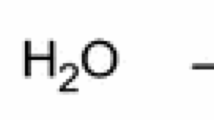Abstract
A novel flame-retardant agent, guanidyl- and phosphorus-containing polysiloxane (GPPDMS), was synthesized. The combustion properties of the cotton fabrics treated with GPPDMS were evaluated by cone calorimeter. The results showed that the TTI value of the treated fabric increased and the value of HRR, THR, EHC, and Mass loss decreased. It demonstrated that the treated cotton fabric with GPPDMS generated less combustion heat and obtained better flame retardancy which can be confirmed by the increase of FPI value and the decrease of CO/CO2 ratio. It also revealed that the cotton fabric treated with 18.6 % (add-on) of GPPDMS had highest reduction in heat of combustion. The chemical structures and morphologies of the residues, and the thermal decomposition behavior of cotton fabrics were investigated by FT-IR, scanning electron microscopy, and thermogravimetric analysis, respectively. The results showed that GPPDMS played a protective role on the degradation of cotton fabrics, hindered the formation of volatile species and favored the formation of char. Furthermore, EDS analysis results showed that remarkable amount of Si and P elements were still present on the surface of fibers after combustion; it indicated that the concurrent presence of Si and P in flame retardant effectively enhances the flame retardancy of cotton fabric.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fire safety has been attracting more attention due to the severe damages, injuries, and deaths caused by fire [1, 2]. The major reason for the fire may be due to the combustible materials surrounding us, such as wood, paper, plastics, and cotton fabric. Among these materials, the flame retardancy of cotton fabric has been a hot topic due to its close relation with our daily life. Low thermal stability, easy ignition, and rapid combustion of cotton fabrics represent their weaknesses and limitations. Many studies have been reported about the flame retardancy of cotton fabric [2, 3].
Although many new techniques have been developed in these years such as the sol–gel technique [4, 5] and the layer-by-layer (LbL) assembly [6, 7], the application of flame retardants is also a major approach to impart flame retardancy to cotton fabric. Many types of flame retardants as one-component agents or as mixtures have been employed in cotton fabric including halogen-containing flame retardants, phosphorus-containing flame retardants, nitrogen-containing flame retardants, and silicon-based flame retardants. The most effective flame retardancy can be achieved by the employment of phosphorus-containing flame retardants and halogen-containing flame retardants.
Halogen-containing compounds are gradually eliminated due to toxic gases they generate when combusting. At the same time, silicone-based flame retardants have been attracting more attention as one of the most promising and environmental friendly flame retardants due to their very good thermal stability [8, 9]. Furthermore, the synergistic effect of different flame retardant-elements has been a focus in researches [10, 11]. Researchers have found that concurrent presence of silica, phosphorus, and nitrogen can significantly enhance both the thermal and thermo-oxidative stability and flame retardancy of cotton fabric [12].
In this study, the combustion properties of cotton fabric treated with a flame retardant-agent, guanidyl- and phosphorus-containing polysiloxane (GPPDMS), were evaluated by cone calorimeter. The thermal decomposition behavior and the combustion behavior of the cotton fabrics were investigated by thermogravimetric (TG) analysis. In addition, morphologies and structure of the residues were investigated by scanning electron microscopy (SEM) and Fourier transform infrared (FT-IR). Furthermore, the elemental composition of the cotton fabrics was analyzed using energy dispersive X-ray spectrometer (EDS).
Experimental
Materials
Sourced and bleached 100 % plain-woven cotton fabric (14.75tex × 14.75tex, 122 g m−2) was supplied by Weifang Qirong Textiles Co., Ltd.
3-Aminopropyltriethoxysilane (purity grade 98 %) was supplied by Qufu HuaRong Chemistry Engineering New Material Co., Ltd. (China). Dicyandiamide (C2H4N4, purity grade 99.5 %) was supplied by Chengdu Kelong Chemical Reagent Factory (China). P2O5 (purity grade 98.0 %) was purchased from TianJin Damao Chemical Reagent Co., Ltd. (China). 2-Phosphonobutane-1,2,4-tricarboxylic acid (PBTCA) was supplied by Shandong Taihe Water Treatment Co., Ltd. (China).
