Abstract
Fly ash has been widely used as supplementary cementitious material in concrete industry. Hydration mechanism of composite binder containing fly ash is much more complicated due to the mutual effect of the hydration of cement and the pozzolanic reaction of fly ash. This paper involves the hydration kinetics of composite binder containing up to 65 % of fly ash and comparison of the results with data on composite binder containing slag that are previously published. The hydration heat evolution rate and cumulative hydration heat of composite binder containing fly ash were measured at 298, 318 and 333 K with an isothermal calorimeter. Based on the hydration kinetics model, three hydration processes, namely nucleation and crystal growth (NG), interactions at phase boundaries (I) and diffusion (D) were characterized, the relationship between the hydration rate and hydration degree was discussed at different stages, and kinetics parameters, n, K and E a, were calculated and analyzed. Results show that the hydration heat evolution rate and cumulative hydration heat of composite binder obviously decrease with increasing the replacement ratio of fly ash. Elevated temperatures promote the hydration process, especially for composite binder containing high amount of fly ash. The kinetics model could simulate the hydration process of composite binder containing no more than 65 % of fly ash, whose hydration process sequence is NG → I → D at 298 and 318 K, but it becomes NG → D at 333 K. Fly ash has relatively smaller effect on the overall reaction of composite binder than slag. The reaction rates of composite binder containing fly ash at different stages are higher than those of composite binder containing slag at the same replacement ratio. The value of E a for the overall reaction of composite binder decreases first and then increases with increasing the content of fly ash, and it is lower than that of composite binder containing slag at the same replacement ratio.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fly ash has been widely used as a supplementary cementitious material in modern concrete industry due to its benefits in improving properties of concrete and reducing carbon dioxide emissions, which promotes eco-friendly construction. The hydration of composite binder containing fly ash includes two interrelated processes: the hydration of Portland cement and the pozzolanic reaction of fly ash [1]. Ca(OH)2 is produced by the hydration of cement, and it consumes by the pozzolanic reaction of fly ash. The two reactions may occur simultaneously, and they are influenced by each other. Besides the chemical effect (i.e., pozzolanic reaction), fly ash also has physical effect that promotes the hydration of cement. The hydration rate and reaction degree of composite binder are also affected by elevated temperature [2, 3]. Due to the different chemical compositions and hydration activity, the hydration kinetics of composite binder containing fly ash is different from that of composite binder containing slag.
The hydration kinetics of composite binder containing fly ash has been studied extensively. Narmluk et al. [4] found that the hydration kinetics of composite binder containing fly ash depended on the replacement ratio of fly ash and curing temperatures. At 20 and 35 °C, fly ash retarded the hydration of cement in early period and accelerated the hydration of cement in later period. But at 50 °C, for composite binder containing high amount of fly ash, the hydration of cement retarded at later ages. Deschner et al. [5, 6] investigated the hydration kinetics of composite binder containing 50 % of fly ash, and the results were compared with a reference composite binder containing 50 % of inert quartz powder. It was found that the influence of fly ash on the hydration was mainly related to the filler effect until 2 days and the pozzolanic reaction was observed from 7 days on. The elevated temperatures accelerated both the hydration of cement and the reaction of fly ash. In general, replacement of cement by fly ash leads to a retarding effect on the hydration [2, 7–9]. Dittrich et al. [10] found that a retarding effect of fly ash on the silicate reaction was detected due to the adsorption of Ca2+ ions on the surface of fly ash and the aluminate reaction was influenced by fly ash due to the additional nucleation sites provided. Nocuń-Wczelik [11] pointed out that the hydration heat evolution rate and cumulative hydration heat of composite binder containing 5 % of fly ash were almost unchanged compared to Portland cement, but when the replacement ratio of fly ash was larger than 30 %, the hydration process is severely retarded. Kumar et al. [3] revealed that for composite binder containing 20 % of fly ash, the hydration heat evolution rate decreased at 35 °C due to the dilution effect. Morever, the induction period and the appearing time of the second exothermic peak were prolonged. But at 45 °C, the rate of hydration increased obviously and the time of hydration was also shortened. Langan et al. [12] found that fly ash retarded cement hydration more significantly at high water-to-binder ratio. Furthermore, numerous kinetics models have been proposed to quantify the hydration kinetics of cement [13]. Wang et al. [14, 15] predicted the hydration of composite binder containing fly ash based on the shrinking-core model. The mutual interactions between the hydration of cement and the reaction of fly ash are considered through the available amounts of calcium hydroxide and capillary water in the system. Thomas [16] applied boundary nucleation and growth (BNG) theory to characterize the hydration kinetics of tricalcium silicate and alite. Scherer et al. [17] used this model for the hydration of cement, and it was shown good fits to calorimetric and chemical shrinkage data with the assumption that nucleation and growth rates are constant. Furthermore, he suggested that BNG of C–S–H is more likely to occur within confined pores [18]. Krstulovic and Dabic [19, 20] investigated the hydration kinetics of cement according to the hypothetical mathematical model, which provides for the fact that the process takes place in a heterogeneous system involved three basic processes: nucleation and crystal growth, interactions at phase boundaries and diffusion.
