Abstract
This paper reports thermolysis of diaminoglyoxime (DAG) as a ballistic modifier, and its effects on the thermal behaviors, non-isothermal decomposition reaction kinetics, and burning rates of the homogeneous double-base propellant formulations. Thermal analysis studies were performed by thermogravimetric analysis and differential thermogravimetry (TG-DTG) and differential scanning calorimetry techniques. According to the resulted data, it was found that DAG could change the thermal decomposition mechanism function, thermokinetic parameters and kinetic equation of the propellants. Evaluation of DAG as a ballistic modifier in double-base propellant formulations indicated that it brings down the pressure index to 0.068 compared to 0.24 for the control composition in the pressure range 5–7 Mpa. The results showed that the main exothermal decomposition reaction of the propellant sample in the absence of DAG has the mechanism function of \( f(\alpha ) = \frac{3}{2}\left( {1 - \alpha } \right)^{4/3} [\left( {1 - \alpha } \right)^{ - 1/3} - 1]^{ - 1} \) and the kinetic equation of \( {\raise0.7ex\hbox{${{\text{d}}\alpha }$} \!\mathord{\left/ {\vphantom {{{\text{d}}\alpha } {{\text{d}}t}}}\right.\kern-0pt} \!\lower0.7ex\hbox{${{\text{d}}t}$}} = 1.60 \times 10^{17} \left( {1 - \alpha } \right)^{4/3} [\left( {1 - \alpha } \right)^{ - 1/3} - 1]^{ - 1} e^{{ - 2.21 \times 10^{4} /{\text{T}}}} \), while modified propellant with DAG has a different function mechanism and kinetic equation as following: \( f(\alpha ) = \frac{5}{2}\left( {1 - \alpha } \right)\left[ { - { \ln }\left( {1 - \alpha } \right)} \right]^{3/5} \) and \( {\raise0.7ex\hbox{${{\text{d}}\alpha }$} \!\mathord{\left/ {\vphantom {{{\text{d}}\alpha } {{\text{d}}t }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${{\text{d}}t }$}} = 9.28 \times 10^{40} \left( {1 - \alpha } \right)\left[ { - { \ln }\left( {1 - \alpha } \right)} \right]^{3/5} e^{{ - 4.66 \times 10^{4} /{\text{T}}}} \), respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
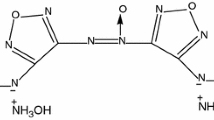
Diaminoglyoxime (DAG) is an explosophoric compound, with the chemical structure illustrated in Scheme 1. The compound is prepared by dehydration of dihydroglyoxime (DHG) in the aqueous sodium hydroxide solution at high temperatures [1–5]. This compound might be utilized as additive in the rocket propellant formulations as coolant [6].
Thermokinetic studies are crucial point in thermal analysis, while the main purpose is definition of the decomposition reaction mechanism and calculating the Arrhenius equation parameters, i.e., activation energy and frequency factor [5, 7–10]. The resulted thermoanalytical data could provide valuable information about shelf-life and safe conditions for storage of the materials [11, 12]. Prediction of these parameters for energetic materials is especially meaningful in order to elucidate miscibility/compatibility and their effects on thermal stability of the resulted composition [5, 13–16].
Until today, thermolysis of DAG and its utilization in the composite double-base propellant have been investigated [11] and the results revealed positive role of this compound in modification of double-base propellant. Stoner and Brill [12, 17] observed that thermolysis of DAG at the propellant burning surface leads to formation of a thermally stable melamine. Performing the rapid thermolysis experiments on decomposition reaction of DAG exhibited that formation of highly thermal stable cyclic azines accomplished by the evaluating low molecular weight gases, i.e., NH3, HCN, CO2, and N2O. Meanwhile, Williams et al. [17, 18] found that DAG forms a thermally stable cyclic azine, which is stable up to 700 °C in the superficial reaction layer at the burning surface of the propellant. Formation of these thermal stable products due to the decomposition of the propellant caused retarding the heat transferring from the gas phase to the condensed segment of propellant. In this paper, the influences of DAG as a ballistic modifier on the thermal behaviors, decomposition reaction kinetics and burning rate of the homogeneous double-base (DB) propellant were investigated under the non-isothermal conditions. To the best of our knowledge, there is no information on the decomposition reaction kinetics of the double-base (DB) propellant containing DAG as a ballistic modifier.
