Abstract
In this study, Langmuir–Hinshelwood and Michaelis–Menten kinetic models are applied to describe the kinetic behaviour of the Co–B catalyst in the hydrolysis of 0.12 M aqueous solutions of boron hydrides at temperatures from 22 to 60°C. Boron hydrides are selected as sodium borohydride (NaBH4, 10 wt % NaOH) and ammonia borane (NH3BH3). Based on the Langmuir–Hinshelwood kinetic approach, it is found that under the same reaction conditions the NaBH4–Co–B catalyst interaction is more effective than that of the NH3BH3–Co–B. According to the Langmuir–Hinshelwood model, apparent activation energies (Ea) for the hydrolysis of NaBH4 and NH3BH3 over Co–B catalysts are calculated to be 45.38 and 57.37 kJ/mol, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Catalytic hydrolysis of boron hydrides for hydrogen production has been reported a long time ago. The first study was published in 1953 by Schlesinger et al. who described the sodium borohydride (NaBH4) hydrolysis at ambient conditions and found that the hydrogen generation rate is a function of the concentration of BH\(_{4}^{ - }\) and pH value of BH\(_{4}^{ - }\) solution [1].
NaBH4 belongs to the class of boron hydrides with a hydrogen content of 10.8 wt %. It easily reacts with water and releases 4 moles of H2 for every one mole of the hydride. For this reason, its solutions are prepared at high pH by alkali agents such as NaOH, KOH and catalysts are used to activate NaBH4 solutions [2]. By dissolving NaBH4 in the water BH\(_{4}^{ - }\) anions are formed that react with water molecules to generate hydrogen gas. The total hydrolysis of NaBH4 is given by
Ammonia borane (NH3BH3) is the latest popular hydrogen storage material for hydrogen production from boron hydrides. It is very competitive and alternative to NaBH4 with its higher hydrogen content (19.5 wt %). NH3BH3 is stable in aqueous solutions and this property is advantageous from the practical point of view. NH3BH3 reacts with two moles of water to generate three moles of hydrogen molecules as seen below [3].
Hydrolyses of these promising boron hydrides have been investigated by many researchers using different noble [4, 5] and base [6–8] metal catalysts. Among the variety of catalysts, Co catalysts have a great potential due to their good to excellent activities and cost-effectiveness [9, 10]. Metals [11], nanoparticles [12], salts [13], oxides [14, 15], borides [9, 10] and Co based catalysts [16] were repeatedly used to hydrolyse boron hydrides.
Kinetic modelling is a powerful tool used to investigate kinetics of heterogeneous reaction of metal catalysed hydrolyses of boron hydrides. Various kinetic models have been proposed as power law models. Among these are zero-order, first-order second-order and nth-order bimolecular kinetic models based on Langmuir–Hinshelwood, Michaelis–Menten and the semi-empirical kinetics [17].
The Langmuir–Hinshelwood mechanism assumes that the heterogeneous reaction takes place between species adsorbed on the catalyst active sites and a surface reaction yields products and by-products, which are desorbed into the bulk medium. The reaction mechanism includes three steps such as (i) equilibrated adsorption of BH4 and H2O, (ii) the reaction of the adsorbed BH4 and H2O and (iii) desorption of products and by-products from the catalyst surface [18]. In addition, this model at low temperatures and concentrations obeys a zero order kinetic law, while the first order kinetics is relevant to description at higher temperatures [19]. Based on this knowledge, we can depict the hydrolysis mechanism as shown in Scheme 1.

Scheme 1 . The Langmuir–Hishelwood and Michaelis–Menten mechanism of catalytic hydrolysis.
Originally, the Michaelis–Menten equation is proposed to outline the mechanism governing the enzymatic sugar inversion, and it is also of use for describing heterogeneous catalysed reaction. The mechanism involves a three-step cycle.
(1) Reactant is adsorbed on the catalyst surface.
(2) Catalyst first reacts reversibly with the reactant, forming a catalytic intermediate and a second reactant interacts with catalytic intermediate.
(3) Intermediate decomposes to give the catalyst, product (H2) and by-products (NaBO2/(NH4)BO2). At this irreversible step the product and by-products are desorbed from catalyst surface.
Based on this model (see Scheme 1), BH\(_{4}^{ - }\) ions are adsorbed on the active sites of catalytic surface and MBH4 intermediate complex forms that reacts with four water molecules from the aqueous phase to produce B(OH)4 species, desorption of which releases 4 moles of H2. Michaelis–Menten model satisfactorily describes the NaBH4–Co–B interaction at high BH\(_{4}^{ - }\) concentrations, but is hardly applicable at lower concentrations of BH\(_{4}^{ - }\) [20].