Based on the current literatures, it is apparent that the investigation of hydration kinetics of composite binder containing fly ash is mainly on calcium hydroxide, and like the reaction of slag, the stoichiometric coefficients of reaction of fly ash is still dubious and poorly understood [21]. Moreover, the development of the degree of hydration of composite binder containing fly ash has not been fully elucidated [4]. For BNG model, it must only be used during the period when the reaction is dominated by a single phase [17], which is not applicable for composite binder containing fly ash. There is little information about the apparent activation energy of composite binder containing fly ash in the current literatures, which is important to understand the hydration mechanism.
The purpose of this work is, therefore, to investigate the hydration kinetics of composite binder containing fly ash based on the kinetics model proposed by Krstulovic et al. [19] and compare the results with data on hydration kinetics of composite binder containing slag that are previously published [22]. The samples were examined using an isothermal calorimeter. The kinetics model and the methods of determination of the kinetics parameters have been detailedly described in the literature [22]. The kinetics analysis was mainly on the overall apparent hydration process.
Experimental
Materials
P.I 42.5 Portland cement and Class I fly ash conforming to Chinese National Standards GB 175-2007 and GB/T 1596-2005, respectively, were used in this paper. The chemical compositions of cement and fly ash determined by X-ray fluorescence (XRF) are given in Table 1.
The water requirement ratio of fly ash is 95 %. The specific surface area of cement is 350 m2 kg−1. The particle size distributions of cement and fly ash measured by a laser particle size analyzer (MASTER SIZER 2000) are shown in Fig. 1. It is apparent that fly ash is finer than cement. The median particle diameters of cement and fly ash (i.e., D50) are 17.17 and 9.77 μm, respectively.
The water-to-binder ratio for all samples is 0.4. The mix proportions of pastes are presented in Table 2.
Test methods
The hydration heat evolution rate and the cumulative hydration heat of composite binder containing fly ash were measured with an isothermal calorimeter (TAM Air from TA instruments). The tests were performed at three constant temperatures (298, 318 and 333 K) within 72 h. After stirring evenly, the samples were immediately placed into the chamber. Then the hydration heat evolution rate and cumulative hydration heat could be monitored continuously as a function of hydration time. The cumulative hydration heat released during initial period and induction period accounts for few percentage [23], which could be negligible. Therefore, the hydration kinetics of composite binder containing fly ash was studied since the ending time of induction period.
Results and discussion
Hydration heat characteristics of composite binder containing fly ash
The hydration heat evolution rate curves of composite binder containing fly ash at 298, 318 and 333 K are shown in Fig. 2. It can be seen from Fig. 2a that the hydration process of cement is significantly affected by the curing temperatures. An increase in temperature accelerates the hydration heat evolution rate. The peak value of the second exothermal effect increases by more than two times from 298 to 318 K and by more than four times from 298 to 333 K. The ending time of induction period and the appearing time of the second exothermal peak obviously reduce. The intension hydration shortens from more than 20 h at 298 K to about 10 h at 333 K. As shown in Fig. 2b–e, the trend of hydration heat evolution rate curves of composite binder containing fly ash is similar to that of cement (Fig. 2a). But the peak value of the second exothermal peak evidently decreases at three examined temperatures with increasing the replacement ratio of fly ash. Moreover, the ending time of induction period and the appearing time of the second exothermal peak increase significantly, which indicates that fly ash has a retarding effect on the hydration. The results are consistent with previous studies [3, 7–12]. Compared to composite binder containing slag [22], incorporation of fly ash could obviously retard the hydration of cement. The reactivity and the water absorption of fly ash are lower than those of slag, and then the ion concentration of pore solution reaches supersaturation for long time. It is noted that there is a third exothermal peak on the hydration heat evolution rate curves of composite binder containing 50 or 65 % of fly ash (Fig. 2d, e). The third exothermal peak is obviously observed at 333 K. The exothermal effect is caused by the pozzolanic reaction of fly ash. For composite binder containing high amount of fly ash, the initial hydration of cement is significantly promoted. The alkalinity of pore solution reaches to a sufficient value that could break down the glass phase of fly ash, and then hydration heat is released. Increasing temperature to 333 K, the depolymerizing ability of vitreous fly ash increases [2]. Thus, the hydration heat evolution rate increases significantly. For composite binder containing small amount of fly ash, the dominant reaction is the hydration of cement, and the exothermal effect of reaction of fly ash is not obvious.
