Abstract
In this work, solifenacin succinate (SOLS), flavoxate HCl (FLXHC) and tolterodine tartrate (TOLT) drugs were investigated using thermal analysis (TA) measurements in comparison with electron impact mass spectral fragmentation at 70 eV. Also chemical purity, melting point (using differential scanning calorimetry), activation energy and enthalpy in the process of characterizing medicines were important requirements evaluated in quality control of the pharmaceutical industry. The thermal decomposition of these drugs revealed a moderate stability up to 161, 215 and 195 °C for SOLS, FLXHC and TOLT drugs, respectively, before a complete decomposition in the temperature ranges of 161–800, 215–650 and 195–650 °C. The initial decomposition can be accounted for the loss of C7H12NO molecule, followed by loss of C20H20NO5 molecule for SOLS, loss of HCl, followed by loss of C24H25NO4 molecule for FLXHC, and loss of C23H30NO7 molecule followed by loss of C3H7 for TOLT drug. On the other hand, the molecular ion can easily fragmented by succinate, HCl and tartrate loss followed by loss of C2H5, C4H8 and C2H4 for SOLS, FLXHC and TOLT drugs, respectively. This is the best-selected pathway comparable with decomposition using TA. In addition, computational method including molecular docking was carried out to investigate the E. coli bacterial RNA (4p20) binding to the drug compounds under study. Molecular docking calculation indicated the existence of hydrogen bond and π-interaction between active sites of E. coli bacterial RNA (4p20) and O and or N and aromatic ring in all drug compounds which lead to their stabilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solifenacin succinate (SOLS) is a urinary antispasmodic (anti-muscarinic class) drug. It acts as a direct antagonist at muscarinic acetylcholine receptors in cholinergically innervated organs. Its anti-cholinergic parasympatholytic action decreases the tonus of smooth muscle in the bladder, effectively reducing the number of required voids, urge incontinence episodes and urge severity and improving retention, facilitating increased volume per void [1].
Flavoxate HCl (FLXHC) is an antispasmodic drug that works by counteracting smooth muscle spasms of the urinary tract through directly acting on the muscle. It prevents spasms in the urinary tract and relieves painful or difficult urination, urinary urgency, excessive nighttime urination, pubic area pain, frequent urination and the inability to hold urine caused by urinary tract infections [2].
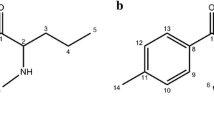
Tolterodine tartrate (TOLT) is an anti-muscarinic drug used in the treatment of urinary urge incontinence and other symptoms of an overactive bladder (Fig. 1) [3].
Literature survey revealed that few chromatographic methods for SOLS [4,5,6,7,8,9,10,11,12,13,14,15], FLXHC [16,17,18,19] and TOLT [20,21,22,23,24,25,26,27], spectrophotometric methods for SOLS [28, 29], FLXHC [30, 31] and TOLT [32,33,34], potentiometric method for the determination of FLXHC [35, 36], spectrofluorimetric method for the determination of FLXHC [37] and voltammetric methods for the determination of FLXHC [38] and TOLT [39, 40] were reported for their determination in bulk, dosage forms and biological fluids.
Thermal decomposition measurements are used to study the physical and chemical changes that occur in the sample under temperature-controlled program. Therefore, one can explain a thermal analysis curve by relating the features of the property against temperature with possible chemical or physical events that have taken place in the system under observation. In thermogravimetric analysis (TG), the mass of sample was measured as a function of temperature at a predetermined rate where the sample may either lose mass to the atmosphere or gain mass by reaction with the atmosphere. In dynamic thermogravimetry (TG), the sample was subjected to continuous temperature changes under usually linear time. Meanwhile, in static thermogravimetric, the sample was maintained at a constant temperature for a period of time where any changes in mass are recorded. Derivative thermogravimetry (DrTG) can be obtained from TG, and it is used to know the decomposition steps of compounds.
Differential thermal analysis (DTA) is the measurement of the difference in temperature between a sample and a reference as heat applied to the system. It provides information on the chemical reaction, phase transformation and structure changes that occur in a sample during a heat-up or a cool-down cycle. The DTA measures the differences in energies released or absorbed and the changes in heat capacity of materials as a function of temperature. All materials behave in certain predictable ways when exposed to certain temperature.
