; Wang et al. 2012).
Brownian motion dependents on the temperature and size of nanoparticles and nanoclusters. By increasing nanocluster size, Brownian motion decreases. Different researchers have studied the effects of Brownian motion on the thermal conductivity of nanofluids. In some studies, Brownian motion enhances the thermal conductivity of nanofluids. Some other researchers have shown that Brownian motion has a negligible effect on the thermal conductivity of nanofluids. Based on the results of Jang and Choi (2004), Brownian motion in molecular level and nanoscale is a key mechanism in thermal behavior of nanoparticles-fluid suspensions. Prasher (2005) has shown that Brownian motion causes convection. This is a main reason for the enhancement of thermal conductivity of nanofluids. Azizian et al. (2012) have shown that Brownian motion is an important mechanism in the enhancement of thermal conductivity of titanium dioxide and alumina/water nanofluids. Evans et al. (2006) and Babaei et al. (2013) have shown that the micro-convection due to Brownian motion does not bring any significant effect on the thermal conductivity of nanofluids.
The review of the mentioned literature shows that Brownian motion and clustering are two interrelated parameters. Therefore, by considering the effect of clustering on thermal conductivity, the effect of Brownian motion must also be considered.
Stability of nanofluids can be assessed by investigation on clustering effects on nanofluid. Sedimentation and centrifugation, dynamic light scattering method (DLS), and UV–vis spectrum are common methods to evaluate the stability of nanofluids (Jiang et al. 2003; Tsai et al. 2004; Hwang et al. 2007; Li et al. 2007; Zhu et al. 2007, 2009; Farahmandjou et al. 2009; Huang et al. 2009; Leron 2009; Wang et al. 2009; Xian-Ju and Xin-Fang 2009; Wei and Wang 2010).
Wei and Wang (2010), Li et al. (2007), Zhu et al. (2007), investigated the stability of nanofluids using the sedimentation method. This method is simple but has disadvantages, e.g., it requires a long time to study nanofluids stability, also; this method does not give any information about size of the formed nanoclusters.
As reported in the literature, zeta potential is an important criterion for stability of nanofluids (Lee et al. 2006; Xian-Ju and Xin-Fang 2009; Ghadimi et al. 2011). This method is based on electrophoresis behavior of suspension (Lee et al. 2006; Xian-Ju and Xin-Fang 2009) and under zeta potential greater than 40 mV or less than −40 mV, the nanofluid possesses good stability (Leron 2009). Mahbubul et al. (2007) investigated the effect of ultrasonic mixing time on colloidal structure of alumina nanoparticles in water. The nanofluids were sonicated for duration of 0–180 min. It was found that zeta potential increased with ultrasonic mixing time and nanofluids are more stable by using ultrasonic mixing times of 90 min or longer.
Generally, zeta potential is the best method to evaluate the dispersion characteristics of nanofluids and nanoparticle behavior in spite of the fact that it is more expensive in comparison with the other techniques.
Huang et al. (2009) have studied the stability of alumina–water and copper–water nanofluids using DLS and UV–vis spectrum methods at different pH values. Their results show that at a certain pH, the values of UV absorbency and zeta potential becomes maximum, which corresponds to a stable nanofluid.
Xian-Ju et al. (2011) investigated on the stability of alumina–water and titanium oxide–water nanofluids under different pH values. Different dispersants have been used in their work. Their results were similar to the results of Huang et al. (2009).
Jiang et al. (2003), Hwang et al. (2007) and Habibzadeh et al. (2010) studied the stability of different nanoparticles such as carbon nanotubes, fullerene, copper oxide and tin oxide in different base fluids using the Uv–vis spectrum method. They obtained a linear relation between UV absorption at the maximum wavelength and the concentration of nanoparticles. According to the well-known theory of Mie, UV absorbency is related with the size of nanoparticles in nanofluids (Kreibig and Genzel 1985). The size of gold nanoparticles is predicted by Haiss et al. (2007) using UV–vis spectrum and Mie’s theory.
Based on the available studies in the literature, a comprehensive study including UV–vis spectrum by considering the size of nanoclusters has not yet been carried out to investigate the nanofluid stability.