Figure 3 presents the cumulative hydration heat curves of composite binder containing fly ash at 298, 318 and 333 K. As shown in Fig. 3a, an increase in temperature evidently increases the cumulative hydration heat of cement. It can be seen from Fig. 3b–e that the cumulative hydration heat curves of composite binder containing fly ash present similar trend to that of cement. However, the cumulative hydration heat significantly decreases with increasing the content of fly ash at all studied temperatures in this paper. Due to the filler effect and pozzolanic reaction of fly ash, the decreased ratio of hydration heat is lower than the replacement ratio of fly ash. An increase in temperature from 298 to 333 K increases the 72-h cumulative hydration by 30.78, 31.38, 31.76 and 32.03 % for samples FA20, FA35, FA50 and FA65, respectively, while it increases by 21.78 % for cement. It is indicated that elevated temperature has a great effect on the hydration of composite binder containing fly ash, especially for composite binder containing high amount of fly ash. Thus, an exothermal peak is observed on the deceleration period of hydration heat evolution rate curves for samples FA50 or FA65 (Fig. 2d, e). But compared to composite binder containing slag [22], the promoting effect of temperature on the composite binder containing fly ash is relatively small. Due to the high activity of slag, an obvious exothermal peak of slag reaction is observed at 298 K, and it is overlapped by the main exothermal peak at 318 and 333 K. The 72-h cumulative hydration heat of composite binder containing no more than 50 % of slag is almost same as that of Portland cement at 333 K. However, the cumulative hydration heat of composite binder containing fly ash is still lower than that of Portland cement at elevated temperatures.
Kinetics of hydration process of composite binder containing fly ash
The hydration rate curve, dα/dt, and the simulated curves, F 1(α), F 2(α) and F 3(α), of composite binder containing fly ash at 298, 318 and 333 K are shown in Figs. 4–6, respectively. As shown in Fig. 4, curves, F 1(α), F 2(α) and F 3(α), could well segmentally simulate the hydration rate curve of composite binder containing fly ash at 298 K. The hydration process of composite binder containing no more than 65 % of fly ash successively experiences NG, I and D, elucidating that the reaction of hydration is controlled by a multiple reaction mechanism.
It can be seen from Fig. 4 that the simulation error has a little increase with increasing the replacement ratio of fly ash. The hydration of composite binder containing fly ash is similar to that of composite binder containing slag, which consists of the rapid hydration of cement and the slow reaction of fly ash. The influence of fly ash on the overall reaction increases with increasing fly ash content. However, fly ash has relatively small effect on the reaction of composite binder compared with slag at the same replacement level [22]. The early-age reaction of fly ash is very slow at ambient temperatures, and after long hydration time (≥7 days), fly ash starts to react with Ca(OH)2 [24–26]. Thus, the contribution of reaction of fly ash to the overall hydration is relatively small in 72-h hydration at 298 K. But a certain amount of slag has reacted at early age, which has a great influence on the overall reaction. After mixing composite binder with water, the changes in hydration kinetics are dominated by the filler effect of fly ash. Incorporation of fly ash increases the water-to-clinker ratio that results in more space for the hydration products of cement. Moreover, the particle size of fly ash is finer than cement (Fig. 1) and the surface of fly ash provides additional sites for the precipitation of hydration products of cement. Thus, the reaction of cement is accelerated. Some researchers also found that the amount of Ca(OH)2 had a certain increase at early stage of hydration [8, 24, 26–28]. Therefore, the simulation value of hydration rate for NG process is a little lower than the actual hydration rate and this phenomenon becomes obvious for composite binder containing 65 % of fly ash. After that, the hydration process enters phase-boundary controlled process (i.e., I process). The controlled time by I process prolongs with increasing the replacement ratio of fly ash (Fig. 4). It is due to the retarding effect of fly ash on the hydration. The exothermal rate of composite binder decreases significantly, and the reaction lasts for a long time (Fig. 2b–e). The hydration products gradually generate in the system, and the controlling mechanism changes smoothly. In the later period of hydration, a thick layer of hydration products is formed around the unhydrated particles. Water and ions react with unhydrated particles by diffusion. The hydration kinetics is dominated by D process. Due to the small exothermal effect generated by the pozzolanic reaction of fly ash, there is a small deviation between the simulation value and actual value of hydration rate (Fig. 4d, e).
