Abstract
The article describes the thermolysis process of sodium borohydride dihydrate in thermoanalytical experiments. The reaction was carried out without solid catalyst and with catalyst as cobalt boride Co2B. It has been found out that in both cases the process starts after the peritectic reaction of the starting compound and forms a liquid phase. The enthalpy of peritectic reaction is ΔHreact = 19 ± 2 kJ mol−1. When thermolysis proceeds in acetonitrile solution without a catalyst intermediate hydroxyborohydride NaBH3OH and/or Na(BH3)2OH is formed according to the NMR experiment data. The formation of similar complexes in the solid phase is confirmed by experiments on the oxidation of the thermolysis products. Thermolysis process with solid catalyst proceeds with an intense exothermic effect at lower temperatures. The kinetics of the non-catalytic process is described by the model of two consecutive reactions, and reaction with the solid catalyst model is approximated by two parallel reactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The hydrolysis of complex alkali metal borohydrides (NaBH4, LiBH4, etc.) has long been used to produce enough hydrogen for technical purposes [1]. Reasons for using hydrides as a hydrogen source are high volumetric density of hydrogen in hydrides and their relative simplicity of obtaining these compounds. Among hydrides, sodium borohydride (NaBH4) holds a special place because of the high content of hydrogen (10.8 mass%), affordable price and stability of its alkaline solutions. Hydrolysis of sodium borohydride is a promising way of obtaining high-purity hydrogen. The half of hydrogen is released from the water [2,3,4,5]. Purely formally hydrolysis process can be represented by the following equation:
Unfortunately, the reaction by Eq. (1) is practically not realized. The main problem is formation of fairly stable borates, like NaB(OH)4·H2O and NaB(OH)4. It should be noted that research into the process of hydrolysis of sodium borohydride is actively carried out now. In last 2 years not less than 25 papers have been published. In general, the research deals with search and investigation of the hydrolysis catalysts. For example, see papers [6, 7]. The use of catalysts enables hydrogen production in the temperature range from − 40 to + 85 °C. Catalysts accelerate the H2 generating process, and prevent the by-products formation. They also allow easy control of the hydrogen generation process according to the load, as well as stop and start it on consumer request.
It should be noted that sodium borohydride dihydrate exists as an individual compound in the system sodium borohydride–water.
The ratio of components in this compound corresponds to Eq. (1), so this compound can be used in a solid form for the production of hydrogen during thermolysis. However, there is hardly any information about this process. Therefore, the main purpose of this paper is thermoanalytical and NMR study of the thermolysis NaBH4·2H2O, both without a catalytic process and using a solid catalyst. Cobalt borides obtained as described in [8] were used as a catalyst.
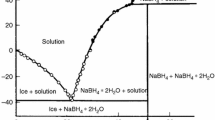
The polyterm of solubility binary system NaBH4–H2O [9] shows that at a temperature of 36.4 °C the anhydrous sodium borohydride is in equilibrium with a saturated solution. Invariant point (36.4 °C. 45.2 mass% NaBH4) corresponds to the peritectic reaction
Consequently, thermolysis process initially proceeds in a liquid phase.
The original binary phase diagram of the NaBH4–H2O system copied from Ref. [9] is shown in Fig. 1.
Diagram of the phase composition in binary system NaBH4–H2O at different temperatures and mass% of the borohydride. The diagram is reprinted from Malceva and Khain [9]
Experimental
Synthesis of sodium borohydride dihydrate
The starting anhydrous sodium borohydride (99% “Acros”) dissolved in 0.1 N NaOH solution (~ 50 g NaBH4 in 50 mL of 0.1 N NaOH) was stirred at 35–36 °C and then filtered under nitrogen. The clear solution in a sealed flask was placed in a refrigerator for 24 h at 0 °C. The precipitated crystals were separated from the mother liquor by filtration, and well wrung out between sheets of filter paper. These crystals were used in all studies presented. The compound was stored in an airtight plastic container in the refrigerator. The composition of the sample was evaluated with respect to X-ray analysis and PMR spectroscopy (proton magnetic resonance).
X-ray powder patterns of solid samples were registered with a Siemens Kristalloflex diffractometer (Cu-Kα radiation) with a graphite diffracted beam monochromator and Ni filter. Data were collected in the range of 2Θ = 5°–60° with a scan step 2Θ = 0.02° and scan rate of 18 min−1. Phase identification was performed using ICDD PDF-2 database and data [10].
