Abstract
The present research is focused on investigation of torrefied biomass combustion process and kinetic analysis. Two wood biomass samples (B1 and B2) were torrefied in a specially designed set-up under 1.0 h of residence time, 230, 260 and 290 °C temperatures, and argon atmosphere. The studied materials were characterised in terms of their chemical composition and calorific value. The results showed the improvements of biomass properties towards higher carbon content and low moisture content fuel. The behaviour and comparison of raw and torrefied biomass during the combustion process was investigated by thermal analysis (TG, DTG and DTA). The samples were heated at an ambient temperature up to 700 °C at constant rates: 10, 20 and 40 °C min in air flow. The MS technique was also used simultaneously with TG to determine gaseous products from combustion process (namely NO, CH4, CO2, and H2O). The kinetic parameters were calculated for torrefied biomass combustion using three isoconversional methods: Friedman, Kissinger–Akahira–Sunose and Flynn–Wall–Ozawa. The isoconversional methods were used to find dependency of the activation energy of studied processes on the conversion degree. The kinetic data for raw and torrefied biomass indicates that torrefaction process reduces the activation energy of the studied biomass. The average values of activation energy for biomass combustion e.g. TB1 are E a = 111; 105.2 and 110.4 kJ mol−1 calculated by Friedman, KAS and FWO methods, respectively. For all studied biomass samples, the slight differences between the values of activation energies calculated by Friedman, FWO and KAS methods were obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of renewable energy and alternative fuel for environmental sustainability has become a crucial topic currently. Nowadays, biomass is a versatile energy source, which stands out as one of the most important renewable energy sources. Biomass energy or bioenergy is an important third energy resource after oil and coal in the world, and it has been extensively applied, because of its high reactivity and volatility. Biomass can be utilised in the forms of solid, liquid and gas for energy. Therefore, biomass is studied in a variety of conversion techniques such as physical, thermal, chemical and biological methods due to obtain clean and sustainable source of energy for electricity and industrial applications [1]. Due to its variety of species and compositions which depend on its origin, several operating problems occur during conversion process. Thus, it is necessary to continuously enhance the biomass properties such as hydrophilic nature, low heating value and high energy required in grinding to improve its efficiency of utilisation. The pretreatment process, as torrefaction, has been found to improve the biomass properties. Torrefaction, so-called mild pyrolysis, is a process where raw biomass is heated under an inert atmosphere at a range of temperature 200–300 °C. During this process, the hydroxyl groups are removed producing a hydrophobic material. Torrefied biomass in contrast to raw one is enhanced by a higher calorific value and energy density, a lower O/C ratio and moisture content. The obtained solid product is easier to grind, because it is brittle. Moreover, the torrefied biomass is more resistant on biological degradation getting safety storage and easier transport. The great advantage is the significant size reduction of torrefied biomass [2, 3]. During biomass torrefaction process, devolatilisation, depolymerisation and carbonisation of lignocellulose components take place. Besides solid product, water, organic, lipids, alkali, CO, CO2, H2, CH4 and primary tars are also formed [4]. Gaseous and liquid products have to be minimised by capturing, and the remaining emissions consist only of CO2, H2O, NOx, CH4 and SOx. The gaseous biomass conversion products contain pollutants. Although NOx emissions is low due to low temperature, it is important to know that nitrogen oxides are environmentally the most harmful substances [5, 6]. Sulphur dioxide is considered to be one of the fundamental air pollutants from combustion process. It is the main constituent of the London type smog and acid rain. But, in the case of torrefaction process, SO2 can be negligible due to least sulphur contents of the lignocellulosic biomass.
Torrefied biomass is successfully applied as a biofuel for combustion, co-gasification or gasification processes [1]. It is worth to mention that torrefied biomass in the form of pellets is very attractive with respect to its enhanced properties. Torrefaction of biomass causes loss of a great amount of volatiles so there are less remaining for combustion. Nevertheless, torrefied biomass has good physical properties; unfortunately, during combustion, pollutants are produced as ash, chlorine, hydrogen fluorides, sulphur and alkaline production, sulphides and nitrates.
