Abstract
Organically modified montmorillonite (OMMT) was used as synergist to enhance the flame-retardant and mechanical properties of poly(butylene succinate)/intumescent flame retardant (PBS/IFR) composites. The flame-retardant, thermal degradation and combustion properties of PBS and its flame-retardant composites were characterized by limiting oxygen index (LOI) test, vertical burning (UL-94) test, thermogravimetric analysis, cone calorimeter and scanning electron microscopy, respectively. The results indicate that PBS/IFR composites exhibit excellent flame retardance when OMMT is at an appropriate content. PBS/IFR composite with 20 wt% IFR and 1.5 wt% OMMT has an LOI of 40.1% and can pass the UL-94 V0 rating. The synergistic effect between OMMT and IFR on the flame-retardant properties of PBS depends on the content of OMMT, and excessive OMMT diminish this synergistic effect. The possible flame-retardant mechanism of OMMT on PBS/IFR composite is proposed. The results of mechanical test also indicate that OMMT can effectively increase the notched impact strength of PBS/IFR composites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an alternative to conventional nonbiodegradable polymers, poly(butylene succinate) (PBS) has attracted great interest and been expected to be one of the most competitive biodegradable polymers due to its outstanding biodegradability, superior processability, economical advantages, good thermal and mechanical properties [1–4]. PBS has been widely used in many fields, such as agricultural films, biomedical materials, packing, fibers and injection-molded products [5–7]. However, PBS is highly combustible and seriously dripping during combustion [8], which restrict its applications in the fields of flame retarding, such as textile industry, automotive components and electrical industry. Thus, it is necessary to improve the flame-retardant property of PBS [9–11].
Recently, various flame retardants have been used to improve the flame retardance of PBS [12–16], among which intumescent flame retardant (IFR) has received extensive attention due to the advantages of low smoke, low toxicity and no dripping during combustion [12–14]. IFR systems, which consist of an acid source, a carbonization compound and a blowing agent, can produce upon heating a swollen char on the surface of the burning polymer, and slow down the heat and mass transfer between gas and condensed phases [17]. However, the sole utilization of IFR usually requires a relatively high loading of flame-retardant additives [12]; moreover, the inferior compatibility between IFR and matrix results in the decrease in mechanical properties of polymer composites as well as flame-retardant efficiency [8]. Thus, development of novel synergistic agents with IFR systems for high efficient flame-retardant PBS is crucial.
Nanoparticles, such as POSS [18], fumed silica [12] and graphene [13], have been used as synergistic agents in combination with IFR systems to improve the flame retardancy of PBS. The incorporation of nanoparticles has obviously reduced the heat release rate of combustion and improved the anti-dripping properties of PBS, resulting from the reinforcement of heat-resistant char [12]. Moreover, the incorporation of nanoparticles exhibited reinforcements on the mechanical properties of PBS/IFR composites, which could be attributed to the strong interfacial interaction between the nanoparticles and PBS matrix [13, 18].
Montmorillonite (MMT) is a class of inorganic nanoparticles with a layer structure and has a positive effect on the flame-retardant properties of polymer [19–21]. A relatively low quantity of MMT in the polymer matrix could create a protective char layer during combustion, and the accumulation of the clay on the surface of the material could act as a protective barrier that limited heat transfer to the material and reduced the volatilization of combustible degradation products, which fed the flame and the diffusion of oxygen into the material [22]. Li et al. [23] studied the synergistic effect of organically modified montmorillonite (OMMT) and IFR system on the flame retardancy and melt stability of PLA and found that the presence of OMMT could improve the melt stability of PLA flame-retardant composites, which suppressed the melt dripping effectively. Moreover, MMT has been proven to strengthen the polymer matrix due to the layer structure [24].