Differential scanning calorimetry (DSC) is used to measure the temperature and heat flows associated with transition in materials as a function of time or temperature. These measurements provide qualitative and quantitative information about the physical and chemical changes that involve endothermic and exothermic processes or changes in heat capacity on DSC technique [41, 42].
The knowledge about drugs interaction mechanisms of drug with plasma proteins as a biomacromolecule was essential requirement to understand pharmacodynamics and pharmacokinetics of drugs [43]. As regards function of plasma proteins as drug carriers, there were a large number of researches about binding of drugs to them [44, 45]. As a result, plasma proteins interact with metal complexes in evaluating the worth drug candidate. Generally, proteins are known as the major targets for most of the drugs in organisms [46]. Their interaction with drug affected on the drug absorption, distribution and elimination in the circulatory system [47] and can prevent their rapid elimination from blood stream [48]. In this work, the behavior of SOLS, FLXHC and TOLT dugs was investigated using electron impact mass spectrometry at 70 eV and thermal analysis techniques such as TG, DrTG, DTA and DSC. These techniques were spirited in material characterization, and many authors have agreed these techniques to characterize the materials [49,50,51,52,53,54]. Therefore, the correct pathway of degradation (TA and MS) knowing this structural session of bonds can be used to predict the active sites of these drugs which responsible for their chemical reactivity. Correlation between the three techniques was discussed in order to throw more light on the degradation behavior of these drugs. Molecular docking of the three drugs under investigation was carried out using molecular docking studies to predict their binding modes toward E. coli bacterial RNA (4p20).
Experimental
Materials
SOLS, FLXHC and TOLT drugs were obtained from Multipharma Pharmaceutical Company (Egypt), Unipharma Pharmaceutical Company (Egypt) and Pfizer Pharmaceutical Company (Egypt), respectively, their purity values were 100.3, 100.5 and 99.72% for SOLS, FLXHC and TOLT drugs, respectively.
Equipment and procedures
Thermal analyses
Thermogravimetric analysis, derivative thermogravimetry and differential thermal analysis measurements were taken by using simultaneous DTA-TG thermal analyzer apparatus (Shimadzu DTG-60H). The mass of samples was ranging from 4 to about 7 mg, using a platinum pan. Measurements were taken from ambient to 800 °C in dynamic nitrogen atmosphere with the flow rate of 30 mL min−1 and heating rate of 10 °C min−1.
DSC curves were obtained using Shimadzu DSC-50 cell. Approximately 3 mg of samples was weighed and placed in a sealed aluminum pan. An empty aluminum pan was used as a reference. The purity determination was performed in temperature range from 10 to 400 °C in nitrogen atmosphere with flow rate of 30 mL min−1 using heating rate of 10 °C min−1. DSC equipment was preliminary calibrated with standard of indium.
Mass spectrometry
The mass spectra of SOLS, FLXHC and TOLT drugs were recorded using Shimadzu GC–MS-Qp 1000 PX quadruple mass spectrometer with electron multiplier detector equipped with GC–MS data system. The sample was ionized by electron beam emitted from the filament, the generated ions being effectively introduced into the analyzer by the focusing and extractor lenses system. The MS was continuously scanned and the spectra obtained were stored. Electron ionization mass spectra were obtained at ionizing energy value of 70 eV, ionization current of 60 mA and vacuum of 10−6 Torr.
Molecular docking simulation
In order to find out the possible binding modes of the drugs under study against E. coli bacterial RNA (4p20), molecular docking studies were performed using Autodock MOA2008.10 software and it is rigid molecular docking software [55] and is an interactive molecular graphics program for calculating and displaying feasible docking modes of drugs and E. coli bacterial RNA (4p20) receptor. It necessitates the drugs and the receptor as input in PDB format. The structure of the drugs in PDB file format was created by Gaussian09 software. The crystal structure of the E. coli bacterial RNA (4p20) receptor was downloaded from the protein data bank (https://www.rcsb.org/pdb/home/home.do).
Results and discussion
Because of their importance as anti-cholinergic drugs, the chemistry and reactivity of SOLS, FLXHC and TOLT drugs have always been of great interest. Knowledge of thermal decomposition mechanism of SOLS, FLXHC and TOLT drugs was very important to understand the chemical processes that exact major fragmentation pathway in EI using conventional MS will be discussed.