Combustion characteristics of torrefied biomass have been studied using thermoanalytical techniques. The influence of temperature and atmosphere has been investigated by thermal analytical techniques [7–10]. Thermogravimetric analysis has a lot of advantages such as a rapid assessment of the fuel value, the temperatures at which combustion starts and ends, the maximum reactivity temperature, the amount of ash and total combustion time. Because thermochemical conversion requires the control of outflow gas emission, chemical composition of exhaust gases should be determined before any industrial application. The combination of MS and TG offers a detailed identification of gaseous species evolved during thermal processes in a real time [11–13].
The most important applications of thermal analysis is kinetic calculations. The kinetic parameters such as activation energy and pre-exponential factor could be obtained by methods for analysing non-isothermal solid-state kinetics. Solid-state kinetic data are the major interest in combustion processes. The kinetic studies of coal, oil and biomass (wood biomass, sewage sludge, etc.) combustion have been studied by many research groups [14–19]. In non-isothermal kinetics, the Friedman (F), Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) methods are the most often used isoconversional methods [20–22].
Generally, the kinetics of reactions in solid-state are described by equation:
where α is conversion defined as follows:
where m i0—the initial mass of the sample, m a—the actual mass, and m f—mass after combustion.
The rate constant k is determined by the temperature and is given by the Arrhenius equation:
where R—gas constant (8.314 kJ mol−1), T—temperature in Kelvin, A (min−1)—pre-exponential factor, and E a—activation energy (kJ mol−1) constants which describe the properties of the material. The combination of these two Eqs. (1) and (3) gives the fundamental expression (4) of analytical methods to calculate kinetic parameters, on the basis of the TG results:
The expression of the function f(α) = (1 − α) and its derivative g(α) = −ln(1 − α) are used to describe solid-state first-order reaction. The equation including reaction order n takes the form:
For non-isothermal TG experiments in which a sample is heated at a constant rate (\(\beta = \frac{{{\text{d}}T}}{{{\text{d}}t}}\)—heating rate); Eq. (5) is given by:
Eq. (6a) expresses the fraction of material conversion during temperature change.
Integration of Eq. (6a) yields:
If x is given by E a/RT and integration limits transformed Eq. (6b) becomes:
Integral p(x) must be approximated as it has no analytical solution [23]. Different approximation formulas [24, 25] give various methods [26–29]. Friedman method [30] is one of the earliest isoconversion methods. The method starts with Eq. (6b):
gives:
Hence, a plot of ln(βdα/dT) versus 1/T at each α gives E a from the slope of the plot.
The Kissinger–Akahira–Sunose [26, 27] method is based on the Coats–Redfern approximation of the p(x) [24]:
and resulting equation is as follows:
The apparent activation energy can be obtained from a plot of ln(β/T 2α ) versus 1/T for a given value of conversion α, where the slope is equal to −E a/R.
Flynn–Wall–Ozawa method is based on the Doyle’s approximation of p(x) [25]:
which results in following equation:
The activation energy can be determined by measuring the temperatures corresponding to fixed values of α from experiments at different heating rates.
In this study, the combustion process of torrefied biomass was investigated by thermogravimetry analysis, and gaseous products were detected using MS.
This study aims to estimate:
-
(a)
Combustion process of torrefied biomass using thermal analysis (TG, DTG) for thermal behaviour and mass spectrometry (MS) to detect gaseous products,
-
(b)
The activation energy (E a) according to conversion degree (α) using the Friedman, KAS and FWO isoconversional methods under non-isothermal conditions.
Authors believe that the experimental results presented in this paper are important and novel with respect to other papers [1–4] because it analyses combustion of torrefied biomass and determines the activation energy of torrefied biomass combustion process using three isoconversional methods.