In view of the advantages of MMT, it is expected that MMT will have a synergistic effect with IFR on the flame retardancy of PBS, and which will reduce the loading of IFR and mitigate the decrease in mechanical properties of PBS. Moreover, the incorporation of MMT may increase the mechanical properties of PBS. However, the application of MMT in PBS/IFR composites has not been reported to the best of our knowledge. The aim of the present work is to study the effect of the organically modified montmorillonite (OMMT) on the flame-retardant and mechanical properties of PBS/IFR composites. The flame-retardant properties were evaluated by limiting oxygen index (LOI), vertical flammability (UL-94) tests and cone calorimeter tests. The thermal properties and the morphology of residual char were investigated using thermogravimetric analysis (TG) and scanning electron microscopy (SEM), respectively. The optimum loading of OMMT, at which PBS/IFR composites had the better flame-retardant and mechanical properties, was investigated. Finally, the possible synergistic effect of OMMT on the flame-retardant mechanism of PBS/IFR composites was proposed.
Experimental
Materials
Poly(butylene succinate) (PBS, weight-average molecular weight: 190,000, hydroxyl end-capped) was purchased from Anqing Hexing Chemicals Co., Ltd. (Anhui, China). Ammonium polyphosphate (APP) and melamine (MA) were provided by Shenzhen Hongtaiji Industrial Co., Ltd. (Guangdong, China). The DK4 Ca-montmorillonite (MMT) modified by cetyl quaternary ammonium was purchased from Zhejiang Fenghong Clay Chemicals Co., Ltd. (Zhejiang, China). The intumescent flame retardant (IFR) was composed of APP and MA (the mass ratio was 2:1).
Sample preparation
All materials were dried in an oven at 60 °C for 12 h. PBS/IFR composites without and with OMMT were prepared using a CTE20 corotating twin-screw extruder (Coperion Keya (Nanjing) Machinery Co., Ltd. China). The blending temperature was set at 130 °C, and the rotation speed was maintained at 80 rpm. All the materials were simultaneously added into the extruder after previous mixing. The compositions for the specimens are listed in Table 1. After compounding, the samples were injection-molded and hot-pressed at about 130 °C into special specimens for analysis.
Characterization
Thermogravimetric analysis (TG) was carried out on 10 mg samples using a TGA Q50 (TA Instruments, USA) at 10 °C min−1 from 50 to 650 °C under air atmosphere at a flow rate of 50 cm3 min−1. The onset decomposition temperatures taken into account were the temperature at 5% of mass loss (T d,−5%) and the temperature of maximum mass loss rate (T max).
The limiting oxygen index (LOI) test was measured using a DRK 304B oxygen index meter (Shandong Drick Instruments Company, China) according to ASTM D2863. The specimens used for the test were of dimensions 100 mm × 6.5 mm × 3 mm.
The vertical test was carried out on a CFZ-2-type instrument (Jiangning Analysis Instrument Company, China) according to the UL 94 test standard. The specimens used were of dimensions 130 mm × 13 mm × 3 mm.
The cone calorimeter (FTT, UK) tests were performed according to ISO 5660 standard procedures. Each specimen of dimensions 100 mm × 100 mm × 3 mm was wrapped in aluminum foil and exposed horizontally to an external heat flux of 35 kW m−2.
Tensile properties were measured using a CMT6103 machine (MTS Systems Corporation, USA) in accordance with GB/T16421-1996. The crosshead speed was set at 50 mm min−1. The tensile modulus of the samples was determined at 0.5% strain. Charpy impact strength was obtained using a JJ-20 impact tester (Changchun Intelligent Instrument and Equipment Co., Ltd. China); the notched specimens was with single-edge V-shaped notch of 2 mm depth milled in the molded specimens with the dimensions of 80 mm × 10 mm × 4 mm. An impact velocity of 2.9 m s−1 was used. All mechanical testing was done at room temperature.
The dispersion of MMT and the surfaces of char residues after LOI test were examined using a Zeiss Ultra 55 field emission scanning electron microscope (SEM, Carl Zeiss, Germany) equipped with energy dispersive spectroscopy (EDS). The accelerating voltage was set at 5 kV.