Thermal analysis decomposition of drugs
Thermogravimetry is a kind of technology to measure the change in the mass of the samples with the temperature [56,57,58,59]. Figure 2 shows the thermal analyses data (TG, DrTG and DTA) for SOLS, FLXHC and TOLT drugs under the goal of this article. The TG data showed two main mass losses for SOLS and TOLT drugs and four main mass losses for FLXHC drug. The first stage occurred at 161–280 °C, 215–254 °C and 195–295 °C as a result of an estimated mass losses of 26.53, 8.51 and 91.78% which may be accounted for the loss of C7H12NO, HCl and C23H30NO7 molecules (calcd. mass loss = 26.22, 8.53 and 90.96%) for SOLS, FLXHC and TOLT drugs, respectively. The second stage occurred at 280–800 °C, 254–307 °C and 295–650 °C as a result of 72.88, 27.043 and 9.14% estimated mass losses which may be due to the loss of C20H20NO5, C9H8 and C3H7 molecules (calcd. mass loss = 73.67, 27.11 and 9.041%) for SOLS, FLXHC and TOLT, respectively. For FLCHC, the third stage occurred at 307–365 °C due to loss amount to 54.47% may be assigned to the loss of C13H13O4 molecule (calcd. mass loss = 54.44%). The fourth stage occurred at 365–650 °C as a result of 9.87% estimated mass losses which may be due to the loss of C2H4N molecule (calcd. mass loss = 9.81%). The thermal degradation patterns of these drugs are shown in Schemes 1, 2 and 3. These mass losses appeared as endothermic and exothermic peaks as given by DTA data. The first mass loss appeared at 152, 236 and 210 °C as endothermic peaks due to melting point of SOLS, FLXHC and TOLT drugs, respectively. Thus, these drugs melt with decomposition. The second mass loss appeared in DTA as endothermic peaks at 255 and 338 °C for SOLS and at 300 and 238 °C for FLXHC and TOLT, respectively. There were also exothermic peaks at 364, 401, 444 and 510 °C for SOLS and at 281, 313, 366, 451, 488 and 535 °C for TOLT. The third and fourth mass losses appeared in DTA as endothermic peak at 331 °C and exothermic peak at 511 °C for FLXHC.
The thermal analysis results showed that FLXHC, SOLS and TOLT drugs started to decompose at 215, 195 and 161 °C, respectively. Therefore, the order of drugs under investigation was FLXHC > TOLT > SOLS according to their stability.
Mass spectral fragmentation
Electron ionization (EI) mass spectra of SOLS, FLXHC and TOLT drugs at 70 eV were recorded and investigated (Fig. 3). Schemes 4, 5 and 6 show the mass fragmentation patterns of the studied drugs, the loss of C7H12NO molecule to produce C20H20NO5 (m/z = 355, R.I. = 32%) for SOLS and the loss of HCl to produce C24H25NO4 (m/z = 392, R.I. = 53%) and C9H8 to produce C15H17NO4 (m/z = 275, R.I. = 42%) for FLHXC which agree with the results of thermal analysis of these drugs. The important ions are represented in Table 1.
Determination of purity of SOLS, FLXHC and TOLT drugs
For the determination of purity of drugs, DSC technique was utilized. It was based on the assumption that the impurities will lower the melting point of a pure substance. The melting transition of pure substance (100% crystalline) should be infinitely sharp, but impurities or defects in the crystal structure will broaden the melting range and lower the melting point [60]. In a system that contains impurities, Van’t Hoff equation approximately held and allowed the purity value to be calculated as follows: Tf = T0 − [(R T 20 × /ΔH f)· 1/F], where T f is the melting temperature of the sample, T 0 is the melting point of a pure substance in Kelvin (K), R is the gas constant, ΔH f is the heat of fusion, F is fraction of sample melted at T f and x is mole fraction of impurities in the original sample. Figure 4 shows the DSC curves of SOLS, FLXHC and TOLT drugs. It was obvious that very strong and sharp endothermic peaks appeared at 147.5, 236.2 and 213.70 °C which may be attributed to the melting of SOLS, FLXHC and TOLT drugs, respectively. Table 2 shows melting point values and degree of purity of SOLS, FLXHC and TOLT which agree with those of the reported methods.