Experimental
Sample preparation
DAG as the ballistic modifier of the propellant was synthesized and purified in the organic chemistry laboratory (MUT, Tehran) as proposed previously [19], while the fibrous NC used in the present investigation was of commercial grade with about 12.0 % nitrogen content [20]. The studied double-base propellant sample (control sample or no. P1) was containing of 56 % (mass fraction) NC, 30 % NG, 3 % centralite II, 7 % diethyl phthalate, 4 % other assistant reagents. The propellant sample modified with DAG (no. P2) was composed of similar components, while 2 % DAG was added and the NC content was reduced to 54 %. The details of the propellant formulations are presented in Table 1. The propellant samples were prepared by a solvent-less double-base propellant extrusion technique, including slurry mixing, rolling, and extruding [9, 17, 21].
Instruments and experimental conditions
Infrared spectrum was obtained with a FT-IR spectrophotometer (Perkin-Elmer 1600) using KBR pellets. Analyses for C, H, and N determination were carried out on a Carlo Erba 1108 Elemental Vario EL analyzer. Thermal behaviors of propellant samples were analyzed by differential scanning calorimeter (DSC) and thermogravimetric analysis (TG). The thermal analyses were carried out on a Mettler TA4000 thermal analyzer and a DSC 1 (Mettler Toledo Co., Switzerland), respectively. The operating conditions of TG analyses were as follows: about 3 mg as the sample mass; purging of N2 gas with the flowing rate of 80 mL min−1; heating rates (β) of 10 °C min−1 in a temperature range of 50–600 °C using an α-Al2O3 crucible. The operating conditions of DSC analyses were included: about 1.5 mg as the sample mass; 50 mL min−1 as flowing rate of N2 purging in a temperature range of 50–500 °C using an α-Al2O3 crucible; heating rates of 3, 5, 7, and 9 °C min−1. The burning rates of propellant samples under different pressures were determined in an indigenously fabricated Crawford Bomb strand-burner equipment [15, 17].
Results and discussion
Characterization of DAG
As seen in Fig. 1, the room temperature FTIR spectrum of pure DAG in KBr matrix shows absorption bands corresponding to N–O bond of oxime (951.78 cm−1), –NH2 group (3368.79, 3470.35 and 1680.8 cm−1), and –OH group (2800–3300 and 1447.74 cm−1), while the absorption band observed at 1652.41 cm−1 may be attributed to C=N bond. The resulted data from IR analysis of DAG are listed in Table 2. Elemental analysis (%), calcd. for C2H6N4O2 (%): C, 20.34; H, 5.12; N, 47.44. Found (%): C, 20.54; H, 5.20; N, 47.36.
Thermal properties of DAG
The DSC curve of pure DAG is given in Fig. 2. According to the DSC data, an endothermic peak was observed around 200 °C corresponding to the melting point of DAG. However, DAG was decomposed exothermally at 205.5 °C, after it is melting at the temperature of 200 °C [5].
Thermal behavior of the propellant in the presence of DAG
The TG-DTG curves of the control propellant (sample P1) and the DAG modified propellant (sample P2) at the heating rate of 10 °C min−1 are shown in Fig. 3. Also, the DSC curves at the heating rates of 3, 5, 7 and 9 °C min−1 for both samples are presented in Figs. 4 and 5. As seen in Fig. 3, there are two mass loss stages in the TG curve of the propellant samples. Meantime, two exothermic peaks were observed in the corresponded DTG curves. TG curves of both samples, at β = 10 °C min−1, exhibited that the first stage begins at about 50 °C and stops at about 230 °C, while the peak in the DTG curve was appeared at 199–201 °C accompanying about 84–86 % mass loss, which is close to the total mass of the ester content (NC/NG) of the propellant and hence it likely attributes to the volatilization and decomposition of the NC/NG mixed ester. The second stage begins at about 410 °C and stops at about 550 °C, with the peak in the DTG curve at 508–535 °C, accompanied by 12–13 % mass loss, and it attributes to the reaction of remaining auxiliaries. However, a few remains were observed at the end of the second decomposition stage. In fact, both samples undergo a main exothermic decomposition reaction during their first stage, this results is confirmed by the exothermic peaks observed in the DSC curves of the samples in Figs. 4 and 5. On the other hand, decomposition temperature of the DAG is about 200 °C and this temperature is comparable with the decomposition temperature of the sample at the first stage. On the other hand, the propellant in the presence of DAG decomposes during a single step and the observed exothermic peak in the temperature range of 400–500 °C was not observed. In other word, the presence of the DAG in the formulation has a main influence on the thermal pattern of the propellant and all the ingredients decompose simultaneously during a single peak at a temperature about 200 °C (Fig. 5).
