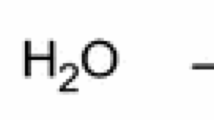Abstract
Alginate fiber, a kind of bio-based fiber, is a type of inherently flame retardant material. Can the addition of alginate fiber to cotton fiber improve flame retardancy of prepared cotton/alginate fabric? To solve this question, in the present work, flammability and thermal degradation properties of the cotton and cotton/alginate fabrics were studied by thermogravimetric analysis (TG), microscale combustion calorimetry (MCC), cone calorimeter (cone) and thermogravimetric analysis coupled with Fourier transform infrared analysis. Compared to cotton fabric, TG results showed that the addition of alginate fiber decreased initial degradation temperature (T initial) and maximum-rate degradation temperatures (T max) of cotton/alginate fabric; however, the addition of alginate fiber improved the char residual amount at higher temperature. MCC and cone results indicated that the addition of alginate fiber reduced the peak heat release rate value and total heat release, showing improvement on flame retardant properties of cotton/alginate fabric. The release amounts of inflammable gases, such as H2O, for cotton/alginate fabric, were almost the same as cotton fabric in the thermal degradation process; however, compared to cotton fabric, the release amounts of flammable gases, such as compounds containing –C–H groups, alcohol, compounds containing carbonyl groups and ethers, were reduced. On the basis of the results mentioned above, the flame retardant properties of cotton/alginate fabric were enhanced. The results obtained in the present study can supply a flame retardant method by the addition of inherently bio-based flame retardant alginate fiber to flame-retard cotton fabric and enlarge the applied fields of alginate fiber.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to its biodegradability, comfortableness, biocompatibility, breathability, capability to absorb moisture, renewability and environmentally friendly properties, cotton fabric has been extensively utilized in both civilian and military fields, such as clothing, firefighter apparel, house furnishing, house decorations and military garments [1–3]. Because of its comfortableness, hydrophilicity, softness and air permeability, cotton fabric is an important bio-based textile over the world [4–6]. However, cotton fabric is easy to be ignited and its flammability is rapid, which seriously limited the application of cotton fabric [7–9]. In the recent decades, one of the main reasons for residential home fires is the ignition and combustion of house decorations and furnishing [10]. Thus, increasing types of flame retardants have been explored to improve the flame retardant properties of cotton fabric, such as phosphorus- [11, 12], nitrogen- [13, 14] and silicon-containing [10, 15, 16] flame retardants.
In the past decade, increasing researchers have been focusing on alginate. It is a type of polysaccharides obtained from algae [17, 18], such as laminaria japonica, laminaria hyperborean, ascophyllum nodosum, laminaria digitata and macrocystis pyrifera [19], and is a linear bio-based polymer which is constituted by α-1, 4-L-guluronate (G) and β-1, 4-D-mannuronate (M) repeating monomeric units. Because of its biodegradability, biocompatibility, and film-forming properties and abundance, alginate has been explored in textile industry, food industry, drug formulations and wound dressings [20, 21]. Sodium alginate, a type of salt alginate, is soluble in water [22] and is a desirable candidate for aqueous processing [23]. On the basis of its gelation properties of alginate, which results from the interaction among carboxylate groups, hydroxyl groups and metal ions in aqueous solutions [24, 25], alginate fibers and films have been prepared [20, 21, 23, 26–30], such as calcium alginate fibers, copper alginate films and so on. The obtained alginate fibers and films are the inherently flame retardant bio-based materials. The effects of Ca2+, Ba2+, Zn2+, Mn2+, Ni2+, Cu2+, Co2+, Mg2+, Fe3+ and Al3+ on flame retardancy, thermal degradation properties and pyrolysis properties of alginate were studied by Zhu [26–30] and Xia [20–22]. The results showed that metal ions enhanced flame retardancy of alginate and reduced the release of gaseous compounds in pyrolysis process. And the addition of metal ions had changed pyrolysis process of alginate, releasing fewer kinds of gaseous compounds.