Preparation of the catalyst
Synthesis of cobalt borides was performed ex situ in aqueous solution by reduction CoC12–6H2O by NaBH4 [8]. The process was carried out at 40 °C with constant stirring of 0.12 M solution of NaBH4. The molar ratio of CoC12–6H2O to hydride was 1:25. The resulting black sample was separated from the mother liquor by using a magnet after completion of gas evolution. It was washed thoroughly with acetone and was dried under vacuum at 70 °C for 3 h and stored in an atmosphere of Ar.
Measurements
Thermal gravimetric analysis (TG) and differential scanning calorimeter (DSC) were used to study thermal behavior, employing Netsch STA Jupiter 449C Instrumentation. The aluminum sample holders were used as crucibles Ø-6 mm and h-8 mm. The sample mass was 3–5 mg. Experiments were performed at four heating rates of 2.5, 5, 7.5 and 10 °C min−1 in a stream of dry air at the rate of 70 mL min−1 in the temperature range 30–550 °C. Thermoanalytical experiments were also carried out in a stream of pure argon. The thermolysis study using a solid catalyst was carried out as follows. The catalyst was placed into the crucible (~ 0.5 mg Co2B), and about 4 mg of NaBH4·2H2O was added there. The components were stirred by a magnet. The catalytic thermolysis was conducted in an atmosphere of dry air and argon also in the temperature range 30–250 °C. The experimental data were processed using the programs package Netzsch company: “Proteus Analysis”, “Peak Separation”, “Thermokinetics”.
NMR spectra of 1H (300 MHz) and 11B (96,3 MHz) were recorded according to standard procedures in the field of 7.04 Tesla spectrometer Bruker AVANCE 300 to 25–70 °C temperature range in standard ampoules 5 mm diameter. The sample temperature with an accuracy of ± 0.10 °C was set using BVT-3200 unit. The directional airflow of 400–500 L min−1 was used to eliminate the temperature gradient along the length of the ampoule. 11B NMR chemical shifts were measured relative to external reference—aqueous solution B(OH)3. 1H NMR shifts relative to TMS. The following samples were studied: anhydrous NaBH4 (I) and NaBH4·2H2O (II), prepared as shown above. The main solvent used was CH3CN and CD3CN. The water content in the main solvent used was under the spectroscopic detection limit. The formations of two fractions were observed when dihydrate was dissolved in acetonitrile at room temperatures. At 50–60 °C, separation was not observed, and the solution was regarded as homogeneous. For anhydrous sample, stratification did not happen.
Results and discussion
Thermolysis without solid catalyst
Figure 2 shows the thermoanalytical curves of the heating process for dihydrate of sodium borohydride in the temperature range 30–150 °C in a dry air atmosphere. At these temperatures, there is a loss of mass (~ 45%), and endothermic effects are observed in DSC curve. It should be noted that in argon the process proceeds similarly. Heating in argon in the temperature range 150–500 °C does not result in a noticeable change in mass and the appearance of thermal effects. If the process took place in accordance with Eq. (1), then significant exothermic effect could be observed [4, 5]. The process begins with the peritectic reaction (see Eq. 2). This process can be represented by the following equation:
Using the data of [10, 11], the enthalpy value of peritectic decay reaction was calculated, which was equal to ΔHreact = 17 kJ mol−1. According to DSC measurements, the enthalpy of the endothermic effect in the temperature range of 30–40 °C was 19 ± 2 kJ mol−1, so the calculated and experimental values coincide. Heating the sample to 550 °C in air leads to exothermic effects and significant mass gain (~ 60%) in the temperature range of 350–520 °C (Fig. 3). We assume that the exo-effects in the area of 350–470 °C are characteristic to the process of oxidation of hydrolysis products. In the temperature range of 150–350 °C, there is no noticeable change in the mass; therefore, in the process of thermolysis there is no formation of hydrates of sodium metaborate, as in the case with hydrolysis in aqueous solution [11] (Fig. 3). Intensive exo-effect at 500 °C is caused by oxidation of anhydrous sodium borohydride, which is confirmed by Fig. 4.
It is known [12] that the hydrolysis of borohydride anion in aqueous solution proceeds according to the scheme:
It is believed that the thermolysis NaBH4·2H2O is through the formation of hydroxy derivatives after the appearance of a liquid phase.
NMR experiment was conducted to identify the nature of intermediates formed in the thermolysis temperature range of 30–150 °C.
NaBH4·2H2O (II) is foamed at T ≥ 40 °C due to the thermolysis. This results in a partial release of material from the HF band coil and thus to disruption of sample homogeneity.
Therefore, further temperature measurements were carried out on the NMR samples (I) and (II) in anhydrous acetonitrile and CD3CN.