It can be seen from Fig. 5 that an increase in temperature from 298 to 318 K increases the simulation error. The hydration kinetics model could be used to well simulate the NG process and D process, but the simulation value of hydration rate is a little lower than actual value for I process. It is apparent that increasing temperature shortens the time controlled by I process. In the initial time, the rate of nucleation and crystal growth of hydration products increases due to the acceleration of hydration at elevated temperature [29]. Much more hydration products generate in a short time and cover on the unhydrated particles that lead to high reaction resistance. The controlling mechanism of the hydration reaction directly transforms from NG process to D process with respect to sample Cem (Fig. 5a). But for composite binder containing fly ash, the hydration process successively experience NG → I → D (Fig. 5b–e). It is related to the small amount of cement and low activity of fly ash, which could not be stimulated fully at 318 K. After intension reaction, the time of hydration entering diffusion controlled process shortens, and the hydration is dominant by D process for a long time.
As shown in Fig. 6, increasing temperature to 333 K significantly increases the rate of hydration. The driving force provided by the elevated temperature promotes plenty of nucleuses generated rapidly. The time of intension reaction of composite binder continues just about 10 h at 333 K (Fig. 2). Hydration products growing from any nucleation site rapidly impinge with adjacent hydration products [30], which results in a dense microstructure. The hydration process might not experience I process and directly controlled by D process. Thus, the hydration process experiences NG → D. From Figs. 5 and 6, it is found that elevated temperatures have a great effect on the hydration mechanism controlled by chemical reaction (i.e., NG process and I process), whose controlled time is long at 298 K but becomes short at 318 and 333 K in 72-h hydration compared to mechanism controlled by diffusion (i.e., D process). For D process, the simulation value is relatively accurate.
Kinetic parameters analysis of composite binder containing fly ash
Table 3 gives kinetic parameters of hydration process of composite binder containing fly ash. An increase in the dosage of fly ash increases the value of the exponent, n. The change rule is different from composite binder containing slag, which presents decreasing trend with increasing the content of slag [22]. It is elucidated that the influences of fly ash and slag on the crystal growth geometry are different. This might be related to the inherent properties of fly ash that has low activity and low water absorption as well as finer particle size than slag. These characteristics promote significantly the rate of nucleation and crystal growth of hydration products. Due to the driving force of elevated temperature, the value of the exponent, n, is large at elevated temperature.
It can be seen from Table 3 that the rate of hydration for NG process is highest during the overall hydration process, and the rate of hydration for I process is higher than that for D process. An increase in temperature increases the difference of rate of hydration among three processes. As discussed above, elevated temperatures have greater effect on the chemical reaction than diffusion. The initial hydration process is controlled by nucleation and crystal growth, which is the autocatalytic reaction. After mixing composite binder with water, many soluble compounds from cement are dissolved and the pore solution reaches supersaturation in a few hours. Then many stable nucleuses formed and started to grow. Due to the driving force provided by the elevated temperatures, the rate of dissolving ions increases and the time of reaching supersaturation shortens. Thus, increasing temperature increases the rate of hydration for NG process (Table 3). When the reactants required to supply the transformation reaction are continuously replenished so that the level of supersaturation is constant, then the hydration kinetics are controlled by phase-boundary reaction [13]. It is apparent that the value of hydration rate for I process increases with increasing temperature due to the shortened time reaching a certain constant supersaturation. For D process, the rate at which reactants are supplied to region is the rate-controlling step. Increasing temperature to 318 or 333 K promotes the movement of water and ions to approach the surface of unhydrated particles, then the rate of hydration increases.
As shown in Table 3, the change rule of hydration rate of composite binder containing fly ash is not similar to that of composite binder containing slag [22]. The values of \( K_{1}^{\prime } \) and \( K_{2}^{\prime } \) increase first and then decrease with increasing the replacement ratio of fly ash at three temperatures. Fly ash acts as inert material at early ages. When the content of fly ash is small, the decrease of cement content has little influence on the overall reaction. Meanwhile, owing to the filler effect of fly ash, the hydration rate of cement is promoted. However, when the dosage of fly ash is large, the reduction of cement content is significant. Many calcium ions are absorbed on the surface of fly ash that leads to a reduction of calcium concentration in the first hours [31]. Then the nucleation and crystallization of Ca(OH)2 and C–S–H are delayed [32]. The time of pore solution reaching to a constant supersaturation is also prolonged; thus, an increase in fly ash content decreases the value of \( K_{2}^{\prime } \). However, the inverse trends of \( K_{3}^{\prime } \) are observed. The value of \( K_{3}^{\prime } \) increases with increasing the dosage of fly ash at 298 K. Addition of fly ash results in a loose microstructure due to the small mass fraction of cement and low reactivity of fly ash at 298 K. Moreover, the effective diffusion coefficient of C–S–H around cement particles increases. It facilitates water diffusion through the C–S–H layer [33]. Thus, the low diffusion resistance of reactants makes high reaction rate in composite binder containing fly ash. The value of \( K_{3}^{\prime } \) is almost same for all the samples at 318 K. It is indicated that elevated temperature stimulates the activity of fly ash and the hydration products fill the pores that leads to dense microstructure as Portland cement. At 333 K, the value of \( K_{3}^{\prime } \) for composite binder containing no more than 35 % of fly ash is lower than Portland cement, which further confirms that the hydration of cement and the pozzolanic reaction of fly ash are obviously promoted by elevated temperature. It is noted that the hydration rate of composite binder containing 50 or 65 % of fly ash is relatively high during D process. It might be related to the pozzolanic reaction of fly ash during D process (Figs. 2d, e, 6d, e).