Results and discussion
Dispersion of OMMT in PBS matrix
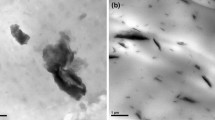
SEM was employed to investigate the morphology of OMMT and the fractured surfaces of Charpy impact samples to evaluate the dispersion of OMMT in PBS matrix, and the SEM microphotographs are shown in Fig. 1. Figure 1b shows that OMMT microsheets disperse homogeneously in PBS matrix although they do not form an exfoliated-intercalated nanostructure. The interfaces between OMMT sheets and PBS matrix are blurry, which indicates that the interfacial interaction between PBS matrix and OMMT microsheets is improved mainly due to the modification of MMT by cetyl quaternary ammonium. The well dispersion of OMMT microsheets and improved interfacial interaction between PBS matrix and OMMT microsheets are expected to effectively improve the flame-retardant and mechanical properties of PBS/IFR composites [12, 25].
Thermogravimetric analysis (TG)
The TG and DTG curves for pure PBS and its flame-retardant composites under air atmosphere are presented in Fig. 2 and the related data are listed in Table 1. As portrayed in Fig. 2, pure PBS shows relatively good thermal stability. The initial decomposition temperature (T d,−5%) of pure PBS is about 306 °C, and the temperature of maximum rate of mass loss (T max) is around 389 °C, and the char residue at 650 °C is about 1.5 mass%. With the addition of 20 mass% IFR, the T d,−5% and T max of PBS matrix are reduced to 294 and 358 °C, respectively. The lower thermal stability of PBS/IFR composite is attributed to the premature decomposition of IFR, which can further catalyze the decomposition of PBS and promote the formation of intumescent char layer [18]. Thus, the char residue at 650 °C increases with the addition of IFR, and the more loading of IFR, the more char residue.
The thermal stability of PBS/IFR20 is further reduced by the presence of OMMT, which is ascribed to the decomposition of cetyl quaternary ammonium organomodifier of OMMT. However, after addition of OMMT, the char residues at 650 °C increase obviously as compared to PBS/IFR20, especially when the loading of OMMT is 1.5 mass%, the char residues at 650 °C of which is more than that of PBS/IFR30. It indicates that OMMT can help to promote the formation of a more effective char, which is generally attributed to the strongly protonic catalytic sites created by the decomposition of the organomodifier and the Bronsted and Lewis acid sites present on the clay lattice [22, 26, 27] and also the barrier effect of the OMMT layers towards polymer decomposition products ablation [24]. Thus, there exists a synergistic effect between IFR and OMMT to catalyze the char formation of PBS. However, further addition of OMMT to 3.0 mass% does not increase the char residue at 650 °C.
Flame-retardant properties
Limiting oxygen index (LOI) and vertical burning (UL-94) test
LOI measurements and UL-94 vertical burning tests were used to evaluate the flame-retardant properties of pure PBS and its flame-retardant composites, and the main results obtained are shown in Table 1. Pure PBS exhibits a LOI value of 22.4% and presents some flame retardance because of the melt dripping during combustion, which can remove the molten polymer material from the pyrolysis zone and the heat from the surface [24]. However, the melt dripping ignites the absorbent cotton and it is not classified in the UL-94 test. When 20 mass% IFR was added, the LOI value raises to 32.6%, but the composite still can only achieve the UL-94 V2 rating due to the melt dripping. The LOI value goes up to 38.8%, and the composite can achieve the UL-94 V0 rating, as the content of IFR increases to 30 mass%.
Addition of OMMT increases the LOI value and improves the UL-94 rating of PBS/IFR composites. The LOI value of PBS/IFR20-1.0 goes up to 36.3%, and it can achieve the UL-94 V0 rating without any melt dripping. The LOI value increases to 40.1% with the OMMT loading of 1.5 mass%, which is higher than that of PBS/IFR30. This indicates that there will be a synergistic effect between IFR and OMMT to improve the anti-dripping and flame-retardant properties of PBS. It is worth noting that the LOI value declines to 37.9% with the addition of 3.0 mass% OMMT, suggesting that there will be an appropriate content for OMMT to improve the flame-retardant property of PBS/IFR composites.