It is well known that the behaviour of the boron hydrides hydrolysis depends on the nature and amount of catalysts and boron hydrides. Depending on reaction conditions, hydrolysis of boron hydrides in the presence of Co based catalysts shows zero- and first-order reaction kinetics. Fernandes et al. tested CoB catalysts prepared by the chemical reduction method in an aqueous NH3BH3 solution and reported Ea of the system as equal to 44.00 kJ/mol [3]. Qui et al. prepared Co–B catalysts in situ via chemical reduction and carried out the catalytic hydrolysis of NH3BH3 to generate hydrogen with activation energy of 34.10 kJ/mol [13]. Metin et al. synthesized SiO2 embedded Co catalysts for hydrolysis of NH3BH3 solution and reported Ea of 42.00 kJ/mol [21]. Also Luo et al. performed NH3BH3 hydrolysis in the presence of co-precipitation Co@M41S catalyst with Ea found to be 55.00 kJ/mol [22]. Similarly, Ye et al. have prepared Co–αAl2O3 catalysts and estimated Ea= 51.50 kJ/mol for NaBH4 hydrolysis [23]. Similarly, Xu et al. prepared Co–αAl2O3 catalysts by the conventional impregnation method and tested for it in the hydrolysis of NH3BH3 with an activation energy of 62.00 kJ/mol of Ea [24]. Sahiner et al. prepared p(AMPS)–Co catalyst using adsorption and chemical reduction methods and reported Ea = 42.00 kJ/mol for the NH3BH3 hydrolysis [12]. In our previous study, we synthesized Co–B amorphous catalysts via solid-state reaction and Ea was determined to be 48.07 kJ/mol [25].
The present study is concerned with kinetic investigations of the hydrolysis of boron hydrides with the Co–B catalyst. Two different molecular kinetic models were applied to shed some light into the kinetics of hydrolysis of two different boron hydrides (NaBH4 and NH3BH3). The results were compared to characterize and describe the Co–B catalysts behaviour in the presence of 0.12 M aqueous solutions of NaBH4 and NH3BH3. In addition, by using Langmuir–Hinshelwood and Michaelis–Menten kinetic models, the kinetic mechanism of catalytic reaction between catalyst and boron hydrides was analysed in detail. It was found that under the same conditions the NaBH4–Co–B catalyst interaction was more effective than that of the NH3BH3–Co–B system.
THEORETICAL
Now we can consider how Langmuir–Hinshelwood and Michaelis–Menten models can be used to explain the mechanism governing the hydrolysis of boron hydrides in the presence of the Co–B catalyst. In accord with the kinetic model of Langmuir–Hinshelwood, reactants are initially adsorbed on the surface of the catalyst and then the reaction between adsorbed species occurs as a second step to generate hydrogen and at the last step hydrogen is evolved and by-products are desorbed from the catalyst surface. Since water was in excess in the hydrolysis reaction medium, its concentration was assumed to be constant. Based on these assumptions, the kinetic models were applied with simplified representation of the adsorbed species [2, 26].
Hydrogen generation rate can be expressed as:
where \({{K}_{{\text{a}}}}\), \(C\), \(k\) and \({{r}_{{{\text{LWCH}}}}}\) are the adsorption constant, concentration of hydride, Arrhenius rate constant and reaction rate, respectively. Calculation of \({{K}_{{\text{a}}}}\) (Eq. (2)) is fulfilled by minimizing the correlation coefficients derived from the data recorded at 40 and 60°C.
Integrating Eq. (1) gives the reaction rate:
Michaelis–Menten kinetic model envisages a reversible adsorption on the active sites. The main step in this model is kinetically controlled adsorption of the reactant on the catalyst surface [20, 27]. The reaction responsible for hydrogen generation can be represented by the equation:
where \({{v}_{{\max }}}\) is the maximum H2 generation rate in logarithmic scale of the hydrolysis reaction and KM is Michaelis–Menten constant. In this method, the use of the Lineweaver–Burk plot makes it easier to determine these values and the Eq. (4) becomes:
EXPERIMENTAL
In the present study, Co–B catalyst was prepared using a sol–gel method according to the procedure described in our previous study. Amorphous to X-ray Co–B catalyst (Co : B molar ratio ≈0.29) had a surface area of 122.70 m2/g with particle size <500 nm [28].