Molecular docking studies
The method of molecule simulation is an efficient way to predict the correct binding mode and the interaction region. Targeting the minor groove of DNA through binding to a small molecule has long been considered as an important tool in molecular recognition of a specific DNA sequence. It was confirmed that the major factor affecting on the distribution, metabolism, the free concentration and the elimination rate of drugs was the binding affinity of drugs to E. coli bacterial RNA (4p20). Moreover, the half-life of drugs depends on their E. coli bacterial RNA (4p20) binding that affected on the dosage of drugs. Therefore, the study of interaction between E. coli bacterial RNA (4p20) and SOLS, FLXHC and TOLT drugs was essential to investigate the pharmacokinetics and availability of drugs in tissues. The study of interaction of SOLS, FLXHC and TLOT molecules and E. coli bacterial RNA (4p20) can express carcinogens’ carcinogenic properties, contributing to further understanding of the molecular mechanisms of drug, as well as the drug–DNA interactions in rational drug design. The big and small grooves are the mainly binding sites of DNA. The interaction of binding mode can be non-covalent binding, covalent binding and long-range assembly. The first step is to explore the interaction of the binding model of the series of compounds and DNA molecule. Then, the binding energy must be calculated.
Molecular docking studies for the investigated drugs have been performed using MOE 2008.10. software [55] to predict their binding modes toward E. coli bacterial RNA (4p20) [61,62,63]. Figure 5 shows the binding models of SOLS, FLXHC and TLOT to the bacterial RNA. The 2D interaction maps (Fig. 6) showed the different modes of interactions between each drug and the bacterial RNA (Table 3).
The O14 and six-membered ring of SOLS compound were the active sites and bound with O2P(Adenosine31, B) and N4(Cytosine32, B) active sites of E. coli bacterial RNA (4p20) via hydrogen bond and π-interaction, and the binding energy was found to be −3.6 and −1.5 kcal mol−1, respectively. The minimum binding energies of the FLXHC compound with DNA were −3.1, −1.1 and −0.7 kcal mol−1. There are a H-bond and π-interaction between N4 (Cytosine8, A), N4 (Cytosine32, B) and N4 (Cytosine10, A) of E. coli bacterial RNA (4p20) and O27 (pyrone ring), N48 (piperidine ring) and six-membered ring of FLXHC compound, respectively. The minimum energy docked pose revealed that the TLOT compound fitted into the curve contour of the targeted DNA in the groove. Moreover, –C=O group (O32, carbonyl group) and N34 (quinuclidine) of the TLOT compound acted as strong H-bond donors or acceptors and were engaged in hydrogen-bonding interactions with N4 (Cytosine30, B) and N6 (Adenosine29, B) in the DNA. The binding energy was found to be −1.3 and −1.2 kcal mol−1, respectively.
Conclusions
This study provided further insights into application of experimental TA and MS techniques on SOLS, FLXHC and TOLT drugs. From the application of these techniques, it was concluded that there were some similarities in TA and MS fragmentation pathways for SOLS and FLXHC drugs but there was difference in TA and MS fragmentation pathway for TOLT drug. DSC technique can be used to determine purity of drugs giving compatible results with the results obtained using the reported methods. This work revealed the importance of the thermal analysis and DSC techniques for the quality control of SOLS, FLXHC and TOLT drugs. The way of interaction between the active a sites in the drugs with E. coli bacteria was studied and confirmed using molecular docking simulation program.
References
Smulders RA, Krauwinkel WJ, Swart PJ, Huang M. Pharmacokinetics and safety of solifenacin succinate in healthy young men. J Clin Pharmacol. 2004;44:1023–33.
Pedersen E. Studies on the effect and mode of action of flavoxate in human urinary bladder and sphincter. Urol Int. 1977;32:202–8.
Gillberg PG, Sundquist S, Nilvebrant L. Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists. Eur J Pharmacol. 1998;349:285–92.
Reddy BVR, Reddy BS, Raman NVVSS, Subhash RK, Rambabu C. Development and validation of a specific stability indicating high performance liquid chromatographic methods for related compounds and assay of solifenacin succinate. J Chem. 2013;2013:1–10.
Annapurna MM, Sowjanya G, Naidu MS, Lohithasu D. A validated liquid chromatographic method for the determination of solifenacin succinate (urinary antispasmodic) in tablets. Chem Sci Trans. 2014;3:602–7.
Vijayasree V, Kumar DA, Rao JVLNS. Validated RP-HPLC method for the estimation of solifenacin succinate in tablet dosage forms. Pharmanest. 2013;4:206–12.