Materials and methods
The two kinds of wood biomass representing Polish wood feedstock were studied. Both, B1 and B2 biomass samples, were taken from two different sawmills. B1 chips were crushed, milled and sieved (sieve size was 5 mm). The B1 wood biomass was much more heterogeneous (leaves, stumps, barks and roots milled altogether) than B2, which was obtained in powdery form of the size ≤1 mm, bright in colour. The physical and chemical properties of biomass were analysed (Table 1). Moisture content of studied biomass was determined using the AXIS ATS precise moisture analyser. The ash content was determined according to EN 15403:2011 European Standard. In the case of sludge, EN 15935:2012 was applied. The volatile matter was determined by using EN 15402:2011 method. Elemental Truespec CHN and SC-144DR Leco analysers were used to determine the carbon, hydrogen, nitrogen and sulphur contents. The higher heating value (HHV) of the studied samples was calculated from the ultimate analyses by means of the Friedl et al. [31, 32] equation.
The chemical composition and volatile matter, ash and moisture of biomass samples as well as the higher heating value are shown in Table 1.
The torrefaction of biomass was performed using of a reactor presented in Fig. 1. The reactor made of a quartz tube reactor, 500 mm of length and 40 mm of diameter, and an electrical furnace with 30 mm of ceramic fibre insulation. Two NiCr–NiAl (K) thermocouples were installed in the reactor: one between the quartz tube surface and an immersion heater and the second inside the tube reactor, and collected with temperature controller. The studied samples 2 g in mass in the ceramic rectangular crucible were placed in the middle of a quartz tube reactor. The electrical furnace was heated up to 230, 260 and 290 °C. Then, the samples were inserted to the heated furnace and torrefied for the 1.0-h residence time; 2.5 L min−1 flow rate of argon was constant through the reactor to keep the inert atmosphere inside. When the process was over, the crucible was taken out from the tube reactor and holds for 5 min on metal surface and then put in a desiccator for cooling down to room temperature. The torrefied samples were furthermore analysed to characterise their physical and chemical properties.
Schematic diagram of biomass torrefaction unit [33]
The thermogravimetric analyses were conducted using a Mettler Toledo TGA/SDTA 851 apparatus. The instrument was calibrated using indium, zinc and aluminium. Its accuracy was 10−6 g. For the thermal analyses (TG/DTG/DTA), the samples were placed in alumina crucibles. Approximately 15 mg of sample was heated from ambient temperature to 700 °C at a constant rate of 10 °C min−1 in a 40 mL min−1 flow of air and argon. Each sample had to be measured under exactly the same conditions, including temperature range, atmosphere and heating rate, to determine the correct conditions. The TG curves for each of the samples were obtained as the outputs for combustion. The ordinate on the TG curves was the percentage ratio of the instantaneous mass of the sample to the initial mass. DTG curves are the result of mathematical transformation (dm/dt = f(t), where m—mass of sample, t—time). DTG curves have allowed to determine and compare the combustion characteristics of raw and torrefied biomass samples. The peak temperature (DTG) is the point where the rate of mass loss is at its maximum.
The evolved gaseous products from decompositions were identified online using a ThermoStar GSD300T Balzers quadruple mass spectrometer (QMS). It was operated in the electron impact mode (EI) using a channeltron as a detector. Screening analyses were performed in the selected-ion monitoring (SIM) mode. The following ions, which are characteristic of the molecules of interest, were monitored: 2, 15, 17, 18, 30, 44 and 64 for H2, CH4, OH (NH3), H2O, NO (C2H6), CO2 and SO2, respectively. The QMS spectrum of mass 17 can represent not only NH3, but also OH-fragment of H2O fragmentation, and that of mass 30 can represent both NO and C2H6. All these gaseous molecules are important from the point of view of air pollution and energy value.
Results and discussion
The biomass composition is a fundamental information that determines properties, quality and thermal application of that type of fuel. That is why the detailed description of ultimate and proximate analysis is presented. Both studied biomass samples have similar chemical composition. Generally, biomass has proximate analysis of 80 % volatile matter and less than 20 % fixed carbon (moisture free and ash free basis). Typically, raw biomass usually has high moisture content resulting in a relatively low calorific value of the fuel. It is well known that moisture content of biomass affects its combustion properties; thus, less moisture content accelerates the maximum combustion temperature and decreases the necessary residence time of feedstock in a combustion chamber, and in consequence results in complete combustion and decreased emissions related to it. Regarding two discussed biomass samples moisture and volatile matter contents were at similar level. The ash, because of its complex character, is one of the most interesting characteristics of biomass, because it originates simultaneously from inorganic, organic and fluid matter during biomass combustion. In studied samples, ash amount in B2 was two times less than in B1 resulting in higher obtained FC. Carbon is the main component of biomass in both samples. The concentration of oxygen is up to 50.5 mass%. Nitrogen and sulphur exist in biomass; therefore, a very less amount causing low emission of NO x , and SO x pollutants during combustion process is expected to appear. It is well known that the thermochemical properties of solid fuels are related with its elemental analysis, by which HHV was evaluated.