The basic data for the main exothermic decomposition processes of the propellants P1 and P2 are listed in Table 3.
Calculation of non-isothermal reaction kinetics
Thermal decomposition kinetic, Arrhenius parameters, i.e., the activation energy (E a) and the pre-exponential constant (A) and the most probable kinetic model function corresponding to the studied propellant samples was explored. The DSC curves resulted from the samples at the heating rates of 3, 5, 7, and 9 °C min−1 were employed to examine their compatibility with two well-known integral methods (i.e., Coats Redfern and Flynn–Wall–Ozawa) (Eqs. (1–2) in Table 4] and also two differential methods (i.e., Kissinger and Starink) [Eqs. (3–4) in Table 4] [21–31]. The values of E a were predicted by the Ozawa method using the isoconversional DSC curves at different heating rates, while the relations between E a and α for both propellant samples are given in Fig. 6. As seen in this figure, the values of activation energy change slightly in the range of 0.35–0.475 (α) for the control sample (P1), and 0.225–0.425 (α) for the DAG modified propellant sample (P2). These ranges were chosen to calculate the non-isothermal reaction kinetics of the samples. Forty-one types of the proposed kinetic model functions in Refs. [32–40] were examined, while the original DSC data were utilized as input of the integral and differential equations. The values of E a, log A, and the linear correlation coefficient (r) for both propellant samples were calculated and listed in Table 5. The most probable mechanism function could be selected using the better values resulted for r. The data in Table 5 exhibit that the values of E a and log A computed from the non-isothermal DSC data have good agreement with the calculated values via Flynn–Wall–Ozawa and Kissinger and Starink methods. Therefore, the mechanism function (listed in Table 6) might be determined by substituting the values of E a/(kJ mol−1) and A/s−1 into Eq. (1):
where \( f(\alpha ) \) represents the differential model function, t symbolizes time, and R is the universal gas constant. The corresponding kinetic equation to the decomposition reaction of the propellant samples was determined and presented in Table 6. As seen in this table, the addition of DAG to the propellant formulation could change the decomposition reaction mechanism function in comparing with the control propellant.
Thermal safety studies
Self-accelerating decomposition temperature (T SADT)
The self-accelerating decomposition temperature (SADT) is defined as the lowest ambient temperature at which an organic substance or self-reactive compound undergoes the self-accelerating decomposition. Determining of this parameter is essential for the safe packaging or transportation purposes in various materials especially in energetics [41]. In this study, the values of the onset temperature (T e) corresponding to \( \beta \) → 0 or T e0 were obtained by Eq. (2), and then, the self-accelerating decomposition temperature (T SADT) for the propellant samples was computed by Eq. (3) [36–40].
While, b and c are coefficients.
The resulted values for the studied propellant samples are given in Table 7.
Critical temperatures of thermal explosion
Critical temperature of thermal explosion (T b) is defined as the lowest temperature to which a specific charge might be heated without undergoing any thermal runaway [30]. This vital parameter for energetic materials usually is predicted to insure safe processing and storage. T b could be calculated by considering the ignition theory and corresponding thermo-kinetic parameters, namely the pre-exponential factor, activation energy, and heat of decomposition reaction. The critical temperatures of thermal explosion for both propellant samples were obtained from Eq. (4) [33, 38] and given in Table 7.
The presented data in Table 7 show that the value of T bp for the DAG modified double-base propellant in comparison with the control sample is high, which confirms the difficulty in happening of transition from thermal decomposition to a thermal explosion in the modified propellant [38].