Krishna SR, Rao BM, Rao NS. A validated rapid stability indicating method for the determination of related substances in solifenacin succinate by ultra fast liquid chromatography. J Chromatogr Sci. 2010;48:807–10.
Desai N, Syed SH, Vasanthraju SG, Karthik A, Udupa N. Development and validation of stability indicating method for determination of solifenacin in bulk formulations. Int J Pharm Pharm Sci. 2011;3:70–4.
Israel DS, Krishnachaitanya K, Gowrisankar D. RP-HPLC method for the estimation of tamsulosin and solifenacin in bulk and its dosage forms. Int J Pharm Sci Res. 2013;4:4343–50.
Paliwal N, Jain P, Dubey N, Sharma S, Khurana S, Mishra R, Paliwal SK. Liquid chromatography tandem mass spectrometry method for quantification of solifenacin in human plasma and its application to bioequivalence study. Int J Drug Dev Res. 2013;5:91–101.
Babu PS, Parveen SKR, Chandrasekar KB, Challa BR, Awen BZSh. In vitro-in vivo correlation studies of modified release solifenacin tablet dosage form. J Sci Res Rep. 2014;3:1905–15.
Puttagunta SB, Shaik RP, Bannoth CK, Challa BSR, Awen BZSh. Bioanalytical method for quantification of solifenacin in rat plasma by LC-MS/MS and its application to pharmacokinetic study. J Anal Sci Tech. 2014;5:1–8.
Oki T, Sato S, Miyata K, Yamada S. Muscarinic receptor binding, plasma concentration and inhibition of salivation after oral administration of a novel antimuscarinic agent, solifenacin succinate in mice. Br J Pharmacol. 2005;145:219–27.
Desai D, Mehta G, Ruikar D, Jain RA, Rajput SJ. Development and validation of stability indicating HPTLC method of solifenacin succinate. Asian J Pharm Biol Res. 2011;1:310–6.
Wankhede SB, Somani K, Chitlange SS. Stability indicating normal phase HPTLC method for estimation of alfuzosin and solifenacin in pharmaceutical dosage form. Int J Chem Tech Res. 2011;3:2003–10.
Attimarad MV, Harsha NS, Setty RS. Simultaneous determination of ofloxacin and flavoxate hydrochloride in human plasma by RP-HPLC. J Liq Chromatogr Rel Tech. 2012;35:768–77.
El-Gindy A, Abdel-Salam ARA, Sallam S. High-performance liquid chromatographic determination of flavoxate hydrochloride and its hydrolysis product. Drug Dev Ind Pharm. 2008;34:1311–22.
Mahesh A. Liquid chromatographic determination of flavoxate HCl in pharmaceutical formulation. J Young Pharm. 2010;2:280–3.
El-Shaheny RN, El-Enany NM, Belal FF. A green HPLC method for the analysis and stability study of flavoxate HCl using micellar eluent. Anal Methods. 2014;6:1001–10.
Kumar SA, Debnath M. Rao JVLNS. Method development and validation of tolterodine tartarate in bulk as well as in pharmaceutical formulation by using RP-HPLC. Int. J Pharm Pharm Sci. 2013;5:665–71.
Parveen SKR, Babu PS, Chandrasekar KB, Challa BR. Analytical method development and validation of tolterodine in pharmaceutical dosage forms by RP-HPLC. Der Pharmacia Lett. 2014;6:246–54.
Ramathilagam N, Meeradevi M, Solairaj P, Rajesh SC. Development and validation of HPLC method for the estimation of tolterodine tartrate in tablets. Int J Pharm Bio Sci. 2012;2:332–7.
Shetty SK, Shah A. Development and validation of tolterodine by RP-HPLC method in bulk drug and pharmaceutical dosage forms. Int J Pharm Tech Res. 2011;3:1083–7.
Krishna SR, Rao BM, Rao NS. A validated stability indicating HPLC method for the determination of related substances and assay of tolterodine tartarate. Rasayan J Chem. 2009;2:144–50.
Mhamunkar SM, Vyavaharkar RY, Bhoir S. RP-HPLC method development and validation for the simultaneous estimation of tamsulosin HCl and tolterodine tartrate in pharmaceutical dosage form. Int J Pharm Pharm Sci. 2012;4:319–22.
Rao MVB, Reddy BC, Rao TS. J.R. Mohanty JR. Determination of tamsulosin hydrochloride 0.2% and tolterodine tartrate 0.2% combination pellets by RP-HPLC method. Res. J. Pharm. Bio. Chem Sci. 2010;1:136–40.