As shown in the literature [22] and Table 3, compared to composite binder containing 50 % of slag, the values of \( K_{1}^{\prime } \), \( K_{2}^{\prime } \) and \( K_{3}^{\prime } \) for composite binder containing 50 % of fly ash are large. For NG process, as mentioned above, the fly ash could not involve into the reaction due to its low activity at 298 K. Furthermore, the reaction degree of fly ash is still low at elevated temperatures [2]. Thus, much of the water is provided for cement hydration. But a small amount of slag is activated by the alkali released by cement hydration and temperature rise during the early hydration process provides energy to activate alkali-hydroxide attack on the slag particles [34]. The reaction of slag will consume the water that leads to relatively low water content provided for cement hydration at early age. But the hydration rate of slag is lower than that of cement; thus, the overall reaction rate, \( K_{1}^{\prime } \), is low. From Fig. 2d in this paper and Fig. 3c in the literature [22], it is apparent that the peak values of the second exothermal effects of samples SL50 and FA50 are 3.90 and 5.60 J g−1 h−1, respectively. The experimental results also verify that. When the temperature is rising to 318 and 333 K, the third exothermic effect of slag reaction is overlapped by the second exothermic effect of cement hydration [22] that results in the peak value of the second exothermal effect of sample SL50 which is larger than that of sample FA50. The morphology of hydration product of slag appears to be finer than that of cement [35], and a large amount of hydration products generates and interweaves, which leads to the decreased surface area of crystal phases. Meanwhile, the degree of supersaturation of pore solution decreases due to the consumption of OH− for the reaction of slag. Then the value of \( K_{2}^{\prime } \) is small. However, for composite binder containing 50 % of fly ash, the degree of reaction of fly ash is limited during I process. The growth space of hydration products is large. The disperse effect of fly ash on the hydration of cement increases the contact area of cement and water. Thus, the degree of supersaturation increases due to the continuous dissolution of ions from cement particles, which increases the value of \( K_{2}^{\prime } \). Owing to the high activity of slag, plenty of hydration products generated by the hydration of cement and the pozzolanic reaction of slag makes the microstructure dense. But the loose microstructure is observed for the composite binder containing fly ash due to the low activity of fly ash [33]. The reduction of diffusion resistance of water and ions results in large value of \( K_{3}^{\prime } \). It should be noted that the values of \( K^{\prime } \) at different stages are the average rate of reaction. Although the value of \( K^{\prime } \) of composite binder containing slag is lower than that of composite binder containing fly ash at the same replacement ratio, but due to the high activity of slag, the cumulative hydration heat of composite binder containing slag is relatively high [22].
α 1 and α 2 represent the transition points of NG → D and I → D, respectively. From Table 3, it can be seen that the values of α 1 and α 2 increase with increasing the replacement ratio of fly ash. It is elucidated that the transformation of controlling mechanism occurs at high degree of hydration for composite binder containing fly ash. It is related to the gentle reaction of composite binder containing fly ash. An increase in temperature decreases the values of α 1 and α 2. It is indicated that the intension reaction makes the transformation of controlling mechanism which occurs at low degree of hydration. The results are in agreement with previous studies that elevated temperatures promote the early-age hydration of composite binder, but it is harmful to the later-age hydration performance [36–38]. It can be seen from Table 3 in this paper and Table 3 in the literature [22] that the values of α 1 and α 2 of sample FA50 are larger than those of sample SL50. As previously discussed, the activity of fly ash is very low at early age. The filler effect of fly ash leads to a transformation of the controlling mechanism at a higher degree of hydration. However, the reaction of slag occurs at early age and the reaction is enhanced by the elevated temperature. The intense reaction makes the transformation of the controlling mechanism at a lower degree of hydration. The promoting effect of elevated temperature on the hydration of composite binder containing fly ash is relatively small compared to composite binder containing slag; thus, the transformation of the controlling mechanism is relatively gentle at elevated temperatures.