All reagents used in this research were of analytical reagent grade. NH3BH3 (97% purity) from Aldrich was used as received. NaBH4 with a purity level ≥96% was supplied by Fluka. Sodium hydroxide NaOH was purchased from Labor Technic, and used as a stabilizer for NaBH4 solutions. To gain insight into the mechanistic aspects of the Co–B catalysed hydrolysis of 0.12 M NaBH4 in an alkaline medium and 0.12 M NH3BH3 the kinetics of the reaction was investigated by hydrogen generation measurements. Two 0.12 M boron hydride solutions were prepared by dissolving boron hydrides in deionized water. In addition, to stabilize NaBH4, basic aqueous solution was prepared with 10 wt % NaOH. A constant amount of the catalyst (19 wt %) was maintained in all hydrolysis runs.
The Co–B catalysed hydrolysis tests of boron hydrides were performed at temperatures from 22 to 60°C in a 15-mL hydrolysis-glass reactor, which was immersed in a water bath with a temperature controlled system (±2°C). The generated hydrogen was collected by the water displacement procedure. Output of reactor was connected with a piece of rubber hose to transfer the evolved hydrogen to the water-filled inverted burette in order to follow with the burette the increase in volume of H2 generated during the hydrolysis. Hydrogen evaluation time was measured simultaneously with the chronometer. The hydrolysis-glass reactor was kept under magnetic stirring at 500 rpm during the hydrolysis.
RESULTS AND DISCUSSION
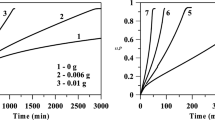
The change in molarity of boron hydride solutions as a function of the hydrolysis time over the Co–B catalyst is shown in Fig. 1. Both boron hydrides showed a similar pattern of behaviour illustrating the temperature dependence of the hydrolysis depth, although their H2 generation rates (HGR) were different. As the temperature rise, the number of boron hydride particles colliding with the Co–B catalyst increases on a pro rata basis. The H2 generation rates given in Fig. 1 are nearly three times higher for NaBH4 than for NH3BH3. At room temperature, induction time (IT), the time needed to develop strong interaction between active sites and boron hydride particles, was longer for NaBH4 (∼150 min) than for NH3BH3 (∼100 min). While lower temperatures (22°C) required more time for the catalyst to react with boron hydrides actively, hydrolysis reaction almost immediately started at higher temperatures (≥40°C).
Langmuir–Hinshelwood kinetic model was applied to our results in an attempt to determine the adsorption constant \({{K}_{{\text{a}}}}\) (Eq. (2)). Although both substrates were hydrolysed in the presence of the same catalyst, the \({{K}_{{\text{a}}}}\) values were determined to be 611.00 and 525.50 L/mol for NaBH4 and NH3BH3, respectively (Table 1). The divergence can be ascribed to different molecular interactions. This means that, under certain conditions, NH3BH3 molecules are stronger adsorbed on the Co–B catalyst surface than NaBH4 molecules. While NH3BH3 is stable in water, stability of NaBH4 is associated with higher pH values and such stabilizers as NaOH or KOH need to be used in aqueous solutions.
According to information published in literature and derived from our earlier studies, the best results for H2 generation were obtained when alkaline NaBH4 solutions containing 10 wt % NaOH were used [29]. Experiments with NaBH4 solutions with a higher or lower NaOH concentration showed a decreased hydrogen generation rate [23]. The hydrolysis of NaBH4 over Co–B catalysts surface occurred in three main steps: (i) adsorption of BH\(_{4}^{ - }\), (ii) hydrolysis reaction of BH\(_{4}^{ - }\) and (iii) desorption of H2 and by-products from the catalyst surface. Adsorption of BH\(_{4}^{ - }\) on the catalyst surface can be considered as the rate-determining step [30]. In NaBH4 solutions stabilized by NaOH BH\(_{4}^{ - }\) ions compete with NaOH for adsorption sites on the catalyst surface [2].
Figure 2 shows application of the Langmuir–Hinshelwood kinetic model to the process of H2 generation by hydrolysing 0.12 M solution of boron hydrides at different temperatures. Arrhenius equation was also used to compare Ea values calculated for different boron hydrides (Fig. 2c). In contrast to \({{K}_{{\text{a}}}}\) values, generation of H2 from NaBH4 proceeds with a lower activation energy (45.38 kJ/mol) than that from NH3BH3 (57.37 kJ/mol).
Langmuir–Hinshelwood kinetics for hydrolysis of light weight complexes in hydride solutions of 10 wt % NaOH + 0.12 M NaBH4 (a) and 0.12 M NH3BH3 (b) for different temperatures: (1) 22°C, (2) 40°C, (3) 60°C. (c) Arrhenius plots for Langmuir–Hinshelwood kinetics calculated for different boron hydrides (◆—NaBH4, ◼—NH3BH3).