Preparation of GPPDMS
In a 500 mL round bottomed flask with a mechanical stirrer, a solution of 3-aminopropylethoxysilane (22.1 g, 0.1 mol) and water (18 g, 1 mol) was bubbled with N2 for 10 min, and then the pH value of the resulting solution was adjusted to 4–5 using dilute acetic acid. The resulting mixture was hydrolyzed at 5 °C for 20 min, and the pH value of the mixture was gradually adjusted to 8–9 for protecting the Si–OH group by adding buffer agent of CaCO3. Then, the mixture was stirred for 2 h at ambient temperature in an inert atmosphere. After that, the pH value of the resulting mixture was adjusted to 7 using dilute acetic acid again, and 16.8 g of dicyandiamide (0.2 mol) was added. The mixture was stirred at 60 °C for 60 min in an atmosphere of N2. Thereafter, 42.5 g of P2O5 (0.3 mol) was added and the mixture was stirred at 60 °C for 50 min. Evaporation of the solvent and other volatile species gave GPPDMS.
The reaction equation is shown in Scheme 1.
FT-IR (KBr) (cm−1): 3310–3230 (vs. N–H), 1640 (vs. C=N), 1160 (vs. P=O), 1070 (vs, P–O–Si, Si–O–Si).
1H-NMR: δ = 1.1 ppm (Si–CH2), δ = 1.7 ppm (Si–CH2–CH2), δ = 3.7 ppm (Si–CH2–CH2–CH2), δ = 5.0 ppm (active hydrogen containing in –NH2 and –OH), δ = 7.3 ppm (C=NH).
Preparation of treated cotton fabrics
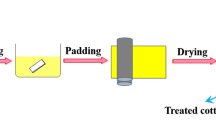
The cotton fabrics were washed in water with 1 mass% NaOH solution and then dried at 100 °C. Then they were soaked in finishing bath containing GPPDMS, 12 mass% PBTCA and 6 mass% urea at room temperature for 30 min, and then passed through a laboratory-scale padder with two dips and two nips to get a wet pick-up of 80 %. Then the samples were rinsed in tap water to remove unfixed agent. After that, the samples were dried at 80 °C for 5 min and cured at 160 °C for 5 min.
The amount (mass% owf) of flame retardant added on cotton fabric was calculated as follows:
where W b and W f represent the mass of cotton fabrics before and after flame-retardant treatment, respectively.
Characterization
The structure of GPPDMS was verified by 1H NMR spectrum (JEOL LA500, Japan). FT-IR analysis was carried out on a Nicolet 5700 FT-IR apparatus (Thermo Nicolet Corporation, US) using the KBr pellet technique.
The combustion of cotton fabrics was investigated using FTT0007 cone calorimeter (Fire Testing Technology Ltd.) under a heat flux of 30 kW m−2 according to ISO 5660. The parameters measured were time to ignition (TTI, s), total heat release (THR, kW m−2), heat release rate (HRR, kW m−2) and the relative peak (PHRR, kW m−2), effective heat combustion (EHC, MJ kg−1), and CO and CO2 yield (kg kg−1). The parameters calculated were fire performance index (FPI, s m2 kW−1) and CO2/CO ratio.
Thermogravimetric analysis (TG) was performed using TGA851 thermal analyzer (Mettler-Toledo International Inc.) at a heating rate of 5 °C/min with a continuous air flow rate of 20 mL min−1 from 23 to 600 °C.
The surface morphology of the residue of the treated cotton fabrics after combustion was investigated by a JSM-6010LA SEM instrument (Japan Electron Optics Laboratory Co., Ltd.). A sputter coater was used to pre-coat conductive gold onto the surface before observing the microstructure at 10 kV beam voltage.
EDS analysis was performed by a JSM-6700F instrument (Japan Electron Optics Laboratory Co., Ltd.).