Yanamandra R, Vadla CS, Puppala U, Patro B, Murthy YLN, Ramaiah PA. A new rapid and sensitive stability indicating UPLC assay method for tolterodine tartrate, application in pharmaceuticals, human plasma and urine samples. Sci Pharm. 2012;80:101–14.
Teja GD, Dasu ChD, Babu PS, Ravisankar P. Quantitative analysis of solifenacin succinate in pharmaceutical dosage form using UV absorption spectroscopy. J Chem Pharm Sci. 2013;6:195–8.
Seetharaman R, Lakshmi KS. Development and validation of first order derivative spectrophotometric method for estimation of solifenacin succinate in pharmaceutical formulation. Int J Res Pharm Biomed Sci. 2011;2:1052–7.
Attimarad M. Simultaneous determination of ofloxacin and flavoxate hydrochloride by absorption ratio and second derivative UV spectrophotometry. J Basic Clin Pharm. 2011;2:53–61.
Attimarad M. Simultaneous determination of ofloxacin and flavoxate hydrochloride by first and ratio first derivative UV spectrophotometry. J Iranian Chem Soc. 2012;9:551–7.
Shetty SK, Shah A. New spectrophotometric method for estimation of tolterodine in bulk and pharmaceutical formulation. Int J Pharm Sci Res. 2011;2:1456–8.
Vanilatha S, Theresa MM, Prasanna N, Kumari DS, Harika B, Sirisha P, Xavier MA, Kumari KGB. New method development and validation of tolterodine using visible spectrophotometer. Int J Sci Inov Disc. 2011;1:288–93.
Fraihat SM, Khatib HS. Indirect spectrophotometric determination of tolterodine tartrate in pure and pharmaceutical preparations. Asian J Chem. 2013;25:1887–90.
Rizk MS, Abdel-Haleem FM. Plastic membrane electrodes for the determination of flavoxate hydrochloride and cyclopentolate hydrochloride. Electrochim Acta. 2010;55:5592–7.
Sakr MM, El Nashar RM. Potentiometric determination of tolterodine in batch and flow injection conditions. Talanta. 2012;96:153–60.
Nassar MW, Attia KA, Abou-Seada HM, Allam AE. Spectroflourimetric determination of tolterodine tartarate in pure form and pharmaceutical preparation. Int J Pharm Sci Res. 2013;4:3845–9.
Ghoneim MM, El-Attar MA, Razeq SA. Voltammetric quantitation at the mercury electrode of the anticholinergic drug flavoxate hydrochloride in bulk and in a pharmaceutical formulation. Central Eur J Chem. 2007;5:496–507.
Kul D. Sensitive and selective determination of tolterodine tartrate and its electrochemical investigation on solid carbon based electrodes. J Anal Chem. 2014;69:970–81.
Macikova P, Skopalova J, Cankar P, Papouskova B, Strakova R, Jirovsky D, Maier V. Electrochemical oxidation of tolterodine. Electroanalysis. 2013;25:205–12.
Khandpur RS. Handbook analytical instrumentation. 2nd ed. New Delhi: Tata McGraw-Hill, India; 2006.
Cazes J. Ewing’s analytical instrumentation handbook. 3rd ed. Florida: Florida Atlantic University Boca Raton; 2005.
Cui F, Qin L, Zhang G, Liu Q, Yao X, Lei B. Interaction of anthracycline disaccharide with human serum albumin: investigation by fluorescence spectroscopic technique and modeling studies. J Pharm Biomed Anal. 2008;48:1029–36.
Cui F, Kong X, Qin L, Zhang G, Liu Q, Lei B, Yao X. Specific interaction of 4′-O-(aL-Cladinosyl) daunorubicin with human serum albumin: the binding site II on HAS molecular using spectroscopy and modeling. J Photochem Photobiol B. 2009;95:162–9.
Khan SN, Islam B, Yennamalli R, Sultan A, Subbarao N, Khan AU. Interaction of mitoxantrone with human serum albumin: spectroscopic and molecular modeling studies. Eur J Pharm Sci. 2008;35:371–82.
Lu Y, Cui F, Fan J, Yang Y, Yao X, Li J. Interaction of human serum albumin with N-(4-ethoxyphenyl)-N′-(4-antipyrinyl) thiourea using spectroscopies and molecular modeling method. J Lumin. 2009;129:734–40.