Burning rate measurement for the studied propellant samples
In order to investigate the possible utilizing of DAG in propellants as the ballistic modifier, the burning rates [u/(mm s−1)] of the both control propellant (P1) and the DAG modified propellant (P2) were measured and compared at different pressures (P/MPa). The resulted burning rates are listed in Table 8. As seen in this table, the addition of about 2 % DAG to the propellant sample has no significant effect on its burning rate, however brought down the n value. The brought down of the n value from 0.24, for the control sample, to 0.068 for the DAG modified sample in the pressure region of 5–7 MPa was observed. In order to evaluate the effects of DAG as the ballistic modifier on the burning rate of the studied double-base propellant, the pressure exponent (n) of the burning rate (u) was computed. Then, the average values of the catalysis efficiency (\( \bar{Z} \)) were compared before and after addition of the ballistic modifier into the propellant formulation. To achieve this aim, the values of n and \( \bar{Z} \) were obtained by Eqs. (5) and (6) [17, 21, 37, 38]:
where a is the coefficient (mm s−1 MPa−1).
For the DAG modified propellant: at 5–8 MPa, u = 4.97P 0.26, \( \bar{Z} \) = 0.9; at 8–12 MPa, u = 8.47P 0.155, \( \bar{Z} \) = 1.17; at 12–15 MPa, u = 15.7P 0.024, \( \bar{Z} \) = 0.99.
As seen, addition 2 % DAG as a ballistic modifier to the propellant formulation has no considerable effect on the burning rate, but reduces efficiently the pressure exponent of the double-base propellant.
Conclusions
The ability of DAG as a ballistic modifier for the application in the homogeneous double-base propellant was investigated. Thermo-kinetic results showed that DAG could enhance the enthalpy of decomposition, apparent activation energy, and change the decomposition reaction mechanism function of the double-base propellant. The mechanisms of the chemical reactions corresponding to the main exothermal decomposition reactions of the control sample and DAG modified propellant are classified, respectively, as \( f(\alpha ) = \frac{3}{2}\left( {1 - \alpha } \right)^{4/3} [\left( {1 - \alpha } \right)^{ - 1/3} - 1]^{ - 1} \) and \( f(\alpha ) = \frac{5}{2}\left( {1 - \alpha } \right)\left[ { - { \ln }\left( {1 - \alpha } \right)} \right]^{3/5} \), while their kinetic equations are: \( {\raise0.7ex\hbox{${{\text{d}}\alpha }$} \!\mathord{\left/ {\vphantom {{{\text{d}}\alpha } {{\text{d}}t}}}\right.\kern-0pt} \!\lower0.7ex\hbox{${{\text{d}}t}$}} = 1.60 \times 10^{17} \left( {1 - \alpha } \right)^{4/3} [\left( {1 - \alpha } \right)^{ - 1/3} - 1]^{ - 1} e^{{ - 2.21 \times 10^{4} /{\text{T}}}} \) and \( {\raise0.7ex\hbox{${{\text{d}}\alpha }$} \!\mathord{\left/ {\vphantom {{{\text{d}}\alpha } {{\text{d}}t }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${{\text{d}}t }$}} = 9.28 \times 10^{40} \left( {1 - \alpha } \right)\left[ { - { \ln }\left( {1 - \alpha } \right)} \right]^{3/5} e^{{ - 4.66 \times 10^{4} /{\text{T}}}} \). Evaluating thermal safety of the propellants samples showed that the DAG modified propellant has a higher resistance to heat and higher thermal safety than the control propellant. Furthermore, DAG could reduce efficiently the pressure exponent of the homogeneous double-base propellant to 0.068 at the pressure of 5–7 MPa.
References
Gunasekaran A, Jayachandran T, Boyer JH, Trudell ML. A convenient synthesis of diaminoglyoxime and diaminofurazan: useful precursors for the synthesis of high density energetic materials. J Heterocycl Chem. 1995;32:1405–7.
Pourmortazavi SM, Kohsari I, Teimouri MB, Hajimirsadeghi SS. Thermal behaviour kinetic study of dihydroglyoxime and dichloroglyoxime. Mater Lett. 2007;61:4670–3.
Komin AP, Street RW, Carmack M. Chemistry of 1,2,5-thiadiazoles. III. [1,2,5] thiadiazolo [3,4-c][1,2,5] thiadiazole. J Org Chem. 1975;40:2749–52.