As a kind of inherently bio-based flame retardant fiber, can alginate fiber be exploited to flame-retard cotton fabric? In order to solve this question, cotton/alginate fabric was prepared and supplied by Qingdao Huajin Co. Ltd. (Qingdao, China), and the content of alginate fiber in cotton/alginate fabric was kept as 5 mass%. In the present work, the flammability and thermal degradation properties of the cotton and cotton/alginate fabrics were investigated by thermogravimetric analysis (TG), microscale combustion calorimetry (MCC), cone calorimeter (cone) and thermogravimetric analysis coupled with Fourier transform infrared analysis (TG-FTIR).
Experimental
Materials
Cotton and cotton/alginate fabrics were kindly supplied by Qingdao Huajin Co. Ltd. (Qingdao, China). The content of alginate fiber in cotton/alginate fabric was 5 mass%.
Measurements
Micro-scale combustion calorimeter
On the basis of ASTM D7309 standard, flammability behaviors of cotton and cotton/alginate fabrics were investigated by a micro-scale combustion calorimeter (MCC) (Fire Testing Technology, UK). 5 ± 0.05 mg of the sample was heated from room temperature to 700 °C under nitrogen atmosphere with a flowing rate of 80 cm3 min−1, and the heating rate was set to be 1 °C s−1. Before getting into a 900 °C combustion furnace, the volatile and anaerobic compounds produced in the thermal degradation process of the sample were mixed in a stream, and the stream flowing rate was set to be 20 cm3 min−1, including 80 % nitrogen and 20 % oxygen. Each sample was done in three times.
Cone calorimeter test
On the basis of the procedures in ISO 5660-1, burning behaviors of cotton and cotton/alginate fabrics were studied with a cone calorimeter (Fire Testing Technology, UK). Specimens, whose sheet dimensions were 100 mm × 100 mm × 1.7 mm, were put into an aluminum foil and irradiated horizontally with a heat flux of 25 kW m−2. And every sample was run in three times.
Thermogravimetric analysis
A thermogravimetric analyzer (Q50, TA Instruments Co., USA) was utilized to explore thermal stability properties of cotton and cotton/alginate fabrics. 10 ± 0.2 mg of the sample was charged and heated from 30 to 700 °C in N2 atmosphere at a heating rate of 10 °C min−1, and the N2 flowing rate was set to be 90 cm3 min−1. Each sample was run in two times. The temperature reproducibility of the instrument was within ±1 °C, and the mass reproducibility was within ±0.1 %.
TG-FTIR
Thermogravimetric analysis, which was coupled with a Fourier transform infrared spectrometry spectrophotometer (iS50, Nicolet Instruments Co., USA) (TG-FTIR), was carried out through a thermogravimetric analyzer (Q50, TA Instruments Co., USA). TG and FTIR were connected through a stainless steel pipe. The pipe and gas cell were set to be 300 and 250 °C, respectively, to minimize secondary chemical reactions and the condensation of volatile products. 10 ± 0.2 mg of the sample was charged and heated from 30 to 700 °C at a heating rate of 10 °C min−1 with a nitrogen flowing rate of 90 cm3 min−1.