McCallum MM, Pawlak AJ, Shadrick WR, Simeonov A, Jadhav A, Yasgar A, Maloney DJ, Arnold LA. A fluorescence-based high throughput assay for the determination of small molecule–human serum albumin protein binding. Anal Bioanal Chem. 2014;406:1867–75.
Li F, Feterl M, Warner JM, Day AI, Keene FR, Collins JG. Protein binding by dinuclear polypyridyl ruthenium (II) complexes and the effect of cucurbit [10] uril encapsulation. Dalton Trans. 2013;42:8868–77.
Yogam F, VethaPotheher I, Jeyasekaran R, Vimalan M, Antony Arockiaraj M, Sagayaraj P. Growth, thermal, and optical properties of l-asparagine monohydrate NLO single crystal. J Therm Anal Calorim. 2013;114:1153–9.
Suresh P, Janarthanan S, Sugaraj Samuel R, JestinLenus A, Thangaraj K. Studies on the growth, spectral, optical, and thermal aspects of a NLO crystal l-tryptophan hydrogen selenite (LTHS). J Therm Anal Calorim. 2014;115:339–43.
Kannan V, Rakhikrishna R, Philip J, Brahadeeswaran S. Studies on thermophysical and mechanical properties of hydrazonium Ltartrate An organic nonlinear optical material. J Therm Anal Calorim. 2014;116:339–40.
Anitha B, Rathakrishnan S, Malliga P, Pragasam AJA. Crystal growth, structural, optical, thermal and electrical properties of organic NLO l-arginine acetamide single crystal. J Therm Anal Calorim. 2015;119:785–9.
Kazemi A, Hayaty M, Mousaviazar A, Samani KA, Keshavarz MH. The synthesis and characterization of polyvinyl nitrate as an energetic polymer and study of its thermal behavior. J Therm Anal Calorim. 2015;119:613–8.
Yang T, Chen L, Chen W, Zhou Y, Gao H, Zhong T. Thermal stability of 2-ethylhexyl nitrate with acid. J Therm Anal Calorim. 2015;119:205–12.
Molecular Operating Environment (MOE 2008.10), Chemical Computing Group Inc., Montreal, QC, Canada.
Santos LB, Ribeiro CA, Capela JMV, et al. Kinetic parameters for thermal decomposition of hydrazine. J Therm Anal Calorim. 2013;113:1209–16.
Tabacof A, de Araujo Maria, Calado V. Thermogravimetric analysis and differential scanning calorimetry for investigating the stability of yellow smoke powders. J Therm Anal Calorim. 2017;128:387–98.
Zhang Y, Du Z, Han Z, Yao Q, Hu Z. Synthesis and thermal analysis of 2-methyl-4,5-dicyano-2H-1,2,3triazole. J Therm Anal Calorim. 2016;124:529–37.
Sangeetha MK, Mariappan M, Madhurambal G, Mojumdar SC. TG–DTA, XRD, SEM, EDX, UV, and FT-IR spectroscopic studies of l-valine thiourea mixed crystal. J Therm Anal Calorim. 2015;119:907–13.
Hatakeyama T, Liu Z. Hand book of thermal analysis. London: Wiley; 1998.
Anthony CM, Osselton MD, Widdop B. Clark’s analysis of drugs and poisons. 3rd ed. London: Pharmaceutical Press; 2004.
Neil MJO. The merck index, an encyclopedia of chemicals, drugs and biological. 14th ed. New Jersey: Merck Research Laboratories, Whitehouse Station; 2006.
Kondo J, François B, Russell RJ, Murray JB, Westhof E. Crystal structure of the bacterial ribosomal decoding site complexed with amikacin containing the γ-amino-α-hydroxybutyryl (haba) group. Biochimie. 2006;88:1027–31.
Acknowledgements
The authors would like to express their gratitude to the National Organization for Drug Control and Research (NODCAR, Egypt) and Faculty of Science, Cairo University, for providing instruments and the means necessary to accomplish this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attia, A.K., Mohamed, G.G. & Ahmed, H.E. Structural investigation, molecular structure and molecular docking of solifenacin succinate, flavoxate hydrochloride and tolterodine tartrate anti-cholinergic drugs. J Therm Anal Calorim 131, 1345–1360 (2018). https://doi.org/10.1007/s10973-017-6592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6592-x