Michaelis–Menten kinetic model was performed by the determination of the logarithmic phase of H2 generation data of 0.12 M boron hydride solutions (Fig. 3). Three different reaction temperatures showed a logarithmic phase in different time and/or concentration range. As it could be seen from Lineweaver–Burk plots, NaBH4 and NH3BH3 also have different values.
Application of Michaelis–Menten kinetic model for hydrolysis of light weight complex in hydride solutions (10 wt % NaOH + 0.12 M NaBH4 (◆) and 0.12 M NH3BH3 (◼)) for different temperatures: 22°C (a), 40°C (b) and 60°C (c). (d) Arrhenius plots for Michaelis–Menten kinetics calculated for different boron hydrides.
Table 2 shows the \({{v}_{{\max }}}\) and \({{K}_{{\text{M}}}}\) values registered for both boron hydride solutions at different temperatures. The Michaelis constant \({{K}_{{\text{M}}}}\) explains the affinity between the reactant and the active phase of catalyst. The value of \({{K}_{{\text{M}}}}\) depends on the nature of the catalyst and the substrate and on operating conditions such as temperature and pH. The influence of temperature on \({{K}_{{\text{M}}}}\) values indicates that this parameter improves catalytic efficiency under the conditions chosen for our experiments. As the temperature was increased from 22 to 40°C and then to 60°C \({{K}_{{\text{M}}}}\) values found for NH3BH3 solutions decreased by ∼26 and ∼32%, respectively. Lower values of \({{K}_{{\text{M}}}}\) characterise more efficient catalysts. The nature of reactants and reaction conditions can also affect the hydrolysis kinetics as evidenced by the results shown in Table 2. Lower \({{K}_{{\text{M}}}}\) values obtained for NaBH4 compared to those found for NH3BH3 apparently suggest that the catalyst affinity of the NaBH4–Co–B system is superior to that of the NH3BH3–Co–B catalyst.
According to the Langmuir–Hinshelwood model, the \(k\) value corresponds to the total number of adsorption sites on the catalyst surface. However in the light of our findings the \(k\) value can be defined as the reaction rate for the elementary step of adsorption (Table 2). Moreover, based on the Michaelis–Menten kinetic model, the hydrolysis of NaBH4 solutions demonstrates higher \({{v}_{{\max }}}\) and lower Ea values than that of NH3BH3 solutions (Table 2). Comparing the applicability of kinetic models, a conclusion can be made that the hydrolysis data of boron hydrides obeyed Langmuir–Hinshelwood kinetic model with 1 and 0.9996 correlation co-factors. From the kinetic results, it can be inferred that hydride complexes initially absorbed on the Co–B catalysts react to generate hydrogen.
CONCLUSIONS
In this study, the heterogeneous reaction of hydrolysis of boron hydrides in the presence of the Co–B catalyst was investigated. Langmuir–Hinshelwood and Michaelis–Menten kinetic models provide two molecular approaches. While Langmuir–Hinshelwood kinetic model suggests a surface reaction between reactants initially adsorbed on active sites, Michaelis–Menten kinetic model envisages reversible adsorption on active sites. In our experiments water was taken in excess and its concentration may be taken constant. Correspondingly, simplifications in our calculations were warranted. According to Langmuir–Hinshelwood and Michaelis–Menten kinetic models, Ea values for NaBH4 and NH3BH3 were found to be 45.38, 57.37 kJ/mol and 37.61, 52.00 kJ/mol, respectively. Comparing results of kinetic investigation carried out at the same temperature and concentration of boron hydrides with allowance for correlation co-factor values, it can be postulated that the Langmuir–Hinshelwood kinetic model provided a more relevant explanation for the behavior of our system.
REFERENCES
Schlesinger, H.I., Brown, H.C., Finholt, A.E., Gilbreath, J.R., Hoekstra, H.R., and Hyde, E.K., J. Amer. Chem. Soc., 1953, vol. 75, no. 1, p. 215.
Retnamma, R., Novais, A.Q., and Rangel, C.M., Int. J. Hydrogen Energy, 2011, vol. 36, no. 16, p. 9772.
Fernandes, R., Patel, N., Miotello, A., Jaiswal, R., and Kothari, D.C., Int. J. Hydrogen Energy, 2012, vol. 37, no. 3, p. 2397.
Abo-Hamed, E.K., Pennycook, T., Vaynzof, Y., Toprakcioglu, C., Koutsioubas, A., and Scherman, O.A., Small, 2014, vol. 10, no. 15, p. 3145.