In this work, UV–vis spectrum and DLS methods were employed to evaluate the dispersion characteristics and stability behavior of γ-Al2O3/water nanofluid. To prepare the nanofluid, the ultrasonic vibrator with different mixing strength and mixing time were used. The effect of ultrasonic mixing time on the UV–vis spectrum was investigated and compared with the results obtained by DLS method. Thermal conductivity of nanofluid was measured under different temperatures and nanoclusters size.
2 Experimental procedure
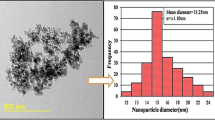
γ-Al2O3/water nanofluid was prepared with nanoparticle volume concentrations of 1, 2 and 3 % via two-step method (Ghadimi et al. 2011). γ-Al2O3 nanoparticles (produced by Nanoamor company) with 25 nm diameter and 180 m2/g specific surface area were dispersed into de-ionized water and an ultrasonic vibrator (Hielsher Up200, 200 Watts, 24 kHz, Germany) was used for better mixing. An isothermal bath (Wisecircu WCL-P8/Korea) was employed to stabilize the temperature within ±0.5 °C during the experiments. Thermal conductivity of nanofluids was measured by hot wire method using thermal property meter KD2 (Decagon Device, Inc., USA). The size of aggregations and zeta potential of nanofluid was measured using zeta sizer (Malvern, HS C1330-3000). Nanofluids with different nanoparticle concentrations were analyzed by UV–vis spectrophotometer. Dilute solutions of nanofluids are needed to use DLS as well as UV–vis. Therefore, 0.1 cc of each sample was mixed with 200 cc of de-ionized water.
3 Results and discussions
3.1 The effect of ultrasonic mixing time on stability of nanofluid
The DLS and UV–vis spectrum methods have been used to investigate the effect of ultrasonic mixing duration on the stability of nanofluids. In this research, DLS method has been used to obtain alternative results and also to compare and validate the results created by UV–Vis spectrophotometer. Nanofluids were prepared with nanoparticle concentrations of 1, 2 and 3 % by volume. Each sample was sonicated for 15, 30, 60, 90, 120, 150 and 180 min. Then, immediately 0.1 cc of sample was diluted with 200 cc of de-ionized water to measure zeta potential and UV–vis spectrophotometer.
3.1.1 Dynamic light scattering (DLS) method
As mentioned earlier, the stability of nanofluid can be evaluated by zeta potential. Figure 1 shows the effect of ultrasonic mixing time on the results of zeta potential. It shows that zeta potential is enhanced by increasing the ultrasonic mixing time. This is due to the reduction of agglomerated nanoclusters sizes and the enhancement of electrostatic charges on the surface of nanoclusters. During nanofluid preparation process, the nanoparticles are agglomerated which results in nanocluster formation with different sizes. The results indicate that zeta potential becomes greater than 50 mV after 150 min of ultrasonic mixing time, which is stable condition for nanofluid (Ghadimi et al. 2011). Therefore, 150 min of ultrasonic mixing time is the most appropriate time for γAl2O3 nanofluid preparation. Based on the results of Fig. 1, the concentration of nanofluid cannot change the value of zeta potential. A comparison between the results of the present study for zeta potential and the data reported by Mahbubul et al. (2007) also is shown in Fig. 1. This figure shows that the trend of zeta potential variation versus ultrasonic mixing time in these works is same. The values of zeta potential in the present study are lower than the values of zeta potential reported by Mahbubul et al. (2007). This is due to the smaller size of primary nanoparticles (13 nm) and clusters (Fig. 2) in this work.
Generally, dispersion of nanoparticles into the base fluid using ultrasonic mixer leads to a reduction in nanocluster sizes. In Fig. 2, the size of nanoclusters is demonstrated versus ultrasonic mixing time for different concentrations of nanofluid. It can be found that the size of nanoclusters decreases by increasing the ultrasonic mixing time. The results of Fig. 2 emphasize that the rate of size reduction during short period of mixing times is high. About 30 min, it varies very slowly over the time, and finally, it becomes constant. This may be interpreted by the fact that the size distribution of the nanoclusters at this time is nearly identical. This trend is also same to the work of Mahbubul et al. (2007).