Torrefaction of biomass samples resulted in higher HHV and higher carbon contents. The HHV of torrefied B1 ranged from 17.8 to 24.4 MJ kg−1 and for torrefied B2 ranged from 20.8 to 26.2. Therefore, it can be said that a significant increase in HHV after torrefaction process is observed compared to raw samples. The high value of HHV results from increased carbon content of torrefied B1 and B2 compared to raw biomass. C.a. 60 % increase in carbon content for biomass B1 is observed at 290 °C (Table 2). Hydrogen content in all torrefied samples usually decreased with temperature of the process. There is an influence of torrefaction conditions observed on the nitrogen content of the samples. The values of nitrogen content increase c.a. 2×. Figure 2 presents the change in the H/C and O/C ratios with the increase in the torrefaction temperature. Decrease in H/C and O/C moves torrefied biomass towards coal properties.
According to the mass loss and heating values of torrefied biomass, the energy yield of torrefaction products can be calculated by Eq. (1):
where: η E—energy yield, η m—mass yield \(\left( {\eta_{\text{m}} = \frac{{m_{\text{torrefied}} }}{{m_{\text{raw}} }}} \right)\), m torrefied—mass of torrefied biomass, m raw—mass of raw biomass, HHV—high heating value.
The energy yield indicates the amount of energy lost during torrefaction. Results show that the torrefaction temperature at fixed residence time (1 h) has significant effects on the mass yield and energy yield (Table 2) of torrefied biomass.
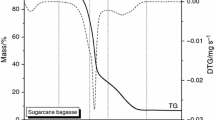
Figures 3 and 4 show the mass changes (%), TG curves and derivative thermogravimetric (DTG) curves for studied biomass (B1, B2) up to 700 °C with heating rate of 10 °C min−1 in air atmosphere. Combustion process of raw biomass resulted in mass loss of 97.1 % for B1 and 98.8 % for B2 at 700 °C. There is negligible mass loss in the case of B1 and B2 up to 150 °C. In the range between 200 and 600 °C, a significant mass loss is seen. In the context of different phenomena that can be observed, similar results were obtained from TG analysis of other kind wood biomass [7]. Wood biomass contains three main organic components: hemicellulose, cellulose and lignin which are in different proportions and decompose at different range of temperatures [9]. The first mass loss is connected with moisture release. For biomass (B1 and B2), maximum mass loss is between 200 and 500 °C due to degradation of their basic organic components such as hemicellulose, cellulose and lignin. Mass loss occurs due to decomposition of hemicellulose (190–320 °C), cellulose (280–400 °C) and lignin (320–450 °C). The majority of hemicellulose mass loss can be observed above 200 °C for wood biomass samples. Hemicellulose produces less tars and char due to its low degradation temperature range compared to that of the cellulose. DTG curves for B1 and B2 characterise two main peaks. For B2, the second peak is less comparing to B1. The slight differences attribute to the concentration of hemicellulose, cellulose and lignin in studied biomass samples.
Figure 5 presents TG profiles for raw and torrefied biomass B1 combustion process. The thermal conversion of torrefied biomass takes place at higher temperatures. TG curves are moved towards higher temperatures what correspond to coal properties. The mass loss connected with moisture release is not observed, thus confirming the hydrophobic properties of torrefied wood. Torrefaction process of biomass leads to water evaporation, and its hemicellulose content reduces, yielding a gas consisting of CO, CO2, H2O and organic compounds.