Figure 3 shows the digital photographs of the specimens after LOI tests. Pure PBS exhibits serious melt dripping and has no char residue. The presence of 20 mass% IFR catalyzes the formation of obvious intumescent char, which prevents the dripping of molten PBS matrix and acts as a superior thermal insulator and mass transport barrier to protect the underlying PBS matrix from further burning and reduce its heat release [12]. In accordance with LOI and UL-94 results, addition of 1.5 mass% OMMT helps to form a glassy coat and a more notable intumescent char, thus increasing the flame retardance of PBS/IFR composites. However, it seems likely that the higher content of OMMT, just as 3.0 mass%, may diminish this effect, and the intumescent char of PBS/IFR20-3.0 is less than that of PBS/IFR20-1.5.
Cone calorimeter test
Cone calorimeter test can provide numerous combustion properties of a material, such as time to ignition (TTI), heat release rate (HRR), peak of heat release rate (PHRR), average heat release rate (AHRR), total heat released (THR), average mass loss rate (AMLR) and char yield (CY) [18, 28]. The corresponding data of cone calorimeter test are presented in Table 2, and Fig. 4 illustrates the HRR, the THR and the mass loss as functions of combustion time for PBS and its flame-retardant composites.
As can be observed from Fig. 4, pure PBS shows a distinct peak on the HRR curve with the peak value of 538 kW m−2 and has a large THR of 64.6 MJ m−2. When incorporated IFR and OMMT, there exist two peaks on the HRR curves of PBS flame-retardant composites. The peak appearing early is attributed to the heat release of combustible gas produced by the pyrolysis of PBS, and the peak appearing later is due to the partial breakage of the char layer from continuous high-temperature oxidation [14].
The incorporation of IFR obviously decreases the PHRR and THR. For example, the PHRR and HRR of PBS/IFR20 are diminished to 340 kW m−2 and 49.3 MJ m−2, respectively, which are 37 and 24% lower than those of pure PBS, respectively. Moreover, addition of OMMT further decreases the PHRR and HRR of PBS/IFR20 and of PBS/IFR20-1.0 to 176 kW m−2 and 32.6 MJ m−2, respectively, which are even less than those of PBS/IFR30. The reduction in HRR and THR implies that OMMT can not only prolong the time of burning but also decrease the total amount of material offered for combustion. However, the HRR of PBS/IFR20-3.0 is 47.2 MJ m−2, which is higher than that of PBS/IFR20-1.0. It suggests that large amount of OMMT reduces the synergistic effect between IFR and OMMT for the flame resistance of PBS matrix.
It can be observed from Fig. 4 and Table 2 that the TTIs of PBS flame-retardant composites decrease as compared to pure PBS, which is probably that the presence of IFR and OMMT may catalyze the PBS decomposition, just as mentioned on TG analysis. The AMLR and CY of PBS flame-retardant composites are obviously reduced and increased, respectively, as compared to pure PBS. These phenomena can be attributed to the carbonization of PBS by the presence of IFR and the enhanced barrier effect of intumescent char by the presence of OMMT. However, the AMLR of PBS/IFR20-3.0 is larger than that of PBS/IFR20-1.0, and the CY of PBS/IFR20-3.0 is even less than that of PBS/IFR20. It indicates that the enhanced barrier effect of intumescent char by OMMT diminishes at high content.
Figure 5 shows the digital photographs of the residue char for PBS and its flame-retardant composites after the cone calorimeter tests. Pure PBS leaves little residue char after burning and PBS/IFR20 has remarkable, but rough and cracked intumescent char residue. Addition of 1.0 mass% OMMT helps PBS/IFR20 produce more notable and perfect char residue, which is continuous and has little crack. Therefore, it can effectively prevent the heat and mass transfer between the flame and the polymer substrate and protect the underlying materials from further burning. However, when added 3.0 mass% OMMT, the char residue of PBS/IFR20 becomes fragmentized and has a poor physical barrier to isolate the heat and mass between the flame and the polymer substrate. As a result, the PHRR, THR and CY values of PBS/IFR20-3.0 are significantly reduced.