Talawar MB, Sivabalan R, Senthilkumar N, Prabhu G, Asthana SN. Synthesis, characterization and thermal studies on furazan- and tetrazine-based high energy materials. J Hazard Mater. 2004;113:11–25.
Kohsari I, Pourmortazavi SM, Hajimirsadeghi SS. Non-isothermal kinetic study of the thermal decomposition of diaminoglyoxime and diaminofurazan. J Therm Anal Cal. 2007;89:543–6.
Kanno H, Yamamoto H, US Patent 5,476,967 (1995).
Shamsipur M, Pourmortazavi SM, Roushani M, Miran Beigi AA. Thermal behavior and non-isothermal kinetic studies on titanium hydride-fueled binary pyrotechnic compositions. Combust Sci Technol. 2013;185:122–33.
Sinditskii VP, Burzhava AV, Chernyi AN, Shmelev DS, Apalkova VN, Palysaeva NV, Sheremetev AB. A comparative study of two difurazanyl ethers. J Therm Anal Calorim. 2016;123:1431–8.
Ravanbod M, Pouretedal HR, Catalytic effect of Fe2O3, Mn2O3, and TiO2 nanoparticles on thermal decomposition of potassium nitrate. J Therm Anal Calorim. doi:10.1007/s10973-015-5167-y
Han Z, Wang D, Wang H, Henkes C. Electrospray formation of RDX/ceria mixture and its thermal decomposition performance. J Therm Anal Calorim. 2016;123:449–55.
Takahashi PM, Netto AVG, Mauro AE, Frem RCG. Thermal study of nickel(II) pyrazolyl complexes. J Therm Anal Calorim. 2005;79:335–8.
Stoner CE Jr, Brill TB. Thermal decomposition of energetic materials 46. The formation of melamine-like cyclic azines as a mechanism for ballistic modification of composite propellants by DCD, DAG, and DAF. Combust Flame. 1991;83:302–8.
Trache D, Khimeche K, Mezroua A, Benziane M, Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. J Therm Anal Calorim. doi:10.1007/s10973-016-5293-1
Pourmortazavi SM, Hosseini SG, Hajimirsadeghi SS, Fareghi Alamdari R. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic systems. Combus Sci Technol. 2008;180:2093–102.
Fathollahi M, Pourmortazavi SM, Hosseini SG. The effect of the particle size of potassium chlorate in pyrotechnic compositions. Combust Flame. 2004;138:304–6.
Wei H, Liming H, Zhongliang M, Yanlia G, The kinetic and viscosity analysis of glycidyl azide polymer spherical propellant. J Therm Anal Calorim. doi:10.1007/s10973-015-5225-5
Talawar MB, Makashir PS, Nair JK, Pundalik SM, Mukundan T, Asthana SN, Singh SN. Studies on diaminoglyoxime (DAG): thermolysis and evaluation as ballistic modifier in double base propellant. J Hazard Mater. 2005;A 125:17–22.
Williams GK, Palopoli SF, Brill TB. Thermal decomposition of energetic materials 65. Conversion of insensitive explosives (NTO, ANTA) and related compounds to polymeric melon-like cyclic azine burn-rate suppressants. Combust Flame. 1994;98:197–204.
Park DJ, Stern AG, Willer RL. A convenient laboratory preparation of cyanogen. Synth Commun. 1990;20:2901–6.
Pourmortazavi SM, Hosseini SG, Rahimi-Nasrabadi M, Hajimirsadeghi SS, Momenian H. Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;162:1141–4.
Yi JH, Zhao FQ, Hong WL, Xu SY, Hu RZ, Chen ZQ, Zhang LY. Effects of Bi-NTO complex on thermal behaviors, nonisothermal reaction kinetics and burning rates of NG/TEGDN/NC propellant. J Hazard Mater. 2010;176:257–61.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Hu R-Z, Gao S-L, Zhao F-Q, Shi Q-Z, Zhang T-L, Zhang J-J. Thermal Analysis Kinetics. 2nd ed. Beijing: Science Press; 2008.
Ma H-X, Song J-R, Hu R-Z. Non-isothermal kinetics of the thermal decomposition of 3-nitro-1,2,4-triazol-5-one magnesium complex. Chin J Chem. 2003;21(12):1558–61.