Results and discussion
Combustion behaviors
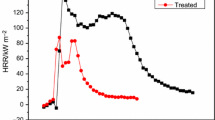
The combustion behavior of untreated sample and treated sample had been investigated by cone calorimeter. The collected data are summarized in Table 1 and plotted in Figs. 1–4. From Table 1, we can know that the time to ignition (TTI) of the treated samples have increased significantly comparing to the untreated sample [9 s (add-on% = 4.7), 16 s (add-on% = 18.6) vs. 2 s]. When samples were ignited, the combustion proceeded: the heat release rate (HRR) increases to a maximum (namely, PHRR) and then decreases down to the flame out (FO). The treatment with GPPDMS reduces the PHRR values of cotton fabrics remarkably and a lower PHRR value is achieved when higher amount of GPPDMS was employed as shown in Fig. 1 [96.31 kW m−2 (add-on% = 4.7), 71.80 kW m−2 (add-on% = 18.6) vs. 144.23 kW m−2]. It is also noteworthy that GPPDMS enhances the flame retardancy of cotton fabrics by decreasing combustion period as confirmed by the FO values and hindering the formation of volatile species as evidenced by the strong decrease of total heat release (THR) values (Fig. 2), EHC values, Mass values, and the increase of FPI values (Table 1). EHC is expected in a fire where incomplete combustion takes place, and it is a parameter corresponded to the heat released from the volatile portion during combustion. From Table 1 and Fig. 3, we can know that the EHC value of cotton fabric has decreased significantly [7.97 MJ kg−1 (add-on% = 4.7), 7.06 MJ kg−1 (add-on% = 18.6) vs. 12.57 MJ kg−1], since the employment of GPPDMS. Mass loss is another parameter reflecting the incomplete combustion of cotton fabrics as shown in Fig. 4: the mass loss of the treated cotton fabric is lower than the untreated ones [6.78 g (add-on% = 4.7), 5.82 g (add-on% = 18.6) vs. 11.53 g]. FPI was calculated as the ratio between TTI and PHRR. The higher FPI indicates the better flame retardancy [13]. From Table 1, we can know that the FPI of treated cotton fabric is higher than the untreated ones [0.10 s m2 kW−1 (add-on% = 4.7), 0.24 s m2 kW−1 (add-on% = 18.6) vs. 0.01 s m2 kW−1]. It is also can be concluded that the higher the add-on of GPPDMS, the better is the flame retardancy of treated cotton fabric.
CO and CO2 are the main components of fire gases, and the analysis of the two species can provide useful information on the mechanism of decomposition of cotton fabrics [14]. Low CO2/CO ratios mean low conversion of CO to CO2 and suggested inefficiency of combustion. Pure cotton fabrics produced CO and CO2 due to two-step reaction: CO including other gases was produced in first step pyrolysis reaction, and CO2 was released due to the oxidation of CO when enough oxygen was available in the second step. When cotton fabrics were treated with GPPDMS, the CO production changed a little, whereas the CO2 production was decreased leading to remarkable decrease of CO2/CO ratio [22.01 (add-on% = 4.7), 16.40 (add-on % = 18.6) vs. 23.30]. This suggested the enhancement of flame retardancy of cotton fabrics.
Thermal behaviors
The thermal and thermo-oxidative stability of untreated and treated cotton fabric was investigated by TG. TG curves of the fabrics under air atmosphere are shown in Fig. 5a. Data from TG and DTG curves of the cotton fabrics are presented in Table 2. It can be seen that, the initial decomposing temperatures of treated cotton fabrics with GPPDMS are 264.2 °C (add-on% = 4.7) and 272.9 °C (add-on% = 18.6), respectively, which are lower than that of the untreated cotton fabric, and the residue of the treated cotton fabrics at 500 °C are higher than that of the untreated cotton fabric. As shown in Fig. 5a and Table 2, the residues increase with the increase of GPPDMS add-on. Compared to the other fabrics, the treated cotton fabric with GPPDMS of 18.6 % has the highest residual char. DTG curves of the fabrics under air atmosphere are shown in Fig. 5b. The untreated cotton fabric has two evident mass loss stages, while the treated cotton fabrics have a major and a minor mass loss stage. This phenomenon could be attributed to the different thermal degradation processes of the cotton fabrics.
The untreated cotton fabric favors formation of flammable volatiles and loses much mass during degradation. As shown in many literature, phosphorus-based flame retardants can evidently lower the decomposition temperature of cotton fabric by accelerating dehydration and producing more char [15, 16]. We also know that silica is an efficient flame retardant for cotton fabric because silica coating on cotton favors the formation of char [3, 4]. The cotton fabric treated with GPPDMS degrades at lower temperature due to the synergistic effect arise from P, N, and Si elements contained in GPPDMS. During combustion, the lower initial decomposing temperature of the treated cotton fabrics is due to the esterification of phosphoryl groups from GPPDMS with hydroxyl groups from cellulose, which finally induces the release of water in a relative low temperature range, and the earlier decomposition of GPPDMS on cotton fabric to form phosphoric and polyphosphoric acid at lower temperature which promotes dehydration of cellulose and reduces the release of volatile species with more char yield at higher temperature. GPPDMS also forms a physical barrier silica-protecting cotton fabric from heat and oxygen transfer and favoring the char formation.