The signals of \({\text{BH}}_{4}^{ - }\) anion in PMR spectra were observed at − 0.12 ppm as a quartet with the coupling constants J (1H–11B) = 81 Hz. Spectra were recorded on samples dihydrate solution in acetonitrile and CD2HCN at 50 °C. The water signals at 3.05 ppm and a residual signal from CD2HCN partially deuterated acetonitrile as a quintet at ~ 1.8 ppm with coupling constants J (1H-2H) = 2.3 Hz. The ratio of the intensities of proton signals of water and tetraborohydride was 1: 1, which corresponds exactly to the original composition. At 70 °C in 11B NMR spectra of the sample (II) was observed a quintet with relative intensities of 1: 4: 6: 4: 1 at − 60 ppm and coupling constants J (11B–1H) = 81 Hz due to borohydride anion \({\text{BH}}_{4}^{ - }\) as the main form with more than 97% (Fig. 5). In addition to signals from \({\text{BH}}_{4}^{ - }\) in NMR 11B spectra, a group of multiplets three quartets were recorded. Its indicates the possibility of formation a hydroxyborohydride anion BH3OH− and/or (BH3)2OH− bridged OH group or a contact ion pair with a cation Na+ (insert at Fig. 5). Formation of such structures is shown in [12, 13]. The singlet at 17 ppm also appears in this temperature range. This appearance is due to the formation of anion metaborate according to reaction (1).
Apparently, similar oxo- and hydroxyforms of borohydride anion are formed during a non-catalytic thermoanalytical experiment, which is confirmed by Fig. 3.
Summarizing the results of thermal analysis and 11B NMR, the process of non-catalytic thermolysis can be described by the following equation:
Non-catalytic thermolysis proceeds mainly through hydrolysis and dehydration.
Thus, endothermic effects in the temperature range of 30–100 °C (Fig. 2) are caused by several processes occurring in the reaction system: first, peritectic reaction; secondly, gradual hydrolysis of borohydride anion; thirdly, dehydration of solid intermediate reaction products. In the temperature range 120–140 °C, the process of mass loss is practically over.
Catalytic thermolysis
Carrying out the thermolysis in the presence of a solid catalyst essentially changes the mechanism of the process (Fig. 6).
The process, as in the case of non-catalytic thermolysis reaction, starts with the formation of liquid phase. At this point, the change in mass, i.e., hydrogen evolution can be observed. It is possible that partial hydrolysis of borohydride proceeds at this period to form hydroxycomplexes. Then there is an intensive exothermic peak due, presumably, to the formation of hydrated sodium metaborate because only in this case, reaction (1) can be highly exothermic [2, 3].
Complete dehydration of NaBH4·2H2O corresponds to 49% of mass loss, and complete hydrolysis to 10.8% of mass loss (Eq. 1). The smaller the mass loss during thermolysis, the more hydrogen is released. In the case of catalytic thermolysis, the mass loss in the temperature range 40–150 °C is two times less than in the non-catalytic process (see Eq. 4). Equation (5) reflects this relationship.
By the temperature of 90 °C, the intensive process practically finished and is transformed into a solid-phase process of dehydration of metaborate, which is completed by 250 °C. The process which takes place below 90 °C may be described by the following equation:
According to Eq. (5), the mass loss due to evolution of hydrogen and water vapor is 23%, which corresponds to the mass loss by 90 °C (Fig. 6). The total mass loss of up to 250 °C is 29%, which includes mass loss by dehydration of sodium metaborate. Therefore, Eq. 5 is consistent with the experimental data (Fig. 6).
Thermolysis kinetics
The thermolysis kinetic analysis was made using the thermogravimetric experiment’s results at different heating rates. The inverse and direct kinetic problems were solved using Netzsch Thermokinetics software. Theoretical basis of the software and practical problems examples are described in [14]. At first, the inverse kinetic problem was solved by model-free methods [15] in order to find the initial estimates for Arrhenius parameters. The kinetic inverse problem solution revealed several stages of the process. Therefore, multistage models should be used for the experimental data fitting. At the next stage, the direct kinetic problem was studied with the use of multivariate non-linear regression. As a result, the statistical analysis revealed the optimal kinetic parameters and the function type (Fig. 7) as the best data approximation. The two-stage model (I) gave the best experimental data description (Fig. 7):
In this model, the reaction 1 was described with Eq. (6) and can be used for thermogravimetric curve approximation:
Reaction 2 was described by Eq. (7):
The obtained model parameters and the statistics are presented in Table 1.