Hu R-Z, Chen S-P, Gao S-L, Zhao F-Q, Luo Y, Gao H-X, Shi Q-Z, Zhao H-A, Yao P, Li J. Thermal decomposition kinetics of Pb0.25Ba0.75(TNR)·H2O complex. J Hazard Mater. 2005;A117:103–10.
Shamsipur M, Pourmortazavi SM, Hajimirsadeghi SS. Investigation on decomposition kinetics and thermal properties of copper fueled pyrotechnic compositions. Combust Sci Tech. 2011;183:575–87.
Ma H-X, Song J-R, Zhao F-Q, Hu RZ, Xiao H-M. Nonisothermal reaction kinetics and computational studies on the properties of 2,4,6,8-tetranitro-2,4,6,8-tetraazabicyclo [3,3,1] onan-3,7-dione (TNPDU). J Chem Phys. 2007;A111:8642–9.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Pourmortazavi SM, Hajimirsadeghi SS, Kohsari I, Fathollahi M, Hosseini SG. Thermal decomposition of pyrotechnic mixtures containing either aluminum or magnesium powder as fuel. Fuel. 2008;87:244–51.
Shamsipur M, Pourmortazavi SM, Hajimirsadeghi SS, Atifeh SM. Effect of functional group on thermal stability of cellulose derivative energetic polymers. Fuel. 2012;95:394–9.
Tompa AS, Boswell RF. Thermal stability of a plastic bonded explosive. Thermochim Acta. 2000;357–358:169–75.
ASTM E698-11, standard test method for arrhenius kinetic constants for thermally unstable materials using differential scanning calorimetry and the Flynn/Wall/Ozawa method, ASTM international, west conshohocken, PA, 2011. http://www.astm.org.
Shamsipur M, Pourmortazavi SM, Fathollahi M. Kinetic parameters of binary iron/oxidant pyrolants. J Energy Mater. 2012;30:97–106.
Pickard JM. Critical ignition temperature. Thermochim Acta. 2002;392:37–40.
Tompa AS, Boswell RF. DSC and TG study of the stability in vacuum of ferrocenyl compounds and their compatibility with ammonium perchlorate. Thermochim Acta. 1984;77:133–50.
Boldyrev VV. Thermal decomposition of ammonium perchlorate. Thermochim Acta. 2006;443:1–36.
Chen P, Zhao F-Q, Luo Y, Hu R-Z, Gao S-L, Zheng Y-M, Deng M-Z, Gao Y. Thermal decomposition behavior and non-isothermal decomposition reaction of copper(II) salt of 4-hydroxy-3,5-dinitropyridine oxide and its application in solid rocket propellant. J Chem. 2004;22:1056–63.
Yi J-H, Zhao F-Q, Wang B-Z, Liu Q, Zhou C, Hu R-Z, Ren Y-H, Xu S-Y, Xu K-Z, Ren X-N. Thermal behaviors, nonisothermal decomposition reaction kinetics, thermal safety and burning rates of BTATz-CMDB propellant. J Hazard Mater. 2010;181:432–9.
Yi J-H, Zhaoa F-Q, Xu S-Y, Zhang L, Gao H, Hu R-Z. Effects of pressure and TEGDN content on decomposition reaction mechanism and kinetics of DB gun propellant containing the mixed ester of TEGDN and NG. J Hazard Mater. 2009;165:853–9.
Weijian N, Xiaopeng C, Linlin W, Jiezhen L, Lingping Z, Zhangfa T. Thermal decomposition kinetics of abietic acid in static air. Chin J Chem. 2013;21(7):724–9.
Chen KY, Wu SH, Wang YW, Shu CM. Runaway reaction and thermal hazards simulation of cumene roperoxide by DSC. J Loss Prevent Proc. 2008;21:101–9.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pourmortazavi, S.M., Farhadi, K., Mirzajani, V. et al. Study on the catalytic effect of diaminoglyoxime on thermal behaviors, non-isothermal reaction kinetics and burning rate of homogeneous double-base propellant. J Therm Anal Calorim 125, 121–128 (2016). https://doi.org/10.1007/s10973-016-5373-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5373-2