Results and discussion
Thermal stability properties of cotton and cotton/alginate fabrics
In order to investigate the effect of alginate fiber on the thermal stability properties of cotton/alginate fabric, thermogravimetric analysis (TG) was utilized. TG and derivative thermogravimetric analysis (DTG) curves of cotton fabric, cotton/alginate fabric and alginate fiber are shown in Fig. 1. The data acquired from these curves, such as the initial degradation temperatures (T initial s) at which 95 % mass retained, the maximum-rate degradation temperatures (T max s), the thermal degradation rate at T max (R max) and the char residual amount at 500, 600 and 700 °C, respectively, are depicted in Table 1. T initial of alginate fiber was 190 °C, and its T max was 196 °C. The main mass loss stage of alginate fiber was from 190 to 350 °C, which was attributed to the dehydration and depolymerization of alginate. It can be seen from Fig. 1 and Table 1 that the char residual amount of alginate fiber was much higher; the char residual amounts at 500, 600 and 700 °C were 51.0, 49.2 and 44.2 %, respectively. From Fig. 1 and Table 1, it is noted that T initial of cotton fabric was about 292 °C. And its T max took place at 374 °C with a thermal degradation rate of 2.0 % °C−1. From Fig. 1a, it can be observed that the main mass loss stage of cotton fabric was from 270 to 420 °C, which was attributed to the dehydration and depolymerization of cellulose [31], forming the volatiles and aliphatic char. The thermal degradation rate decreased when temperature further increased. This step included the further thermal degradation of char residues, releasing CO2 and/or CO. As a result, the char residual amounts of cotton fabric at 500, 600 and 700 °C were 12.2, 10.7 and 9.7 %, respectively. As can be observed from Fig. 1 and Table 1, the addition of alginate fiber reduced T initial and T max of cotton/alginate fabric to 240 and 348 °C, respectively. This result indicated that the addition of alginate fiber decreased the thermal stability properties of cotton fabric, and the reason for this phenomenon was that the thermal stability properties of alginate fiber were worse than those of cotton fiber [26–30]. Or calcium ion, which was from alginate fiber, might result in this phenomenon. Calcium ion might catalyze some chemical reaction happened in the thermal degradation process of cotton/alginate fabric, showing the reduction of T initial. However, the addition of alginate fiber increased the char residual amount at higher temperature zone and decreased the thermal degradation rate at T max of cotton/alginate fabric. And the char residual amounts of cotton/alginate fabric at 500, 600 and 700 °C were 20.0, 18.1 and 16.9 %, respectively, and the thermal degradation rate at T max was reduced to 1.2 % °C−1.
As mentioned above, the addition of alginate fiber to cotton fiber to prepare cotton/alginate fabric reduced thermal stability properties at lower temperature zone, while increased the char residual amount at higher temperature zone. This result indicated that calcium ion, from alginate fiber, might catalyze some chemical reactions in the thermal degradation process of polysaccharide to form more amounts of char residues.
Flame retardancy and flammability of cotton and cotton/alginate fabrics
Flame retardancy versus micro-scale combustion calorimeter test
In order to explore flame retardancy of cotton and cotton/alginate fabrics, micro-scale combustion calorimetry (MCC) was utilized. Heat release rate (HRR) curves of cotton and cotton/alginate fabrics as a function of temperature are presented in Fig. 2. And the acquired data, such as peak heat release rate (PHRR), the temperature (T PHRR) at which PHRR took place, total heat release (THR), heat release capacity (HRC) and residue are depicted in Table 2. Compared to cotton fabric, there existed a significant decrease in PHRR, T PHRR, HRC and THR for cotton/alginate fabric, and there was a serious increase in char residual amount. PHRR value of cotton/alginate fabric decreased from 295 to 145 W g−1, which was relative to about 51 % reduction. Compared to cotton fabric, T PHRR of cotton/alginate fabric reduced from 376 to 341 °C, which was accordant with TG result, and this result also indicated the catalyzed effect of calcium ion introduced by alginate fiber on decreasing thermal stability of cotton fabric. HRC value of cotton/alginate fabric was 136 J k−1 g−1, relating to an approximately 52 % reduction. THR value of cotton/alginate fabric also decreased from 11.9 to 8.6 kJ g−1, amount to about 28 % reduction. However, the addition of alginate fiber improved the char residual amount after burning, from 6.9 to 15.8 %. The improved char residue showed that more cotton fabric participated in the carbonization process because of the catalyzed effect of calcium ion introduced by alginate fiber. Thus, less flammable gases were produced to the gaseous phase to maintain burning; as a result, PHRR, THR and HRC values were obviously reduced.
Flammability versus Cone test
In order to explore the effect of alginate fiber on the flammability of cotton/alginate fabric, a cone calorimeter instrument was utilized. The data acquired from cone calorimeter test, such as average heat release rate (Av-HRR), peak HRR (PHRR), time to PHRR (T PHRR), fire growth rate index (FIGRA), peak smoke production rate (PSPR), total smoke production (TSP), time to ignition (TTI) and total heat release (THR), were also depicted in Table 3.