The apparent activation energies of composite binder containing fly ash at different stages of hydration are shown in Table 4. It is evident that the apparent activation energies for NG process and I process are larger than D process. It is indicated that chemical reaction needs much more energy than diffusion. The apparent activation energies of samples FA20 and FA 35 during NG process are almost same as Portland cement. But for composite binder containing 50 or 65 % of fly ash, the promoting role of fly ash to the hydration of cement is strong due to the high water-to-clinker ratio and sufficient nucleation sites provided by the surface of fly ash. Thus, the reaction resistance is small and E a becomes low. For I process, incorporation of fly ash decreases the apparent activation energy. Fly ash could disperse the cement particles, and then the reaction resistance decreases. But for sample FA65, large amount of fly ash reduces the concentration of pore solution and it should be provided with more driving force for reaching a constant supersaturation. Due to the loose microstructure of composite binder containing fly ash, E a decreases with increasing the replacement ratio of fly ash. The apparent activation energies of composite binder containing fly ash at different stages are low compared to composite binder containing slag [22]. It is elucidates that the hydration of composite binder containing slag is more sensitive to the temperature. The results are in accordance with the hydration heat evolution rate and cumulative hydration heat [22]. Furthermore, it also explains why the rate of reaction of sample FA50 is higher than that of sample SL50.
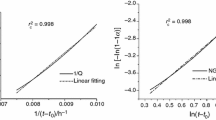
The apparent activation energies of the overall reactions of composite binder containing fly ash in a temperature range of 298–333 K are shown in Table 5. The value of E a for the overall reaction of composite binder containing fly ash decreases first and then increases with increasing the replacement ratio of fly ash. For composite binder containing small amount of fly ash, the interactions of cement hydration and fly ash reaction promote the hydration of composite binder, whose long-term compressive strength is the same or higher than cement [39]. For composite binder containing more than 50 % of fly ash, relatively small quantity of Ca(OH)2 is produced due to the low mass fraction of cement, and the alkalinity could not fully stimulate the activity of fly ash. Thus, the apparent activation energy is larger. It is indicated that raising temperature has a great promoting effect on the hydration of composite binder containing high amount of fly ash. Thus, the exothermal effect of the pozzolanic reaction of fly ash could be obviously observed on the hydration heat evolution rate curves (Fig. 2d, e). The apparent activation energy of the overall reaction of composite binder containing fly ash is lower than that of composite binder containing slag at the same replacement ratio [22]. Therefore, the hydration of composite binder containing slag is evidently promoted by the elevated temperatures.
Some researchers investigated the apparent activation energy of cement, and the average value was about 40 kJ mol−1 [40–43], which is very close to E a determined in this paper (40.01 kJ mol−1). But only little information regarding the apparent activation energy of composite binder containing fly ash is found. Han et al. [44] found that the apparent activation energies of Type II cement and composite binder containing 20 and 30 % of fly ash determined on a basis of the proposed model with water-to-binder ratio of 0.4 are 40.626, 42.226 and 43.017 kJ mol−1, respectively. It is evident that E a obtained for composite binder containing fly ash is relatively higher than that determined in this paper. Bentz [45] found that the apparent activation energies of Type I/II cement and composite binder containing 40 and 60 % of fly ash in a temperature range of 298–313 K are 34.5, 34.5 and 33.2 kJ mol−1, respectively. While in a temperature range of 288–298 K, they become 44.0, 49.5 and 47.4 kJ mol−1, respectively. In the literature [45], the pastes were prepared by volumetric proportion and the water-to-binder ratio by mass is 0.35, which results in the difference with the values determined in this paper.
Conclusions
This study involves the hydration kinetics of composite binder containing up to 65 % of fly ash and comparison of the results with data on the composite binder containing slag, which has been published previously. The main conclusions are as follows.
-
1.
The hydration heat evolution rate and cumulative hydration heat of composite binder decrease with increasing the replacement ratio of fly ash. Elevated temperatures promote the hydration process, especially for composite binder containing high amount of fly ash. But there is still a certain gap between the cumulative hydration heat of composite binder containing fly ash and that of Portland cement.
-
2.
The kinetics model could determine the controlling mechanism of the hydration reaction of composite binder containing no more than 65 % of fly ash studied in this paper. For composite binder, the controlling mechanism of the reaction during hydration process is NG → I → D at 298 and 318 K, and it becomes NG → D at 333 K.