Characterization of the char residue
The refined morphology of carbonaceous char of PBS flame-retardant composites after LOI tests were analyzed by SEM and shown in Fig. 6. The intumescent carbonaceous char of PBS/IFR20 is rough, and there are distinct traces of raised bubbles caused by the volatile gas produced by PBS and IFR on the surface of the carbonaceous char (arrowed in Fig. 6a). At the same time, there are lots of cracks on the surface of carbonaceous char (arrowed in Fig. 6b), it is indicated that the carbonaceous char catalyzed by IFR is not strong enough to trap the vast volatile gas produced by PBS and IFR. The carbonaceous char of PBS/IFR20-1.0 exhibits relatively smooth and has little cracks, and the MMT sheets accumulate and embed in the surface of the carbonaceous char (circled in Fig. 6c). The accumulation of MMT sheets is propelled by the burst of bubbles at the sample surface, exhibiting island-like floccules instead of a continuous net-like protective layer [29]. This perfect carbonaceous char can effectively hinder the diffusion of combustible gas and prevent the propagation of oxygen and heat into the interior matrix, thus improving the flame-retardant property of PBS/IFR composites. However, while the content of OMMT increases to 3.0 mass%, the carbonaceous char of PBS/IFR20 shows rough and has lots of holes, besides which there are a number of cracked MMT aggregates on the surface of carbonaceous char (circled in Fig. 6d).
The chemical components of PBS/IFR composites modified by OMMT before and after LOI tests were investigated by EDS and shown in Table 3. In PBS/IFR composites modified by OMMT, the nitrogen and phosphorus element are related to IFR, and the aluminum and silicon element are introduced by OMMT. It can be seen from Table 3 that the relative phosphorus content of combusted samples are much more than those of uncombusted samples. This is due to the formation of various kinds of phosphorus-containing compounds in the carbonaceous char, which are generated by the pyrolysis of IFR and can cross-link into a network structure to protect the material beneath [12]. The aluminum and silicon content of combusted samples are higher than that of uncombusted samples, and the aluminum and silicon content of combusted PBS/IFR20-3.0 are also higher than that of combusted PBS/IFR20-1.0. This indicates that OMMT accumulates on the surface of carbonaceous char rather than sinking through the molten PBS matrix to strengthen the carbonaceous char and postpone the degradation process which will form combustible substance [30]. However, excessive accumulation of OMMT would embrittle the carbonaceous char, and the carbonaceous chars become easily destroyed by the volatile gas. The conjecture is proven by the hole on the carbonaceous char of PBS/IFR20-3.0 (Fig. 6d) and the less nitrogen element on the carbonaceous char of PBS/IFR20-3.0 as compared to PBS/IFR20-1.0, which indicates the escape of the nitrogen-containing volatile gas from the cracked carbonaceous char.