HRR curves of cotton and cotton/alginate fabrics are presented in Fig. 3a. Cotton fabric burned quickly after ignition and a sharp peak took place with a PHRR value of 174 kW m−2. Then, HRR values reduced gradually to a flat until the flame extinguished. Likewise, cotton/alginate fabric also burned quickly after ignition and a sharp peak took place with a PHRR value of 132 kW m−2, while the HRR values decreased and then increased to the biggest PHRR value of 142 kW m−2. At last, HRR values decreased to a flat. Further, compared to cotton fabric, there was an obvious shift to an earlier time in TTI of cotton/alginate fabric. TTI value of cotton/alginate fabric was 22 s, while TTI value of cotton fabric was 43 s, which was much longer than that of cotton/alginate fabric. This phenomenon might be attributed to the addition of alginate fiber. According to the previous papers [26–30], T max s of alginate fibers and films were lower than 300 °C, and the addition of metal ions further decreased T max of alginate. What is more, the addition of metal ions might catalyze some chemical reactions in the thermal degradation process of alginate and cotton. As a result, TTI value of cotton/alginate fabric was lower than that of cotton fabric. And cotton/alginate fabric released heat when it started burning, resulting in the formation of char layer. The formed char prevented the cotton/alginate fabric from both heat and mass transfer, resulting in the first reduction in heat release rate. However, the char layer cannot protect the matrix for a longer time, and this might result from the fragile and cracked char layer. As a result, the heat release rate increased again.
FIGRA value of cotton fabric was 3.16 kW m−2 s−1, while that of cotton/alginate fabric was 1.89 kW m−2 s−1; and FIGRA was defined as PHRR divided by TPHRR. Compared to that of cotton fabric, there was 40 % reduction for FIGRA value of cotton/alginate fabric. The reduction in FIGRA of cotton/alginate fabric indicated the suppression in a fire spread, decreasing the occurrence possibility of a fire.
Total heat release (THR) curves of cotton and cotton/alginate fabrics are showed in Fig. 3b. At the end of burning, THR values for cotton and cotton/alginate fabrics were 13.2 and 11.5 kJ m−2, respectively. There was 13 % reduction in THR for cotton/alginate (Fig. 3b; Table 3). This suggested that the addition of alginate fiber improve the flame retardancy of cotton fabric.
The main cause of death during fire was total smoke production (TSP) and smoke production rate (SPR) [32]. As a result, the exploration of TSP and SPR for cotton and cotton/alginate fabrics might be beneficial in indicating the possible hazard in well-ventilated conditions [33]. TSP and SPR curves of cotton and cotton/alginate fabric are showed in Fig. 4a, b, respectively. At the end of combustion, compared to cotton fabric, TSP of cotton/alginate fabric was lower than that of cotton fabric, and there was 31 % reduction in TSP. This showed that the addition of alginate fiber caused the complete flammability of cotton fabric, releasing lower amount of smoke. From Fig. 4b and Table 3, it was noted that SPR values of cotton/alginate fabric were a little lower than that of cotton fabric through the flammability process, and peaks of SPR values of cotton and cotton/alginate fabrics were almost the same.
Combined the analysis of MCC and cone tests, it can be made a conclusion that the addition of alginate fiber improved the flame retardant properties of cotton fabric, and alginate fiber can be utilized as a kind of flame retardant to flame-retard cotton fabric.