The TG results indicate that GPPDMS treated on cotton fabric lowers the decomposition temperature of cotton fabric and enhances the formation of char after pyrolysis.
Char structure
After combustion, it was realized that the residue of treated cotton fabric was different from the residue of the untreated cotton fabric. The residue of treated cotton fabric showed a more compact structure. SEM analysis of the residue was carried out to further characterize the surface morphology as shown in Fig. 6. It can be seen that the structure of the untreated fabric has been destroyed seriously after combustion and the surface of the residue is discontinuous (Fig. 6a). Compared to the residue of untreated cotton fabric, the surface of the residue of treated cotton fabric is uniformly continuous with an irregular coating (Fig. 6b) due to the formation of SiO2 matrix in the burning and the formation of polyphosphoric acid and other derivatives of GPPDMS during the thermal decomposition process. The cross-section of the charred fibers of treated cotton fabric as shown in Fig. 6c also demonstrates the existence of separate surface coating, and the bubbles on the separate surface coating may indicate that GPPDMS acts as a kind of intumescent flame retardant. The formed coating may act as a physical barrier inhibiting the transmission of heat, energy, and O2.
Elemental composition
Elemental analysis was performed as shown in Figs. 7–10, and the data were collected in Table 3. The elemental mapping shows that the distribution of Si and P elements on fibers before and after combustion is homogeneous and regular. Furthermore, the fibers after combustion show a wider distribution of Si and P elements than fibers before combustion. From Figs. 9 and 10, it is noteworthy that remarkable amount of Si and P elements is still present on the surface of fibers after combustion. From Table 3, we can know that the mass percentage concentrations of Si element on fibers increased from 1.21 to 2.77 % and the atomic percentage concentrations of Si element on fibers increased from 0.63 to 1.42 %, while the mass percentage concentrations of P element increased from 4.65 to 9.80 % and the atomic percentage concentrations of P element on fibers increased from 2.18 to 4.55 %. The increment of Si and P elements on fibers after combustion evidenced that Si and P elements are the main constituents of flame retardant-agent and demonstrate that SiO2 matrix, polyphosphoric acid, and other derivatives of GPPDMS are formed during the thermal decomposition process. The results are coherent with the SEM analysis of the char that a separate surface coating formed on the surface of fibers after combustion. To further discuss the retardant mechanism of GPPDMS, the residue of treated cotton fabric after combustion was characterized by FT-IR.
FT-IR analysis of the residues after combustion
The chemical structure of the residue of untreated and treated cotton fabric after burning in air are investigated by FT-IR analysis as shown in Fig. 11. Compared to the residue of untreated cotton fabric [spectrum (a)], treated cotton fabric shows some special characteristic peaks [spectrum (b)]. In spectrum (b), the peak at 2,330 cm−1 is attributed to the stretching vibration of C≡N which is produced by the pyrolysis of guanidine groups; the peak at 1,060 cm−1 can be ascribed to the Si–O–Si stretching vibration and the peak at 816 cm−1 is due to the Si–O–Si bending vibration [17] which confirms the formation of SiO2 matrix in the residue; the peak at 1,180 cm−1 is assigned to stretching vibration of P=O [18] which indicates the presence of phosphoric and polyphosphoric acid. These results share same ideas with SEM analysis and elemental analysis that the flame retardancy of treated fabric with GPPDMS is improved due to the formation of phosphoric and polyphosphoric acid which promoting the carbonization of fabric and SiO2 matrix which acts as a physical barrier during burning.
Conclusions
A novel flame-retardant agent, guanidyl- and phosphorus-containing polysiloxane (GPPDMS), was synthesized, and combustion behavior of the treated cotton fabric with GPPDMS was studied. Presence of GPPDMS can increase the value of TTI and FPI and lower both the value of HRR, THR, EHC, and CO/CO2 ratio comparing that without GPPDMS. It demonstrated that the treated cotton fabric with GPPDMS generated less combustion heat and the more add-on of GPPDMS, the less combustion heat was. The TG test results indicate that GPPDMS treated on cotton fabric lowers the decomposition temperature and enhances the formation of char after pyrolysis. The cotton fabric treated with 18.6 % (add-on) of GPPDMS at 500 °C has a char yield of 34.5 %, which is 33.3 % higher than that of the untreated cotton fabric (1.2 %). The chemical structures of the residues were investigated by FT-IR, and the results demonstrated the formation of SiO2 matrix, polyphosphoric acid, and other derivatives of GPPDMS during the thermal decomposition process as evidenced by SEM pictures and EDS analysis results. From the above results, it can be concluded that GPPDMS improved the thermal stability of cotton fabrics effectively by hindering the formation of volatile species and favoring the formation of char.