Possible flame-retardant mechanism
The flame-retardant mechanism of MMT for polymer has been investigated by various researchers. It has been widely accepted that MMT can catalyze the formation of a stable char residue [26, 27], and the accumulation of MMT on the surface of the material can act as a protective barrier that limits heat and oxygen transfer to the material and reduces the volatilization of combustible degradation products [22, 31]. The synergy between MMT and phosphorus-containing flame retardant was proposed by Song [32] that polyphosphoric acid reacted with MMT to develop a glassy coat and a stable carbonaceous–silicious–phosphorated charred layer, which insulated the underlying material. Tang [33] also found that the synergy between MMT and the flame retardant was closely associated with their ratio, an antagonism was observed at high MMT loadings. This phenomenon is explained by the dual counteracting effect of silicates. MMT, reassembling during fire on polymer’s surface, not only hinders the diffusion of oxygen, heat and flammable gases but also hampers the volatilization of ammonia (evolved by APP decomposition) whose role is to foam the char, thus hampering the fused carbonaceous char from swelling [33]. According to our research, it is likely that apart from the above mechanism, the accumulation of MMT may embrittle the intumescent char at higher amount, and thus, the synergistic action between MMT and IFR is diminished. Thus, the possible flame-retardant mechanism of PBS/IFR composites without and with OMMT may be deduced as follow:
When heated, PBS will decompose and produce combustible gas, which diffuses and burns when in contact with oxygen. At the same time, the heat created by combustion will transfer to the matrix, resulting in a serious dripping due to the relatively low melt viscosity of PBS. For PBS/IFR systems, the phosphoric acidic species decomposed from IFR will catalyze the formation of viscous carbonaceous char to encapsulate on the molten matrix. The raised bubbles indicates that the carbonaceous char can trap the combustible gas and provide resistances of both oxygen propagation and heat transfer, thus keeping the underlying materials from further burning and postponing the degradation process. However, the carbonaceous char is not strong enough to effectively prevent the escape of the considerable volatile gas produced by PBS and IFR, which break through and cause the numerous cracks on the carbonaceous char. When added appropriate amount of OMMT, the organic modifier of OMMT will give an additional catalytic effect to the formation of carbonaceous char [22], and the accumulation of OMMT microsheets on the surface of carbonaceous char also provide a physical barrier to mass and heat transfer [27]. What’s more, OMMT microsheets can act as a physical or chemical cross-linking to strengthen the carbonaceous char, providing a more stable shield for the material beneath from the radiated heat and limiting the diffusion of the considerable volatile products available for burning in the gas phase [31]. Thus, the incorporation of OMMT has a synergistic effect with IFR to improve the flame resistance of PBS. However, if added excessive OMMT, more OMMT microsheets accumulate and aggregate on the surface of carbonaceous char, and the carbonaceous char will become brittle and easy to be destroyed by the volatile gas, resulting a diminished physical barrier to both mass and heat transfer. Thus, there will be an appropriate content for OMMT to have a synergistic effect with IFR on the flame-retardant property of PBS.
Mechanical properties
The tensile stress–strain curves of PBS and its flame-retardant composites are shown in Fig. 7, and Table 4 presents the tensile and notched impact properties of PBS and its flame-retardant composites. IFR has an obviously negative effect on the tensile strength, elongation at break and notched impact strength of PBS because of the poor interfacial interaction between IFR and PBS matrix. The tensile strength, elongation at break and notched impact strength of PBS decrease gradually with the increase of IFR. When the content of IFR is 30 mass%, the tensile strength, elongation at break and notched impact strength of PBS decrease from 33.0 MPa, 192% and 8.1 kJ m−2 to 23.1 MPa, 10.6% and 4.1 kJ m−2, respectively. Addition of OMMT slightly decreases the tensile strength of PBS/IFR composites due to a slight plasticizer effect conferred by organomodifier in clay (cetyl quaternary ammonium) [24]. However, the plasticizer effect is positive to the notched impact strength of PBS/IFR composites, which can induce much more deformation of PBS matrix during the fracture process, thus absorb large energy. Thus, the notched impact strength of PBS/IFR20 with different content of OMMT (6.3–7.0 kJ m−2) is higher than that of PBS/IFR20 (4.2 kJ m−2). Addition of OMMT also improves the tensile modulus of PBS/IFR composites due to the good dispersion and layer structure of OMMT.
Conclusions
We have used organically modified montmorillonite (OMMT) as synergistic agent for intumescent flame retardant (IFR) on the flame retardance of PBS and to mitigate the decrease in mechanical properties of PBS/IFR composites. PBS/IFR composite exhibits excellent flame-retardant properties when the OMMT is at an appropriate content. The optimum content of OMMT for PBS/IFR20 is 1.5 mass%, at which PBS/IFR20 have an LOI of 40.1% and can pass the UL-94 V-0 rating. During combustion process, OMMT microsheets tend to migrate and accumulate on the surface of carbonaceous char, and act as physical or chemical cross-linking to strengthen the carbonaceous char, leading to more stable carbonaceous char to effectively prevent the transfer of both mass and heat. This synergistic effect between OMMT and IFR on the flame retardance of PBS depends on the content of OMMT, and excessive OMMT will embrittle the carbonaceous char and diminish the synergistic effect. The results of mechanical test also indicate that OMMT can effectively increase the notched impact strength of PBS/IFR composites.