A formal calculation can be interpreted as follows. Reaction 1 (see Eq. 6) describes processes taking place in the liquid phase. The hydrolysis changes the pH of the liquid phase, i.e., hydroxyborohydride anion serves as a catalyst of the reaction. The calculation demonstrates that the kinetics of the stage is described by the equation of autocatalysis (CnB). The hydrogen evolution is the limiting step of the process. The fraction of reaction 1 (Eq. 6) is about 71% in the entire process and corresponds to the reaction in the liquid phase. The next stage is the solid-phase reaction of nth order. This is the final dehydration of the reacting system and the formation of hydroxycomplexes. The products of this reaction oxidized at high temperature in the air together with NaBH4 (see Fig. 3).
The use of other two-stage process schemes did not lead to a satisfying result. In these cases, the Fisher criterion value (i.e., the variances ratio statistics for the other models) exceeded the Fcrit substantially. Fcrit is the tabular value of the criterion for a given number of degrees of freedom and 95% probability.
Thus, thermolysis process is due to peritectic decay and can be adjusted not only by using of the catalyst but also by changing of the water vapor pressure in the reaction volume.
The catalytic thermolysis kinetics
Similar to the previous section, kinetic analysis for the thermolysis process in the presence of a solid catalyst was carried out. Calculations were performed for the temperature range of 40–80 °C. The calculations have shown that a two-stage model (II) with parallel processes gives the best fit of the experimental data (Fig. 8):
The results of modeling the kinetics of thermolysis of sodium borohydride dihydrate to a solid catalyst of the two-stage model with two parallel processes (see inset in Figure and Table 2). The correlation coefficient R 2 = 0.998, solid line—calculation

In this model, the function type of reaction 1 (Fig. 7) was similar to the function (6) of the previous model. Prout–Tompkins Eq. (8) was chosen as the best fit for reaction 2:
The model parameter’s values and the statistics are presented in Table 2.
This formal approach can be interpreted as follows. Both functions characterizing reactions 1 and 2 are caused by the catalysis with sodium metaborate hydrate being the final solid product in the temperature range of 40–80 °C. Reaction 1 can be assumed to proceed in the liquid phase. Reaction 2 can be assumed to proceed with solid NaBH4 participation as the product of peritectic decay. In both cases, hydrogen evolution occurs.
These models give the opportunity to simulate the time dependence of the concentrations of reactants and products for various temperatures in the condition of the experiment. Thus, Fig. 9 shows such dependence for the process at 70 °C without a catalyst (see Fig. 2).
The figure shows that initial substance (liquid phase) is exhausted in 6 min after the reaction beginning (curve A). This process might correspond to reaction 1. Then conversion reaction (reaction 2) proceeds with an oxoderivative and sodium metaborate formation. The process duration is 15 min.
The situation is dramatically different in the presence of a solid catalyst (Fig. 10).
The entire process duration is 4 min at the same temperature. Both curves have inflection points in 1 min after the process starts. This indicates the increasing impact of the second stage of the process. This might be due to the increasing rate of the sodium metaborate hydrate formation. Two minutes after the process starts, it transforms into a solid phase. Thus, the liquid-phase formation defines the process behavior in case of the catalytic thermolysis also. It should be noted that thermolysis process does not occur at temperatures below 35 °C and under external pressure of water steam ≥ 7 mm Hg even in the presence of a solid catalyst.
Thus, the characteristic feature of thermolysis NaBH4·2H2O is formation of the hydroxyborohydrides in case of non-catalytic process as well in the presence of a solid catalyst.
Calculations of the thermolysis NaBH4·2H2O for isotherm characterizes only the conditions of thermoanalytical experiments. But the joint solution of the kinetic, heat and mass transfer equations will take into account the scale factor.
Conclusions
-
1.
According to DSC measurements, the enthalpy of the endothermic effect in the temperature range of 30–40 °C was 19 ± 2 kJ mol−1. This effect is caused by the peritectic reaction, which resulted in a suspension of anhydrous sodium borohydride in a saturated solution.
Thus, the thermolysis of NaBH4·2H2O proceeds at a significant rate at temperatures above 36.4 °C (i.e., at the peritectic transition temperature) in both cases: either with or without solid catalyst. The change in mass during non-catalytic thermolysis is observed in the temperature range 40–150 °C.
-
2.
NaBH4·2H2O heating in air to a temperature of 550 °C leads to intense exothermic effects at 300–490 °C. The appearance of these effects is due to the oxidation of hydroxycomplexes borohydride anion as the partial hydrolysis products.
-
3.
At 70 °C, in NMR 11B spectra, a group of multiplets three quartets were recorded, which indicates the possibility of formation of a hydroxyborohydride anion BH3OH− or (BH3)2OH−. Consequently, thermolysis of a crystalline NaBH4·2H2O proceeds like hydrolysis in solutions.