The most significant gaseous products from torrefied biomass combustion process are NO, CH4, CO2 and H2O. Mass spectrometry (MS) was used to monitor these gases. Because the profiles of gas emissions for both studied torrefied biomasses (B1 and B2) are very similar, only results for B1 are presented in Figs. 6–9. Representing temperatures for the maximum peaks of gas emission for B1 and B2 are summarised in Table 3.
Nitrogen oxide is one of the chemical compounds that harms the environment. NO was released during combustion process above 250 °C for all studied samples (Fig. 6). The maximum emission was found in c.a. 310 °C for B1 and B2. Taking into account, the residence time of torrefaction process, NO emission takes place in the same range of temperatures for such obtained biomass samples. However, it can be observed that amount of NO depends on temperature of torrefaction. NO emission significantly decreases with the increasing torrefaction temperature. The biomass combustion goes in not every high temperature too. Similar tendency is observed for CH4. Only one peak was observed for all samples (Fig. 7). The maximum peaks of methane (m/z = 16) are observed between 250 and 400 °C for combustion of all studied torrefied biomass samples. The NO and CH4 evolution profiles are similar, and its emission is on the same range.
The formation of CO2 (m/z = 44) during torrefied biomass combustion up to 700 °C has complex mechanism, and it is related to the degradation of organic compounds (Fig. 8). CO2 emission takes places in 250–500 °C range temperatures with two maximum peaks, c.a. 316 and 425 °C, respectively. Below 400 °C, CO2 is released from volatile matter, and as the temperature increases, the polymerisation reaction of coking in solid phase takes place, accompanied by the emission of low concentration CO2. The maximum CO2 emission takes place c.a. 425 °C and corresponds to carbon combustion. The H2O (m/z = 18) is formed in the range 230–500 °C for all studied cases (Fig. 9). The H2O profiles are similar. The main peak (c.a. 310 °C) is related to the combustion of hydrogen contained in cellulose and lignin. Above 400 °C, small amount of H2O is produced (second peak).
TG and MS results taken together provide a comprehensive information about torrefied biomass combustion. TG gives the information about thermal behaviour of fuel, whereas at the same time, MS detects gaseous products of studied process. Although MS allows to detect species, this method does not give quantity information, only approximately one. It is worth to notice that torrefaction temperature plays significant role in the formation of gaseous products, and gas products are generated in two ranges of temperature. H2O, CH4 and NO are produced in the 200–400 °C range, whereas maximum CO2 is produced in the second range of temperatures, from 350 to 500 °C.
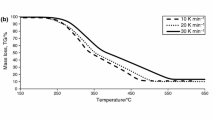
Figures 10 and 11 present the differential thermogravimetric (DTG) curves obtained from combustion processes of torrefied biomass by different heating rate (β = 10, 20 and 40 °C min−1). Figures 10 and 11 show the DTG profiles with mass loss rate peaks for combustion process. With the increasing of heating rate, the studied processes end in higher temperatures. The influence of heating rate is evidently observed on DTG curves, peaks are wider and the rates of the decomposition are higher for both torrefied biomass TB1 and TB2. The maximum mass loss rates (DTG curves) occur in similar temperature ranges c.a. 310 °C. DTG curves can be divided for two regions, first from 270 to 380 °C with one peak, and the second from 400 to 560 °C. The first peak reflects the decomposition of cellulose and less amount of hemicellulose. The second region is attributed to the decomposition of lignin.
The non-isothermal kinetic study of torrefied biomass during the combustion was performed. Differential and integral isoconversional methods, namely Friedman, KAS and FWO, were used to determine activation energies E a and pre-exponential factors A for the zeroth-order conversion at several conversion degrees (α) assuming one-step kinetics. β = 10, 20, 40 K min−1 heating rates were used to examine kinetics of combustion of torrefied biomass. Methods used in analysis of measured data can be applied only within certain ranges of E a/RT as dictated by Doyle’s approximation of exponential integral. The isoconversional methods are considered as a good approach for the investigation of biomass combustion kinetics. As the examples, Figs. 12 and 13 present dependence of ln(βdα/dT) and ln(β/T 2) via 1/T for different conversion degrees (α = 0.1–0.9) and correspond to the data from combustion of torrefied biomass. Linear regression is used to calculate the best fit for the line approximated by the three points obtained from the measured data. The average activation energies are obtained from the slope of the line of appropriate conversion degree α. The pre-exponential factors for zeroth-order conversion are obtained from the lines’ intercepts. The averaged values of energies and factors are summarized in Table 4. All methods used gave similar values. Figures 14 and 15 present the activation energies versus degree of conversion using Friedman, FWO and KAS methods for raw B1 and torrefied biomass TB1. The differences in E a are less in the case of torrefied biomass TB1 combustion.