Experiments for nanofluids with 2 % nanoparticle concentration were carried out to investigate the effect of ultrasonic mixing time on the size distribution of nanoclusters.
Figure 3 presents the intensity of scattering for nanoclusters plotted versus nanocluster size for different ultrasonic mixing times. Based on the results of this figure, the size distribution of nanoclusters is narrowed by increasing the ultrasonic mixing time. Also, by increasing the mixing time, the nanocluster size corresponding to the maximum intensity reduces to a smaller size. Based on the results after 30 min of ultrasonic mixing time, the range of distribution size of nanoclusters becomes narrower. For better understanding of the effect of ultrasonic mixing time on the size distribution of nanoclusters, the polydispersity index (PDI) can be used. PDI is a key parameter in DLS method which shows the quality of the size distribution on nanoclusters in suspensions (Alan 2007). PDI lower than 0.3 and with a single peak in size distribution curve, results in monodisperse-sized dispersion. In Fig. 4, PDI is plotted versus ultrasonic mixing time. It is clear that the PDI decreases over ultrasonic mixing time. PDI reduction means with more ultrasonic mixing, the large nanoclusters break into the smaller nanoclusters. According to the results, PDI equals to 0.3 when the ultrasonic mixing time is 30 min. By comparison of Figs. 2, 3, 4 and 5, it can be concluded that the time needed to get monodispersion is more than 30 min. This confirms the results obtained from Figs. 2 and 3. It is also clear that the concentration of nanofluid does not have a noticeable effect on PDI.
3.1.2 UV–vis spectrum method
In this study, UV–vis spectrum was used to evaluate the nanofluid stability. In order to study the effect of the ultrasonic mixing time on UV absorbency, several experiments were carried out using Al2O3/water nanofluid with concentration of 2 %. The result is shown in Fig. 5. In this figure, UV–vis spectrum is plotted for various ultrasonic mixing times. It is found that maximum absorbency is occurred in the wavelength of 195 nm (λ max). By increasing the ultrasonic mixing time from 0 to 30 min, the maximum absorbency increases, then for time longer than 30 min, this value decreases.
By decreasing the nanocluster size, UV absorption decreases more under the higher wavelength and vice versa. Also, the wavelength of the maximum absorption (λ max = 195 nm) is constant. Therefore, the nanocluster size change does not happen. By increasing the ultrasonic vibration time or decreasing the size of nanoclusters, the absorption under λ max increases. Then, for sonication time longer than 30 min, the absorbency value decreases.
Figure 6 presents maximum absorbency plotted versus ultrasonic mixing times in nanofluid with concentrations of 1, 2 and 3 % by volume. The trend of absorbency versus mixing time is similar to Fig. 5. It shows that for 150 min of ultrasonic mixing time, maximum absorbency does not change; therefore, this time is considered as an optimum mixing ultrasonic time. It can be found that maximum absorbency increases by increasing the nanofluid concentration. The maximum absorbency for 30-min mixing time for nanofluid with 3 % concentration is higher than those of other concentrations.
Comparison between the results of DLS method and UV–vis spectrum indicates that the results of these two methods are consistent.
3.2 Investigation of nanofluid stability after ultrasonic mixing
According to the mentioned results, to create a stable nanofluid, ultrasonic mixing time of 150 min is an appropriate time. At this time, zeta potential is higher than 50 mV and UV absorbency is minimum. Several experiments were carried out to investigate the stability of nanofluid under the appropriate ultrasonic mixing time (150 min). Nanofluids with concentrations of 1, 2, and 3 % were used. The test samples were kept in an isothermal bath under the temperature of 35 °C for 1 month. Every week the size distribution, zeta potential, and UV absorbency of nanofluids were measured. The results are shown in Figs. 7 and 8. Figure 7 shows the size of nanoclusters versus time by DLS method. It shows that the size of nanoclusters increases over time and maximum UV absorbency gradually decreases. The nanocluster size and reduction in maximum absorbency are higher for nanofluids with higher concentrations. By increasing the nanocluster size, the settling of nanoclusters leads to a decrease of maximum UV absorbency over time.