References
Tachibana Y, Masuda T, Funabashi M, Kunioka M. Chemical synthesis of fully biomass-based poly(butylene succinate) from inedible-biomass-based furfural and evaluation of its biomass carbon ratio. Biomacromolecules. 2010;11:2760–5.
Mohanty AK, Misra M, Hinrichsen G. Biofibres, biodegradable polymers and biocomposites: an overview. Macromol Mater Eng. 2000;276:1–24.
Gan ZH, Abe H, Doi Y. Crystallization, melting, and enzymatic degradation of biodegradable poly(butylene succinate-co-14 mol ethylene succinate) copolyester. Biomacromolecules. 2001;2:313–21.
Gigli M, Negroni A, Soccio M, Zanaroli G, Lotti N, Fava F, Munari A. Influence of chemical and architectural modifications on the enzymatic hydrolysis of poly(butylene succinate). Green Chem. 2012;14:2885–93.
Sinha Ray S, Okamoto K, Okamoto M. Structure–property relationship in biodegradable poly(butylene succinate)/layered silicate nanocomposites. Macromolecules. 2003;36:2355–67.
Shah AA, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics: a comprehensive review. Biotechnol Adv. 2008;26:246–65.
Bin T, Qu JP, Liu LM, Feng YH, Hu SX, Yin XC. Non-isothermal crystallization kinetics and dynamic mechanical thermal properties of poly(butylenes succinate) composites reinforced with cotton stalk bast fibers. Thermochim Acta. 2011;525:141–9.
Hu WZ, Wang BB, Wang X, Ge H, Song L, Wang J, Hu Y. Effect of ethyl cellulose microencapsulated ammonium polyphosphate on flame retardancy, mechanical and thermal properties of flame retardant poly(butylene succinate) composites. J Therm Anal Calorim. 2014;117:27–38.
Kuan CF, Kuan HC, Ma CCM, Chen CH. Flame retardancy and nondripping properties of ammonium polyphosphate/poly(butylene succinate) composites enhanced by water crosslinking. J Appl Polym Sci. 2006;102:2935–45.
Nie SB, Liu XL, Dai GL, Yuan SJ, Cai F, Li BX, Hu Y. Investigation on flame retardancy and thermal degradation of flame retardant poly(butylene succinate)/bamboo fiber biocomposites. J Appl Polym Sci. 2012;125:E485–9.
Jin TX, Zhang XY, Tao YF, Wang D, Chen F, Fu Q. A novel biodegradable phosphorus-containing copolyester with preferable flame retardancy and mechanical properties. RSC Adv. 2015;5:61364–70.
Chen YJ, Zhan J, Zhang P, Nie SB, Lu HD, Song L, Hu Y. Preparation of intumescent flame retardant poly(butylene succinate) using fumed silica as synergistic agent. Ind Eng Chem Res. 2010;49:8200–8.
Wang X, Song L, Yang HY, Lu HD, Hu Y. Synergistic effect of graphene on antidripping and fire resistance of intumescent flame retardant poly(butylene succinate) composites. Ind Eng Chem Res. 2011;50:5376–83.
Yang HY, Song L, Tai QL, Wang X, Yu B, Yuan Y, Hu Y, Yuen RKK. Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites. Polym Degrad Stab. 2014;105:248–56.
Liu YJ, Mao L, Fan SH. Preparation and study of intumescent flame retardant poly(butylenes succinate) using MgAlZnFe–CO3 layered double hydroxide as a synergistic agent. J Appl Polym Sci. 2014;131:8964–73.
Chen Q, Wen X, Chen H, Qi YL, Gong J, Yang HF, Li YH, Tang T. Study of the effect of nanosized carbon black on flammability and mechanical properties of poly(butylene succinate). Polym Adv Technol. 2015;26:128–35.