The average activation energies for raw biomass ranged from 152.6 to 162.3 and 214.6–232.2 kJ mol−1 for B1 and B2, respectively, whereas for torrefied biomass, average activation energies were from 105.2 to 111 and 182.8–189 kJ mol−1 for TB1 and TB2, which were significantly lower than for the raw biomass. The present kinetic data for raw and torrefied biomass show the difference in the activation energies between the raw and torrefied biomass. The reduction of activation energy emphasises the importance of torrefaction process. This is the consequence of changes in structure and chemical composition of biomass sample. Torrefied biomass characterises coal properties.
Conclusions
In this study, the gaseous products of torrefied biomass (B1 and B2) combustion have been investigated. The TG–MS tests were done taking into account three different torrefaction temperatures at which torrefied biomass was obtained under specific residence time. The most evident impact of torrefaction on thermal properties of biomass was observed in HHV values. Comparing the HHV for raw and torrefied biomass, the significant increase was obtained (from 15.70 to 24.4 MJ kg−1 for B1 and 17.60–26.20 for B2, respectively). The gaseous fraction detected during torrefied biomass combustion contains primary CO2, NO, CH4 and H2O. The both studied torrefied biomasses (B1 and B2) released during combustion are similar in profiles and shapes of CO2, CH4 and H2O. The main gaseous products of torrefied biomass combustion process are CO2 and H2O (NO and CH4 emission are very low) what confirms the carbon and hydrogen are the only significant compounds of torrefied biomass. The amount of gases decreases with the increasing torrefaction temperature, probably because of the gasses removal during torrefaction process. The NO emission in both cases is insignificant due to the low processing temperature and should be negligible. The SO2 emission was not detected due to extremely low content of sulphur in raw B1 and B2 biomass. Besides the MS investigations, TA tests were conducted. It can be concluded from the results of this work that torrefaction process had significantly improved the thermal properties of both wood biomass samples (B1 and B2). However, more relevant results were obtained for homogeneous material (B2). The effect of temperature was greater on torrefaction than residence time in all studied samples.
The kinetic analyses demonstrated that torrefaction process leads to the decrease in the activation energy of the combustion process. The average activation energy changed from 152 (raw biomass) to 111 kJ mol−1 (torrefied biomass) for Friedman methods. For raw biomass, additional energy from hemicellulose conversion is required. The temperature range of hemicellulose decomposition is 190–320 °C. The majority of hemicellulose mass loss can be observed above 200 °C for wood biomass samples, being the torrefaction temperature. The novelty of this study was applied the three non-isothermal isoconversional methods: Friedman, KAS and FWO to obtain activation energy values for combustion of raw and torrefied biomass. E a values obtained for combustion of torrefied biomass are lower than corresponding raw biomass what confirmed better fuel properties of torrefied material.
References
Prins MJ, Ptasinski KJ, Janssen FJJG. More efficient biomass gasification via torrefaction. Energy. 2006;31:3458–70.
Van der Stelt MJC, Gerhauser H, Kiel JHA, Ptasinski KJ. Biomass upgrading by torrefaction for the production of biofuels: a review. Biomass Bioenerg. 2011;35:3748–62.
Ciolkosz D, Wallace R. A review of torrefaction for bioenergy feedstock production. Biofuel Bioprod Bioref. 2011;5:317–29.
Chew JJ, Doshi V. Recent advances in biomass pretreatment—torrefaction fundamentals and technology. Renew Sust Energ Rev. 2011;15:4212–422.