References
Brushlinsky NN, Sokolov SV, Wagner P, Hall JR. World fire statistics. Report No. 10. Centre of fire statistics, international association of fire and rescue service; 2006.www.ctif.org.
Mostashari SM, Mostashari SZ. Combustion pathway of cotton fabrics treated by ammonium sulfate as a flame-retardant studied by TG. J Therm Anal Calorim. 2008;91:437–41.
Horrocks AR. Flame retardant challenges for textiles and fibres: new chemistry versus innovatory solutions. Polym Degrad Stab. 2011;96:377–92.
Alongi J, Ciobanu M, Carosio F, Tata J, Malucelli G. Thermal stability and flame retardancy of polyester, cotton and relative blend textile fabrics treated by sol–gel process. J Appl Polym Sci. 2011;119:1961–9.
Alongi J, Ciobanu M, Malucelli G. Sol–gel treatments for enhancing fire stability of cotton fabrics: optimization of the process and evaluation of durability. Cellulose. 2011;18:167–77.
Alongi J, Carosio F, Malucelli G. Influence of ammonium polyphosphate-/poly(acrylic acid)-based Layer by Layer architectures on the char formation in cotton, polyester and their blends. Polym Degrad Stab. 2012;97:1644–53.
Alongi J, Malucelli G. Cotton treated with novel oxidic phases acting as effective smoke suppressants. Carbohydr Polym. 2012;90:251–60.
Yang SN, Lv GP, Liu Y, Wang Q. Synergism of polysiloxane and zinc borateflame retardant polycarbonate. Polym Degrad Stab. 2013;98:2795–800.
Chen XB, Zhou SX, You B, Wu LM. Mechanical properties and thermal stability of ambient-cured thick polysiloxane coatings prepared by a sol–gel process of organoalkoxysilanes. Prog Org Coat. 2012;74:540–8.
Hu S, Hu Y, Song L, Lu HD. The potential of ferric pyrophosphate for influencing the thermal degradation of cotton fabrics. J Therm Anal Calorim. 2012;109:27–32.
Chen YZ, Peng HQ, Li JH, Xia ZX, Tan H. A novel flame retardant containing phosphorus, nitrogen, and sulfur: synthesis and application in thermoplastic polyurethane. J Therm Anal Calorim. 2014;115:1639–49.
Alongi J, Colleoni C, Rosace G, Malucelli G. Thermal and fire stability of cotton fabrics coated with hybrid phosphorus-doped silica films. J Therm Anal Calorim. 2012;110:1207–16.
Schartel B, Bartholmai M, Knoll U. Some comments on the main fire retardancy mechanisms in polymer nanocomposites. Polym Adv Technol. 2006;17:772–6.
Nazarè S, Kandola B, Horrocks AR. Smoke, CO and CO2 measurements and evaluation using different fire testing techniques for flame retardant unsaturated polyester resin formulations. J Fire Sci. 2008;26:215–42.
Kandola BK, Horrocks AR, Price D, Coleman GV. Flame-retardant treatments of cellulose and their influence on the mechanism of cellulose pyrolysis. J Macromol Sci. 1996;36:721–94.
Tsafack MJ, Levalois-Grützmacher J. Flame retardancy of cotton textiles by plasma-induced graft-polymerization(PIGP). Surf Coat Technol. 2006;201:2599–610.
Brancatelli G, Colleoni C, Massafra MR, Rosace G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym Degrad Stab. 2011;96:483–90.
Wang CS, Shieh JY, Sun YM. Phosphorus containing PET and PEN by direct esterification. Eur Polym J. 1999;35:1465–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, C., Lu, Z., Zhu, P. et al. Combustion behaviors of cotton fabrics treated by a novel guanidyl- and phosphorus-containing polysiloxane flame retardant. J Therm Anal Calorim 119, 349–357 (2015). https://doi.org/10.1007/s10973-014-4154-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4154-z