Bourbigot S, Le Bras M, Duquesne S, Rochery M. Recent advances for intumescent polymers. Macromol Mater Eng. 2004;289:499–511.
Wang X, Hu Y, Song L, Yang HY, Yu B, Kandola B, Deli D. Comparative study on the synergistic effect of POSS and graphene with melamine phosphate on the flame retardance of poly(butylene succinate). Thermochim Acta. 2012;543:156–64.
Janowska G, Mikołajczyk T, Olejnik M. Effect of montmorillonite content and the type of its modifier on the thermal properties and flammability of polyimideamide nanocomposite fibers. J Therm Anal Calorim. 2008;92:495–503.
Kiliaris P, Papaspyrides CD. Polymer/layered silicate (clay) nanocomposites: an overview of flame retardancy. Prog Polym Sci. 2010;35:902–58.
Saad GR, Naguib HF, Elmenyawy SA. Effect of organically modified montmorillonite filler on the dynamic cure kinetics, thermal stability, and mechanical properties of brominated epoxy/aniline formaldehyde condensates system. J Therm Anal Calorim. 2013;111:1409–17.
Laoutid F, Bonnaud L, Alexandre M, Lopez Cuesta JM, Dubois P. New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng, R. 2009;63:100–25.
Li SM, Yuan H, Yu T, Yuan WZ, Ren J. Flame-retardancy and anti-dripping effects of intumescent flame retardant incorporating montmorillonite on poly(lactic acid). Polym Adv Technol. 2009;20:1114–20.
Fukushima K, Murariu M, Camino G, Dubois P. Effect of expanded graphite/layered-silicate clay on thermal, mechanical and fire retardant properties of poly(lactic acid). Polym Degrad Stab. 2010;95:1063–76.
Bian JJ, Han LJ, Wang XM, Wen X, Han CY, Wang SS, Dong LS. Nonisothermal crystallization behavior and mechanical properties of poly(butylene succinate)/silica nanocomposites. J Appl Polym Sci. 2010;116:902–12.
Xie W, Gao Z, Pan WP, Hunter D, Singh A, Vaia R. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem Mater. 2001;13:2979–90.
Bellucci F, Camino G, Frache A, Sarra A. Catalytic charring–volatilization competition in organoclay nanocomposites. Polym Degrad Stabil. 2007;92:425–36.
Schartel B, Hull TR. Development of fire-retarded materials e interpretation of cone calorimeter data. Fire Mater. 2007;31:327–54.
Kashiwagi T, Harris RH Jr, Zhang X, Briber RM, Cipriano BH, Ragharan SR, et al. Flame retardant mechanism of polyamide 6-clay nanocomposites. Polymer. 2004;45:881–91.
Tang Y, Hu Y, Wang S, Gui Z, Chen Z, Fan W. Intumescent flame retardant–montmorillonite synergism in polypropylene-layered silicate nanocomposites. Polym Int. 2003;52:1396–400.
Zeng QH, Yu AB, Lu GQ, Paul DR. Clay-based polymer nanocomposites: research and commercial development. J Nanosci Nanotechnol. 2005;5:1574–92.
Song L, Hu Y, Yong Y, Zhang R, Chen Z, Fan W. Study on the properties of flame retardant polyurethane/organoclay nanocomposite. Polym Degrad Stabil. 2005;87:111–6.
Tang Y, Hu Y, Li B, Liu L, Wang Z, Chen Z, et al. Polypropylene/montmorillonite nanocomposites and intumescent, flameretardant montmorillonite synergism in polypropylene nanocomposites. J Polym Sci A Polym Chem. 2004;42:6163–73.
Acknowledgements
The project was supported by Guangdong Provincial Key Laboratory of High Performance Resin-Based Composites.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, S., Wu, X. et al. Effect of montmorillonite on the flame-resistant and mechanical properties of intumescent flame-retardant poly(butylene succinate) composites. J Therm Anal Calorim 128, 1417–1427 (2017). https://doi.org/10.1007/s10973-017-6092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6092-z