Williams A, Jones JM, Ma L, Pourkashanian M. Pollutants from the combustion of solid biomass fuels. Prog Energy Comb Sci. 2012;38:113–37.
Magdziarz A, Wilk M, Zajemska M. Modelling of pollutants concentrations from the biomass combustion process. Chem Process Eng. 2011;32:423–33.
Magdziarz A, Wilk M. Thermal characteristics of the combustion process of biomass and sewage sludge. J Therm Anal Calorim. 2013;114:519–29.
Varol M, Atimtay AT, Bay B, Olgun H. Investigation of co-combustion characteristics of low quality lignite coals and biomass with thermogravimetric analysis. Thermochim Acta. 2010;510:195–201.
Chen WH, Kuo PCh. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by thermogravimetry. Energy. 2010;35:2580–6.
Skreiberg A, Skreiberg O, Sandquist J, Sorum L. TGA and macro-TGA characterisation of biomass fuels and fuel mixtures. Fuel. 2011;90:2189–97.
Magdziarz A, Werle S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014;34:174–9.
Calvo LF, Sanchez ME, Moran A, Garcia AI. TG–MS a technique for a better monitoring of the pyrolysis, gasification and combustion of two kinds of sewage sludge. J Therm Anal Calorim. 2004;78:587–98.
Nocquet T, Dupont C, Commandre J-M, Grateau M, Thiery S, Salvador S. Volatile species release during torrefaction of wood and its macromalecular consistuents: part 1—experimantal study. Energy. 2014;72:180–7.
Sanchez ME, Otero M, Gomez X, Moran A. Thermogravimetric kinetic analysis of the combustion of biowastes. Renew Energy. 2009;34:1622–7.
Scott SA, Dennis JS, Davidson JF, Hayhurst AN. Thermogravimetric measurements of the kinetics of pyrolysis of dried sewage sludge. Fuel. 2006;85:1248–53.
Ji S, Zhang S, Lu X, Liu Y. A new method for evaluating the sewage sludge pyrolysis kinetics. Waste Manag. 2010;30:1225–9.
Grammelis P, Basinas P, Malliopoulou A, Sakellaropoulos G. Pyrolysis kinetics and combustion characteristics of waste recovered fuels. Fuel. 2009;88:195–205.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
White JE, Catallo WJ, Legendre BL. Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis. 2011;91:1–33.
Ozawa T. Estimation of activation energy by isoconversion methods. Thermochim Acta. 1992;203:159–65.
Font R, Fullana A, Conesa J. Kinetic models for the pyrolysis and combustion of two types of sewage sludge. J Anal Appl Pyrolysis. 2005;74:429–38.
Mothe CG, de Miranda IC. Study of kinetic parameters of thermal decomposition of bagasse and sugarcane straw using Friedman and Ozawa–Flynn–Wall isoconversional methods. J Therm Anal Calorim. 2013;113:497–505.
Flynn JH. The ‘temperature integral’—its use and abuse. Thermochim Acta. 1997;300:83–92.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Doyle CD. Series approximations to the equation of thermogravimetric data. Nature. 1965;207:290–1.
Akahira T, Sunose T. Joint convention of four electrical institutes. Research report (China Institute of Technology). Sci Technol. 1971;16:22–31.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B: Polym Lett. 1966;4:323–8.
Friedman HL. Kinetics of thermal degradation of char-foaming plastics from thermogravimetry: application to a phenolic resin. J Polymer Sci. 1965;6C:183–95.
Friedl A, Padouvas E, Rotter H, Varmuza K. Prediction of heating values from elemental composition. Anal Chim Acta. 2005;544:191–8.
Bridgeman TG, Fones JM, Williams A, Waldron DJ. An investigation of the grindability of two terrified energy crops. Fuel. 2010;89:3911–8.
Wilk M, Magdziarz A, Kalemba I. Characterisation of renewable fuels’ torrefacion process with different instrumental techniques. Energy. 2015;87:259–69.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magdziarz, A., Wilk, M. & Straka, R. Combustion process of torrefied wood biomass. J Therm Anal Calorim 127, 1339–1349 (2017). https://doi.org/10.1007/s10973-016-5731-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5731-0