The addition of alginate fiber might reduce the release of combustible gases; as a result, less combustible gaseous compounds were produced to the gas phase to support burning; thus, THR and PHRR values were significantly decreased. To prove it, the gaseous ingredients of cotton and cotton/alginate fabrics were explored by TG-FTIR test. 3D FTIR images of cotton and cotton/alginate fabrics are presented in Fig. 5a, b. From Fig. 5a, b, it was observed that the addition of alginate fiber did not obviously change the absorption position of FTIR bands, indicating the similar thermal degradation products of cotton and cotton/alginate fabric. However, the temperature ranges of thermal degradation for cotton and cotton/alginate fabrics were different, indicating that the addition of alginate fiber changed the thermal degradation process of cotton. The peaks from 3500 to 3630 cm−1 were assigned to the stretching vibration of gaseous H2O and all the other kinds of –OH groups; peaks from 2788 to 2864 cm−1 and from 2956 to 3000 cm−1 were attributed to stretching vibration of the aliphatic C-H bonds from all type of alkanes; the peaks at 2340 and 2394 cm−1 were due to the stretching vibration of CO2; the peak at 1746 cm−1 was ascribed to the stretching vibration of C=O groups from various aldehydes, ketones and esters; the peak at 1180 cm−1 was attributed to the absorbance of C–O–C groups from ethers; the peak at 1080 cm−1 was ascribed to the stretching vibration of –C–OH groups.
To further study the effect of alginate fiber on the flame retardant mechanism of cotton fabric, several FTIR spectra of selected groups versus temperature are depicted in Fig. 6, which included gaseous H2O, C–H groups, CO2, C=O groups, C–O–C groups and –C–O–H groups. The specific wavenumbers of the selected spectra peaks were depicted as follows: H2O, 3570 cm−1; –C–H, 2974 cm−1; CO2, 2360 cm−1; C=O, 1746 cm−1; C–O–C, 1180 cm−1 and –C–O–H, 1080 cm−1. From Fig. 6, it was observed that the thermal degradation gaseous compounds for cotton and cotton/alginate fabrics were mainly divided into two types: one was the inflammable gases, H2O and CO2; other was the flammable gases, such as ethers, alcohol, compounds containing C–H groups and compounds containing carbonyl groups. On the basis of Lambert–Beer law, the concentration of a specific gaseous compound was linearly dependent on the absorbance intensity of the accordant wavenumber [34]. As can be observed from Fig. 6, the absorbance intensities of H2O, C=O, –C–O–C–, –C–O–H and –C–H groups obtained from the thermal degradation process of cotton/alginate fabric were lower than those of cotton fabric. What is more, the absorbance intensities of –C–O–C- and –C–H groups were much lower than those of cotton fabric. However, the absorbance intensities of CO2 obtained from cotton/alginate fabric were higher than that of cotton fabric from 60 to 370 °C, which diluted the concentration of the combustible gaseous compounds. The phenomena mentioned above indicated that the addition of alginate fiber reduced the release of H2O, alcohol and compounds containing carbonyl groups, and seriously decreased the production of compounds containing –C–H groups and ethers. And the addition of alginate fiber increased the release of CO2 from 60 to 370 °C. The reduced release amount of the flammable gases, compounds containing –C–H groups, alcohol, compounds containing carbonyl groups and ethers, resulted in less “fuel” to support the flame, performing the decrease in HRR and THR observed from cone test.
In cotton/alginate fabric system, there existed calcium ions, which might catalyze some chemical reaction happening in the thermal degradation process of cotton/alginate fabric to produce less flammable gaseous compounds. As a result, this improved the flame retardant properties of cotton fabric.
Conclusions
Alginate fiber can be utilized as a bio-based flame retardant to flame-retard cotton fabric. The addition of alginate fiber improved the flame retardant properties of cotton/alginate fabric. Compared to cotton fabric, TG results demonstrated that the addition of alginate fiber decreased T initial and T max of cotton/alginate fabric; however, it improved the char residue amount at higher temperature. MCC and cone results showed that the addition of alginate fiber reduced PHRR and THR, indicating the improvement of flame retardant properties for cotton/alginate fabric. The release amounts of inflammable gases, such as CO2, for cotton/alginate fabric were more than that of cotton fabric in the thermal degradation process; however, compared to cotton fabric, the release amounts of flammable gases, such as compounds containing –C–H groups, alcohol, compounds containing carbonyl groups and ethers, were reduced. All of these mentioned above enhanced the flame retardant properties of cotton/alginate fabric. The results obtained in the present study can supply a flame retardant method through the addition of inherently bio-based flame retardant fiber to flame-retard cotton fabric, and enlarge the applied field of alginate fiber.