As mentioned earlier, zeta potential of nanofluid can be used to evaluate the stability of nanofluids. Figure 8 presents zeta potential of nanofluid versus time. This figure shows that zeta potential decreases by increasing time, which is due to the increasing size of nanoclusters. This is more pronounced for nanofluids with higher concentration. Therefore, the stability of nanofluids with higher concentration is reduced more.
The size distribution of nanoclusters varies with time because of nanocluster size growth. To consider the variation of size distribution of nanofluid with time, PDI of samples can be measured at different times. Figure 9 shows the variation of PDI with time. This figure indicates that PDI is gradually enhanced by time. The increase of PDI is due to the aggregation of nanocluster and consequently the formation of larger nanoclusters, which leads the broad size distribution. This figure also indicates that the nanofluid with higher concentration has higher PDI over time. This is due to the fact that higher nanoparticle concentration causes more nanoclusters and consequently less stable nanofluid.
3.3 The effect of ultrasonic mixing time on effective thermal conductivity of nanofluid
In this section, the effect of nanocluster size on thermal conductivity of Al2O3/water nanofluid is explained. In this study, nanofluid with concentrations of 2 and 3 % by volume was prepared. Nanofluids were stirred with ultrasonic vibrator, and time of mixing was 15, 30, 60, 90, 120, and 150 min. Immediately after ultrasonic mixing, nanofluid was inserted in a cylindrical vessel with 1 cm diameter and 10 cm length. The Probe of thermal property meter KD2 was inserted in the centerline of the vessel. The cylindrical vessel was placed vertically in an isothermal bath, and effective thermal conductivity of nanofluids was measured under isothermal conditions. The experiments were carried out under constant temperatures of 15, 25, 35 and 45 °C.
Effective thermal conductivity of nanofluid versus ultrasonic mixing time is shown in Fig. 10. According to Fig. 10, by increasing the ultrasonic mixing time, thermal conductivity first decreases and then gradually increases. Comparison between the results of Figs. 2, 5 and 10 indicates that thermal conductivity decreases by decreasing the size of nanoclusters in nanofluids. This means that the existence of the large-size nanoclusters in nanofluid causes the thermal conductivity to be increased.
The heat is transferred via two ways in nanofluid through the nanoclusters and base fluid. Thermal conductivity of nanoparticles in nanoclusters is higher than base fluid; therefore, heat transfer rate by nanoclusters is higher than that of the base fluid. Effective thermal conductivity of nanofluid is reduced by decreasing size of nanoclusters. Inspecting Fig. 10a, b shows that there is a minimum effective thermal conductivity in which is occurred at ultrasonic time of 30 min. The effective thermal conductivity is enhanced by increasing the ultrasonic mixing time after 30 min. This is due to the increasing of Brownian motions. Brownian motions are enhanced by decreasing of nanocluster size.
It can be found from Fig. 10 that the effect of Brownian motion on thermal conductivity of nanofluid increases by increasing temperature. For example, at the temperature of 15 °C, minimum and maximum thermal conductivity are 0.6316 and 0.6362, respectively, and at temperature 45 °C the corresponding values are 0.6590 and 0.6735, respectively. It is clear that enhancement percentage of thermal conductivity at the temperature of 45 °C is higher. According to Fig. 10, for higher ultrasonic mixing time than 120 min, effective thermal conductivity is almost constant because the size of nanoclusters (Fig. 2) does not change anymore. It can be found from comparison between Fig. 10a, b that in concentration of 3 % the thermal conductivity of nanofluid is higher than that of concentration 2 %. Comparison of the results of this section with the results obtained from DLS method and UV absorbency shows that there is a consistency and good agreement between data obtained by the mentioned two different methods. For example at 30-min ultrasonic mixing time, the results of DLS method showed that bandwidth of size distribution of nanoclusters become narrow. The results of UV absorbency showed that the maximum of λ max is at 30 min ultrasonic mixing time. Also, it is found from the results of thermal conductivity measurement that the minimum thermal conductivity corresponds to 30-min ultrasonic mixing time.