-
3.
Increasing the replacement ratio of fly ash increases the influence of fly ash on the overall reaction. But fly ash has relatively small effect on the overall reaction of composite binder compared with slag at the same replacement level.
-
4.
The value of the exponent, n, increases with increasing fly ash content or temperature. An increase in the content of fly ash increases first and then decreases the values of kinetics parameters, \( K_{1}^{\prime } \) and \( K_{2}^{\prime } \), but increases \( K_{3}^{\prime } \). The rates of reaction of composite binder containing fly ash at different stages are higher than those of composite binder containing slag at the same replacement ratio.
-
5.
The transformation of controlling mechanism occurs at high degree of hydration for composite binder containing fly ash at 298 K, but it occurs at low degree of hydration at 318 and 333 K. Compared to composite binder containing slag, the transformation of controlling mechanism occurs at high degree of hydration for composite binder containing fly ash.
-
6.
The value of E a for NG process is nearly three times as large as that for D process. The value of E a for the overall reaction of composite binder decreases first and then increases with increasing the replacement ratio of fly ash. The value of E a of composite binder containing fly ash is lower than that of composite binder containing slag at the same replacement level.
References
Baert G, Hoste S, De Schutter G, De Belie N. Reactivity of fly ash in cement paste study by means of thermogravimetry and isothermal calorimetry. J Therm Anal Calorim. 2008;94:485–92.
Han FH, Liu RG, Wang DM, Yan PY. Characteristics of the hydration heat evolution of composite binder at different temperature. Thermochim Acta. 2014;586:52–7.
Kumar M, Singh SK, Singh NP. Heat evolution during the hydration of Portland cement in the presence of fly ash, calcium hydroxide and super plasticizer. Thermochim Acta. 2012;548:27–32.
Narmluk M, Nawa T. Effect of fly ash on the kinetics of Portland cement hydration at different curing temperatures. Cem Concr Res. 2011;41:579–89.
Deschner F, Lothenbach B, Winnefeld F, Neubauer J. Effect of temperature on the hydration of Portland cement blended with siliceous fly ash. Cem Concr Res. 2013;52:169–81.
Deschner F, Winnefeld F, Lothenbach B, Seufert S, et al. Hydration of Portland cement with high replacement by siliceous fly ash. Cem Concr Res. 2012;42:1389–400.
Bentz DP. Powder additions to mitigate retardation in high-volume fly ash mixtures. ACI Mater J. 2010;107:508–14.
Lothenbach B, Scrivener K, Hooton RD. Supplementary cementitious materials. Cem Concr Res. 2011;41:1244–56.
Papadakis VG. Effect of fly ash on Portland cement systems part I. Low-calcium fly ash. Cem Concr Res. 1999;29:1727–36.
Dittrich S, Neubauer J, Goetz-Neunhoeffer F. The influence of fly ash on the hydration of OPC within the first 44 h–a quantitative in situ XRD and heat flow calorimetry study. Cem Concr Res. 2014;56:129–38.
Nocuń-Wczelik W. Heat evolution in hydrated cementitious systems admixtured with fly ash. J Therm Anal Calorim. 2001;65:613–9.
Langan BW, Weng K, Ward MA. Effect of silica fume and fly ash on heat of hydration of Portland cement. Cem Concr Res. 2002;32:1045–51.
Thomas JJ, Biernacki JJ, Bullard JW, Bishnoi S, et al. Modeling and simulation of cement hydration kinetics and microstructure development. Cem Concr Res. 2011;41:1257–78.
Wang XY. Properties prediction of fly ash blended concrete using hydration model. Sci China Technol Sci. 2013;56:2317–25.
Wang XY. Modeling the hydration of concrete incorporating fly ash or slag. Cem Concr Res. 2010;40:984–96.
Thomas JJ. A new approach to modeling the nucleation and growth kinetics of tricalcium silicate hydration. J Am Ceram Soc. 2007;90:3282–8.
Scherer GW, Zhang J, Thomas JJ. Nucleation and growth models for hydration of cement. Cem Concr Res. 2012;42:982–93.
Scherer GW. Models of confined growth. Cem Concr Res. 2012;42:1252–60.
Krstulovic R, Dabic P. A conceptual model of the cement hydration process. Cem Concr Res. 2000;30:693–8.
Dabic P, Krstulovic R, Rušic D. A new approach in mathematical modeling of cement hydration development. Cem Concr Res. 2000;30:1017–21.
Merzouki T, Bouasker M, EI Houda Khalifa N, Mounanga P. Contribution to the modeling of hydration and chemical shrinkage of slag-blended cement at early age. Constr Build Mater. 2013;44:368–80.