References
Dong CH, Lu Z, Zhang FJ, Zhu P, Zhang L, Sui SY. Preparation and properties of cotton fabrics treated with a novel polysiloxane water repellent and flame retardant. Mater Lett. 2015;152:276–9.
El-Shafei A, ElShemy M, Abou-Okeil A. Eco-friendly finishing agent for cotton fabrics to improve flame retardant and antibacterial properties. Carbohydr Polym. 2015;118:83–90.
Fang F, Zhang X, Meng YD, Gu Z, Bao C, Ding X, Li SY, Chen XX, Tian XY. Intumenscent flame retardant coating on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf Coat Technol. 2015;262:9–14.
Gao WW, Zhang GX, Zhang FX. Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorous-based flame retardant. Cellulose. 2015;22:2787–96.
Jiang DW, Sun CY, Zhou YN, Wang H, Yan XR, He QL, Guo J, Guo ZH. Enhanced flame retardancy of cotton fabrics with a novel intumescent flame-retardant finishing system. Fiber Polym. 2015;16:388–96.
Nehra S, Hanumansetty S, O’Rear EA, Dahiya JB. Enhancement in flame retardancy of cotton fabric by using surfactant-aided polymerization. Polym Degrad Stab. 2014;109:137–46.
Vasiljević J, Jerman I, Jakša G, Alongi J, Malucelli G, Zorko M, Tomšič B, Simončič B. Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose. 2015;22:1893–910.
Lam YL, Kan CW, Yuen CWM. Effect of zinc oxide on flame retardant finishing of plasma pre-treated cotton fabric. Cellulose. 2011;18:151–65.
Dong CH, Lu Z, Zhang FJ, Zhu P, Wang P, Che Y, Sui SY. Combustion behaviors of cotton fabrics treated by a novel nitrogen- and phosphorus-containing polysiloxane flame retardant. J Therm Anal Calorim. 2015;119:349–57.
Wang X, Romero MQ, Zhang XQ, Wang R, Wang DY. Intumescent multilayer hybrid coating for flame retardant cotton fabrics based on layer-by-layer assembly and sol–gel process. RSC Adv. 2015;5:10647–55.
Liu W, Chen L, Wang YZ. A novel phosphorus-containing flame retardant for the formaldehyde-free treatment of cotton fabrics. Polym Degrad Stab. 2012;97:2487–91.
Nguyen TM, Chang SC, Condon B, Thomas TP, Azadi P. Thermal decomposition reactions of cotton fabric treated with piperazine-phosphonates derivatives as a flame retardant. J Anal Appl Pyrolysis. 2014;110:122–9.
Poon CK, Kan CW. Effects of TiO2 and temperatures on flame retardant finishing of cotton. Carbohydr Polym. 2015;121:457–67.
Shariatinia Z, Javeri N, Shekarriz S. Flame retardant cotton fibers produced using novel synthesized halogen-free phosphoramide nanoparticles. Carbohydr Polym. 2015;118:183–98.
Mohamed AL, EI-Sheikh MA, Waly AI. Enhancement of flame retardancy and water repellency properties of cotton fabrics using silanol based nano composites. Carbohydr Polym. 2014;102:727–37.
Alongi J, Ciobanu M, Malucelli G. Novel flame retardant finising systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol–gel processes. Carbohydr Polym. 2011;85:599–608.
Pathak TS, Yun JH, Lee SJ, Baek DJ, Paeng KJ. Effect of cross-linker and cross-linker concentration on porosity, surface morphology and thermal behavior of metal alginates prepared from algae (Undaria pinnatifida). Carbohydr Polym. 2009;78:717–24.