Sun, D., Mazumder, V., Metin, O., and Sun, S., ACS Nano, 2011, vol. 5, no. 8, p. 6458.
Xu, Q. and Chandra, M., J. Power Sources, 2006, vol. 163, no. 1, p. 364.
Metin, O., Mazumder, V., Ozkar, S., and Sun, S., J. Amer. Chem. Soc., 2010, vol. 132, no. 5, p. 1468.
Yamada, Y., Yano, K., Xu, Q., and Fukuzumi, S., J. Phys. Chem. C, 2010, vol. 114, no. 39, p. 16456.
Wu, C., Wu, F., Bai, Y., Yi, B., and Zhang, H., Mater. Lett., 2005, vol. 59, no. 14, p. 1748.
Ozerova, A.M., Simagina, V.I., Komova, O.V., Netskina, O.V., Odegova, G.V., Bulavchenko, O.A., and Rudina, N.A., J. Alloy Compd., 2012, vol. 513, p. 266.
Cho, K.W. and Kwon, H.S., Catal. Today, 2007, vol. 120, no. 3, p. 298.
Sahiner, N., Ozay, O., Inger, E., and Aktas, N., Appl. Catal., B, 2011, vol. 102, no. 1, p. 201.
Qiu, F.Y., Wang, Y.J., Wang, Y.P., Li, L., Liu, G., Yan, C., and Yuan, H.T., Catal. Today, 2011, vol. 170, no. 1, p. 64.
Groven, L.J., Pfeil, T.L., and Pourpoint, T.L., Int. J. Hydrogen Energy, 2013, vol. 38, no. 15, p. 6377.
Simagina, V.I., Komova, O.V., Ozerova, A.M., Netskina, O.V., Odegova, G.V., Kellerman, D.G., and Ishchenko, A.V., Appl. Catal., A, 2011, vol. 394, no. 1, p. 86.
Kaya, M., Zahmakiran, M., Özkar, S., and Volkan, M., ACS Appl. Mater. Interfaces, 2012, vol. 4, no. 8, p. 3866.
Liu, B.H. and Li, Z.P., J. Power Sources, 2009, vol. 187, no. 2, p. 527.
Zhang, J.S., Delgass, W.N., Fisher, T.S., and Gore, J.P., J. Power Sources, 2007, vol. 164, no. 2, p. 772.
Hung, A.J., Tsai, S.F., Hsu, Y.Y., Ku, J.R., Chen, Y.H., and Yu, C.C., Int. J. Hydrogen Energy, 2008, vol. 33, no. 21, p. 6205.
Demirci, U.B. and Miele, P., C. R. Chim., 2014, vol. 17, no. 7, p. 707.
Metin, Ö., Dinç, M., Eren, Z.S., and Özkar, S., Int. J. Hydrogen Energy, 2011, vol. 36, no. 18, p. 11528.
Luo, Y.C., Liu, Y.H., Hung, Y., Liu, X.Y., and Mou, C.Y., Int. J. Hydrogen Energy, 2013, vol. 38, no. 18, p. 7280.
Ye, W., Zhang, H., Xu, D., Ma, L., and Yi, B., J. Power Sources, 2007, vol. 164, no. 2, p. 544.
Xu, Q. and Chandra, M., J. Alloy Compd., 2007, vol. 446, p. 729.
Coşkuner, B., Figen, A.K., and Pişkin, S., React. Kinet. Mech. Catal., 2013, vol. 109, no. 2, p. 375.
Andrieux, J., Demirci, U.B., and Miele, P., Catal. Today, 2011, vol. 170, p. 13.
Levenspiel, O., Chemical Reaction Engineering, John Wiley & Sons, 1999.
Figen, A.K. and Coşkuner, B., Int. J. Hydrogen Energy, 2013, vol. 38, no. 6, p. 2824.
Kantürk Figen, A., Coşkuner, B., Pişkin, M.B., Dere Özdemir, Ö. J Int Sci Publications: Mater, Methods & Techologies, 2013, vol. 7, no. 1, p. 43.
Zhang, Q., Wu, Y., Sun, X., and Ortega, J., Ind. Eng. Chem. Res., 2007, vol. 46, no. 4, p. 1120.
ACKNOWLEDGMENTS
The authors would like to thank the Yildiz Technical University Research Foundation (Project no. 2012-07-01-GEP01) for its financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
B. Coşkuner Filiz, A. Kantürk Figen The Molecular-Kinetic Approach to Hydrolysis of Boron Hydrides for Hydrogen Production. Kinet Catal 60, 37–43 (2019). https://doi.org/10.1134/S0023158419010075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158419010075