A comparison between the results of the present study for the effective thermal conductivity and the data reported by Das et al. (2003), and Chon and Kihma (2005) is shown in Fig. 11. The effective thermal conductivity ratio (k nanofluid/k base-fluid) of γ-Al2O3–water nanofluid with volume fraction of 2 % versus temperature is shown in this figure. The discrepancy between the reported data is due to different nanocluster sizes through the nanofluids. It is clear that in the present experiments, the thermal conductivity of nanofluid depends on nanocluster size. In fact, different plots for a nanofluid are related to the different nanocluster sizes, which are not considered in the other studies. Different ultrasonic devices (different powers) and different mixing times have a direct effect on the size of nanoclusters in nanofluids. The results of this study show that even under high ultrasonic mixing times, (Fig. 2), the size of the nanoclusters cannot be broken down to the primary size of the nanoparticles. Therefore, the thermal conductivity is strongly dependent on the mixing time, power of ultrasonic devices, method of dispersing, or the size of nanoclusters in nanofluids.
4 Conclusion
In this study, the stability of γ-Al2O3 nanofluid was evaluated using dynamic light scattering (DLS) and UV–vis spectrum methods. The effects of ultrasonic mixing time on zeta potential, mean nanocluster sizes, and UV absorbency were investigated. The results show that increasing mixing time leads to lower cluster size, while further time enhancement causes constant cluster size. Therefore, there is an optimum ultrasonic mixing time to have a stable nanofluid. The results of UV–vis spectrum method show that UV absorbency depends on concentration of nanoparticles and size of clusters in nanofluid. The comparison between the results of DLS methods with the data obtained by UV–vis showing that the results of both methods are consistent. It can be concluded that UV–vis spectrum is a reliable method to evaluate the stability of nanofluid. The results show that with ultrasonic mixing time of optimum value, nanofluid for along times becomes stable. In this work, the effect of nanoclusters size on the effective thermal conductivity of nanofluid also was investigated. This study represents that the effective thermal conductivity reduces by decreasing of clusters size and then enhances. The result also shows that enhancement of effective thermal conductivity under higher temperature is lower.
References
Alan R (2007) Basic principles of particles size analysis. Malvern Instruments Limited
Azizian R, Doroodchi E et al (2012) Effect of nanoconvection caused by Brownian motion on the enhancement of thermal conductivity in nanofluids. Ind Eng Chem Res 51:1782–1789
Babaei H, Keblinski P et al (2013) A proof for insignificant effect of Brownian motion-induced micro-convection on thermal conductivity by utilizing molecular dynamics simulation. J Appl Phys 113:084302
Chon CH, Kihma KD (2005) Thermal conductivity enhancement of nanofluids by Brownian motion. J Heat Transf 127:810
Chon CH, Kihma KD et al (2005) Empirical correlation finding the role of temperature and particle size for nanofluid (Al2O3) thermal conductivity enhancement. Appl Phys Lett 87(7):153107
Das SK, Putra N et al (2003) Temperature dependence of thermal conductivity enhancement for nanofluids. J Heat Transf 125:567–574
Eastman J, Choi U et al. (1997) Enhanced thermal conductivity through the development of nanofluids. Materials research society symposium proceedings, Cambridge University Press, Cambridge
Evans W, Fish J et al (2006) Role of Brownian motion hydrodynamics on nanofluid thermal conductivity. Appl Phys Lett 88:093116
Farahmandjou M, Sebt S et al (2009) Stability investigation of colloidal FePt nanoparticle systems by spectrophotometer analysis. Chin Phys Lett 26(2):027501
Ghadimi A, Saidur R et al (2011) A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf 54(17):4051–4068
Habibzadeha S, Kazemi-Beydokhti A et al (2010) Stability and thermal conductivity of nanofluids of tin dioxide synthesized via microwave-induced combustion route. Chem Eng J 156:471–478
Haiss W, Thanh NT et al (2007) Determination of size and concentration of gold nanoparticles from UV–vis spectra. Anal Chem 79(11):4215–4221