-
4.
Catalytic thermolysis proceeds as an exothermic process in the temperature range 40–90 °C, i.e., at much lower temperatures than non-catalytic thermolysis. Change of mass (~ 23%) indicates an increase in the proportion of hydrogen in the thermolysis products compared to non-catalytic process, where the change in mass is about 45%. Consequently, the catalytic thermolysis decreases the by-products of hydroxyborohydrides.
-
5.
Kinetic calculations showed that the non-catalytic process proceeds as two successive stages. The catalytic process had statistically two significant parallel processes. The values of the kinetic parameters in a catalytic process were substantially more, i.e., thermolysis speed increases as increasing temperature becomes much stronger.
Change history
18 June 2019
The Editor-in-Chief would like to alert readers that due to an administrative error, this article [1] has been republished in the same journal [2]. The correct citations for these articles should be from the original publication [1].
References
Schlesinger HI, Brown HC, Finholt AE, Gilbreath JR, Hoekstra H, Hyde RK. Sodium borohydride, its hydrolysis and its use as a reduction agent and in the generation of hydrogen. J Am Chem Soc. 1953;75:215–9.
Orimo SI, Nakamori Y, Eliseo JR, Zuttel A, Jensen CM. Complex hydrides for hydrogen storage. Chem Rev. 2007;107(10):4111–32.
Amendola SC, Sharp-Goldman SL, Janjua MS, Spencer NC, Kelly MT, Petillo PJ, Binder M. A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst. Int J Hydrog Energy. 2000;25(10):969–75.
Marrero-Alfonso EY, Beaird AM, Davis TA, Matthews MA. Hydrogen generation from chemical hydrides. Ind Eng Chem Res. 2009;48:3703–12.
Beaird AM, Davis TA, Matthews MA. Deliquescence in the hydrolysis of sodium borohydride by water vapor. Ind Eng Chem Res. 2010;49:4596–9.
Yongsheng Wei Ru, Wang Liyuan Meng, Wang Yan, Li Guode, Xin Shigang, Zhao Xinsheng, Zhang Ke. Hydrogen generation from alkaline NaBH4 solution using a dandelion-like Co–Mo–B catalyst supported on carbon cloth. Int J Hydrog Energy. 2017;42(15):9945–51.
Li Qiming, Li Fang, Zhao Shiduo, Xia Xin. Hydrogen generation from hydrolysis of NaBH4 based on high stable NiB/NiFe2O4 catalyst. Int J Hydrog Energy. 2017;42(7):3971–80.
Simagina VI, Komova OV, Ozerova AM, Netskina OV, Odegova GV, Kellerman DG, Bulavchenko OA, Ishchenko AV. Cobalt oxide catalyst for hydrolysis of sodium borohydride and ammonia borane. Appl Catal A. 2011;394:86–92.
Malceva NN, Khain VC. Sodium borohydride. Moscow: Nauka; 1985 (in Russian).
Filinchuk Y, Hagemann H. Structure and properties of NaBH4·2H2O and NaBH4. Eur J Inorg Chem. 2008;20:3127–33.
Marrero-Alfonso EY, Gray JR, Davis TA, Matthews MA. Minimizing water utilization in hydrolysis of sodium borohydride: the role of sodium metaborates hydrates. Int J Hydrog Energy. 2007;32:4723–30.
Khain VC, Malceva NN, Volkov AA. Borohydrides of alkali metals and tetraalkylammonium. Ukhta: Ukhta State University; 2001 (in Russian).
Ruman T, Kushnierz A, Jurkiewicz A, Les A, Rode W. The synthesis, reactivity and 1H NMR investigation of the hydroxyborohydride anion. Inorg Chem Commun. 2007;10:1074–8.
Arkhangelsky IV, Dunaev AV, Makarenko IV, Tikhonov NA, Belyaev SS, Tarasov AV. Non-isothermal kinetic methods. Workbook and laboratory. manual ed. Berlin: Open Access; 2013.
Ozawa T. A new method of analyzing thermo gravimetric data. Bull Chem Soc Jpn. 1881;1965:38.
Acknowledgements
This work was supported by the RFBR under Grant No. 15-03-0750.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arkhangelskii, I.V., Tarasov, V.P., Kravchenko, O.V. et al. Thermoanalytical and NMR investigation of NaBH4·2H2O thermolysis process. J Therm Anal Calorim 131, 2833–2842 (2018). https://doi.org/10.1007/s10973-017-6821-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6821-3