Chen HB, Wang YZ, Sánchez-Soto M, Schiraldi DA. Low flammability, foam-like materials based on ammonium alginate and sodium montmorillonite clay. Polymer. 2012;53:5825–31.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26.
Zhang JJ, Ji Q, Shen XH, Xia YZ, Tan LW, Kong QS. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant calcium alginate fibre. Polym Degrad Stab. 2011;96:936–42.
Zhang JJ, Ji Q, Wang FJ, Tan LW, Xia YZ. Effects of divalent metal ions on the flame retardancy and pyrolysis products of alginate fibres. Polym Degrad Stab. 2012;97:1034–40.
Ross AB, Hall C, Anastasakis K, Westwood A, Jones JM, Crewe RJ. Influence of cation on the pyrolysis and oxidation of alginates. J Anal Appl Pyrol. 2011;91:344–51.
Kong QS, Wang BB, Ji Q, Xia YZ, Guo ZX, Yu J. Thermal degradation and flame retardancy of calcium alginate fibers. Chin J Polym Sci. 2009;27:807–12.
Qin YM. Alginate fibres: an overview of the production processes and applications in wound management. Polym Int. 2008;57:171–80.
Shen W, Hsieh YL. Biocompatible sodium alginate fibers by aqueous processing and physical crosslinking. Carbohydr Polym. 2014;102:893–900.
Liu Y, Li ZF, Wang JS, Zhu P, Zhao JC, Zhang CJ, Guo Y, Jin X. Thermal degradation and pyrolysis behavior of aluminium alginate investigated by TG-FTIR-MS and Py-GC-MS. Polym Degrad Stab. 2015;118:59–68.
Liu Y, Zhao JC, Zhang CJ, Guo Y, Cui L, Zhu P, Wang DY. Bio-based nickel alginate and copper alginate films with excellent flame retardancy: preparation, flammability and thermal degradation behavior. RSC Adv. 2015;5:64125–37.
Liu Y, Zhao JC, Zhang CJ, Guo Y, Zhu P, Wang DY. Effect of manganese and cobalt ions on flame retardancy and thermal degradation of bio-based alginate film. J Mater Sci. 2015;. doi:10.1007/s10853-015-9435-9.
Liu Y, Zhao JC, Zhang CJ, Ji H, Zhu P. The flame retardancy, thermal properties, and degradation mechanism of zinc alginate films. J Macromol Sci B. 2014;53:1074–89.
Liu Y, Wang JS, Zhao JC, Zhang CJ, Ran JH, Zhu P. The flame retardancy and thermal degradation behaviors of trivalent metal-alginate films. Nanomater Energy. 2014;3:3–10.
Shafizadeh F, Bradbury AG, DeGroot WF, Aanerud TW. Role of inorganic additives in the smoldering combustion of cotton cellulose. Ind Eng Chem Prod Res Dev. 1982;27:97–101.
Liu Y, Zhao J, Deng CL, Chen L, Wang DY, Wang YZ. Flame-retardant effect of sepiolite on an intumescent flame-retardant polypropylene system. Ind Eng Chem Res. 2011;50:2047–54.
Wang DY, Liu Y, Ge XG, Wang YZ, Stec A, Biswas B, Hull TR, Price D. Effect of metal chelates on the ignition and early flaming behavior of intumescent fire-retarded polyethylene systems. Polym Degrad Stab. 2008;93:1024–30.
Gao NB, Li AM, Quan C, Du L, Duan Y. TG-FTIR and Py-GC/MS analysis on pyrolysis and combustion of pine sawdust. J Anal Appl Pyrol. 2013;100:26–32.
Acknowledgements
The authors wanted to acknowledge the financial support of National Natural science Foundation of China (51203126) and China Scholarship Council (CSC: 201308420380). This research was also partly supported by Ramon y Cajal fellowship (RYC-2012-10737).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Y., Zhao, JC., Zhang, CJ. et al. Flame retardancy and thermal degradation properties of cotton/alginate fabric. J Therm Anal Calorim 127, 1543–1551 (2017). https://doi.org/10.1007/s10973-016-5418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5418-6