Hong J, Kim D (2012) Effects of aggregation on the thermal conductivity of alumina/water nanofluids. Thermochim Acta 542:28–32
Hong KS, Hong T et al (2006) Thermal conductivity of Fe nanofluids depending on the cluster size of nanoparticles. Appl Phys Lett 88:031901
Huang J, Wang X et al (2009) Influence of pH on the stability characteristics of nanofluids. In: Photonics and optoelectronics, 2009. SOPO 2009. IEEE, Wuhan
Hwang Y, Lee J et al (2007) Stability and thermal conductivity characteristics of nanofluids. Thermochim Acta 455(1):70–74
Jang SP, Choi SUS (2004) Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl Phys Lett 84:4316
Jiang L, Gao L et al (2003) Production of aqueous colloidal dispersions of carbon nanotubes. J Colloid Interface Sci 260(1):89–94
Kreibig U, Genzel L (1985) Optical absorption of small metallic particles. Surf Sci 156:678–700
Lee S, Choi SU et al (1999) Measuring thermal conductivity of fluids containing oxide nanoparticles. J Heat Transf 121(2):280–289
Lee D, Kim JW et al (2006) A new parameter to control heat transport in nanofluids: surface charge state of the particle in suspension. J Phys Chem B 110(9):4323–4328
Leron V (2009) Synthesis and covalent surface modification of carbon nanotubes for preparation of stabilized nanofluid suspensions. Dissertation, McGill University
Li CH, Peterson G (2006) Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticle suspensions (nanofluids). J Appl Phys 99(8):084314–084318
Li X, Zhu D et al (2007) Evaluation on dispersion behavior of the aqueous copper nano-suspensions. J Colloid Interface Sci 310(2):456–463
Mahbubul IM, Chong TH et al (2007) Effect of ultrasonication duration on colloidal structure and viscosity of alumina–water nanofluid. Ind Eng Chem Res 53:6677–6684
Masuda H, Ebata A et al (1993) Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Dispersion of Al2O3, SiO2 and TiO2 ultra-fine particles. Netsu Bussei 7:227–233
Pak BC, Cho YI (1998) Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp Heat Transf Int J 11(2):151–170
Prasher R (2005) Thermal conductivity of nanoscale colloidal solutions (nanofluids). Phys Rev Lett 94(2):025901
Prasher R, Phelan PE et al (2006) Effect of aggregation kinetics on the thermal conductivity of nanoscale colloidal solutions (nanofluid). Nano Lett 6(7):1529–1534
Putra N, Thiesen P et al (2003) Temperature dependence of thermal conductivity enhancement for nanofluids. J Heat Transf 125:567–574
Rao Y (2010) Nanofluids: stability, phase diagram, rheology and applications. Particuology 8(6):549–555
Shalkevich N, Shalkevich A et al (2010) Thermal conductivity of concentrated colloids in different states. J Phys Chem 114(21):9568–9572
Tsai C, Chien H et al (2004) Effect of structural character of gold nanoparticles in nanofluid on heat pipe thermal performance. Mater Lett 58(9):1461–1465
Wang Xj, Li X et al (2009) Influence of pH and SDBS on the stability and thermal conductivity of nanofluids. Energy Fuels 23(5):2684–2689
Wang JJ, Zheng RT et al (2012) Heat conduction mechanisms in nanofluids and suspensions. Nano Today 7:124–136
Wei X, Wang L (2010) Synthesis and thermal conductivity of microfluidic copper nanofluids. Particuology 8(3):262–271
Wu C, Cho TJ et al (2010) Effect of nanoparticle clustering on the effective thermal conductivity of concentrated silica colloids. Phys Rev E 81(1):011406
Xian-Ju W, Xin-Fang L (2009) Influence of pH on Nanofluids’ viscosity and thermal conductivity. Chin Phys Lett 26(5):056601
Xian-Ju W, Hai L et al (2011) Stability of TiO2 and Al2O3 nanofluids. Chin Phys Lett 28(8):086601
Xie H, Wang J et al (2002) Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J Appl Phys 91(7):4568–4572
Yu W, Xie H (2012) A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater 2012:1–17
Zhu H, Zhang C et al (2007) Preparation and thermal conductivity of suspensions of graphite nanoparticles. Carbon 45(1):226–228
Zhu D, Li X et al (2009) Dispersion behavior and thermal conductivity characteristics of Al2O3–H2O nanofluids. Curr Appl Phys 9(1):131–139
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeghi, R., Etemad, S.G., Keshavarzi, E. et al. Investigation of alumina nanofluid stability by UV–vis spectrum. Microfluid Nanofluid 18, 1023–1030 (2015). https://doi.org/10.1007/s10404-014-1491-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1491-y