Han FH, Zhang ZQ, Wang DM, Yan PY. Hydration kinetics of composite binder containing slag at different temperatures. J Therm Anal Calorim. 2015;121:815–27.
Gruyaert E, Robeyst N, De Belie N. Study of the hydration of Portland cement blended with blast-furnace slag by calorimetry and thermogravimetry. J Therm Anal Calorim. 2010;102:941–51.
Lam L, Wong YL, Poon CS. Degree of hydration and gel/space ratio of high volume fly ash/cement systems. Cem Concr Res. 2000;30:747–56.
Papadakis VG. Effect of fly ash on Portland cement systems part II: high-calcium fly ash. Cem Concr Res. 2000;30:1647–54.
Sakai E, Miyahara S, Ohsawa S, Lee SH, Daimon M. Hydration of fly ash cement. Cem Concr Res. 2005;35:1135–40.
Pane I, Hansen W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem Concr Res. 2005;35:1155–64.
Wang A, Zhang C, Sun W. Fly ash effects II. The active effect of fly ash. Cem Concr Res. 2004;34:2057–60.
Tydlitát V, Matas T, Cerný R. Effect of w/c and temperature on the early-stage hydration heat development in Portland-limestone cement. Constr Build Mater. 2014;50:140–7.
Christian JW. The theory of transformations in metals and alloys, part 1. 3rd ed. Oxford: Pergamon Press; 2002.
Fajun W, Grutzeck MW, Roy DM. The retarding effects of fly ash upon the hydration of cement pastes: the first 24 hours. Cem Concr Res. 1985;15:174–84.
Jun-yuan H, Scheetz BE, Roy DM. Hydration of fly ash-portland cements. Cem Concr Res. 1984;14:505–12.
Han FH, Liu RG, Yan PY. Effect of fresh water leaching on the microstructure of hardened composite binder pastes. Constr Build Mater. 2014;68:630–6.
Roy DM, Idorn GM. Hydration, structure, and properties of blast furnace slag cements, mortars and concrete. ACI J. 1982;79:444–57.
Taylor R, Richardson IG, Brydson RMD. Composition and microstructure of 20-year-old ordinary Portland cement-ground granulated blast-furnace slag blends containing 0 to 100 % slag. Cem Concr Res. 2010;40:971–83.
Lothenbach B, Winnefeld F, Alder C, Wieland E, Lunk P. Effect of temperature on the pore solution, microstructure and hydration products of Portland cement pastes. Cem Concr Res. 2007;37:483–91.
Famy C, Scrivener KL, Atkinson A, Brough AR. Effects of an early or a late heat treatment on the microstructure and composition of inner C–S–H products of Portland cement mortars. Cem Concr Res. 2002;32:269–78.
Escalante-García J, Sharp J. Effect of temperature on the hydration of the main clinker phases in Portland cements: part II blended cements. Cem Concr Res. 1988;28:1259–74.
Hannesson G, Kuder K, Shogren R, Lehman D. The influence of high volume of fly ash and slag on the compressive strength of self-consolidating concrete. Constr Build Mater. 2012;30:161–8.
Broda M, Wirquin E, Duthoit B. Conception of an isothermal calorimeter for concrete-determination of the apparent activation energy. Mater Struct. 2002;35:389–94.
Poppe AM, De Shutter G. Cement hydration in the presence of higher filler contents. Cem Concr Res. 2005;35:2290–9.
Ravikumar D, Neithalath N. Reaction kinetics in sodium silicate powder and liquid activated slag binders evaluated using isothermal calorimetry. Thermochim Acta. 2012;546:32–43.
ASTM International. ASTM C1074-11 standard practice for estimating concrete strength by the maturity method. West Conshohocken: ASTM International; 2011.
Sang-Hun H, Jin-Keun K, Yon-Dong P. Prediction of compressive strength of fly ash concrete by new apparent activation energy function. Cem Concr Res. 2003;33:965–71.
Bentz DP. Activation energies of high-volume fly ash ternary blends: hydration and setting. Cem Concr Compos. 2014;53:214–23.
Acknowledgements
Authors would like to acknowledge the National Natural Science Foundation of China (Grant Nos. U1134008 and 51278277), the Postdoctoral Science Foundation of China (No. 2015M580992) and Fundamental Research Funds for the Central Universities (No. FRF-TP-15-108A1).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Han, F., Zhang, Z., Liu, J. et al. Hydration kinetics of composite binder containing fly ash at different temperatures. J Therm Anal Calorim 124, 1691–1703 (2016). https://doi.org/10.1007/s10973-016-5295-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5295-z