Abstract
Growing demands for sustainable land use challenges the management of soil organic matter (SOM). Research on SOM stabilization mechanisms during the last decades opened access to new approaches of SOM assessment. This study tried to empower this trend with a focus on the interrelations between soil components including clay, organic carbon and bound water toward a unifying assessment for practical land use. Soil samples from different regions of the world were collected, air-dried, sieved and equilibrated to 76 % relative humidity prior to analysis. Thermogravimetry was applied to search for a relationship between thermal mass losses corresponding to soil components and mass losses on ignition (MLI) between 110 and 550 °C. The results refer to a predictability of MLI from thermal mass losses in two 10 °C temperature steps (TML), which are both closely correlated with the content of clay and soil organic carbon (SOC), respectively. We found a relationship MLI = 10 × TML130–140 °C + 25 × TML320–330 °C − 2 with R 2 = 0.98, applicable for soils with a wide range of properties. Using previous results, this equation can be rewritten as SOC = 0.48 × MLI–0.12 × clay + 0.2, which is similar to previously published relationship. The application of equation to plots with varying fertilization in long-term field experiments revealed deviations, which could be explained by different amounts of biologically degradable, non-humified, fresh organic residues or similar organic admixtures. This assumption was tested by application to untouched by human activity soils before and after laboratory incubation. The microbiological decay of SOM in incubated samples led to significantly lower differences of calculated and measured MLI, confirming a decrease in the amount of fresh organic matter. We conclude that thermogravimetry is applicable to study interrelations between soil components and to assess soil organic carbon content and quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last years, soil quality and sustainable land use have become more appreciated as key factors for long-term society development [1]. A better understanding of soil function seems necessary for mitigation of climate changes [2] and to safeguard long-term economic welfare [1]. A wide range of scientific publications, reviews and meta-studies reflects the growing attention to these challenges. Many of these works focused on carbon (C) cycling, C sequestration [3], on modeling of C dynamics [4], organic C (OC) storage [5] or on development of new analytical methods [6, 7]. One result is the recognition that storage of soil organic carbon (SOC) is an important ecosystem property [8]. Its sequestration was found to be governed by several mechanisms [9, 10]. This could be an inherent chemical resistance or mutual interactions between soil organic matter (SOM) molecules [11], interactions with pedogenic oxides or clay minerals forming organo-clay complexes [12], bonding to clay by proteinaceous substances [13], physical inaccessibility of SOC in soil aggregates [14–16] and other mechanism depending on environmental conditions and soil-forming factors.
Despite great progress in the understanding the SOC protection mechanisms, there are still unsolved practical questions related to the quality and quantity of SOM in soils under productive land use [17, 18]. One example is shown in Fig. 1, which reports the results of SOC content at two long-term agricultural experiments consisting of both non-fertilized and fertilized plots [18]. The extracted results imply an increasing content of SOC due to fertilization at both experimental sites. Comparing the sites, however, plots with the same amount of long-term fertilizer application are different from each other depending on clay content. As a result, SOC content determination does not indicate the amount of added fertilizers or the SOC stabilization potential. The absence of clearly defined threshold values for SOM challenges the development of sustainable land-use technologies, maintenance of soil fertility, productivity regulation and related aspects.
Organic carbon content of selected plots with different fertilization of two long-term agricultural experiments in Müncheberg and Bad Lauchstädt, sampling depth 0–30 cm (Ap-horizon, data from [43])
In addition to difficulties in quantifying the influence of clay on SOM, answers to these questions may be hampered by scattered information on the temporal and spatial dynamics of SOC [19], incomplete understanding of the interrelations between soil formation factors, soil properties and soil classification [20] as well as an incomplete understanding of the influence of chemical composition of biomass on SOM and carbon sequestration [8, 21].
To model the dynamics of SOC, the rapid and accurate measurement of its content in a wide range of soil types is essential [22]. Measurement of SOC in clay- and carbonate-rich mineral soils is challenging, and several methods have been suggested including acid treatments to remove inorganic carbon (IC), determining organic carbon (OC) and IC separately, measuring only OC, leaving IC intact or measuring simultaneously both OC and IC employing thermal gradient analysis [23].
Although many researchers are interested in solely SOC and soil nitrogen contents, total SOM content is also important. SOM can only be indirectly measured due to its complex structure and interactions with minerals [22]. Its determination seems even more complicated to measure accurately than SOC [24]. Mass loss on ignition (MLI) is often used to directly estimate SOM content [24–27]. In principle, dried soil is heated to its ashing temperature and the MLI is assumed to be the SOM content. To assure the complete removal of organic carbon, the temperature range where the mass losses are measured is typically between 105 and 550 °C (DIN 19128). Nevertheless, the applicability of this method is limited mainly to soils with low clay content (i.e., the higher the clay content, the larger the error) [24]. Indeed, the MLI method leads to the overestimation of SOM due to the loss of hygroscopic water (around 100 °C), inter-crystalline water and hydroxy groups in sesquioxides (above 100 °C), destruction of charcoal, and the decay of carbonates at temperature starting around 550 °C [22, 24]. Due to its simplicity, the MLI determination is still widely used, but a matter of intensive investigations due to its limitations [22, 24–26]. The current trend is to focus on increasing accuracy of SOM determination by overcoming its application limits with a correction for clay content [22], or to deal with detection of thermally stabile carbon [24].
In our previous works [28–33], we focused on the investigation of SOM following recommendations from previous studies, e.g., [34, 35], that intrinsic soil properties can be detected mostly in soils untouched by human activity. This avoids misinterpretations originating from the compositional and structural changes in soils caused by productive land use. As water is an important component involved in soil formation [36] and found to influence SOM stabilization [33], soil samples were additionally conditioned at 76 % relative air humidity after air-drying for comparable water contents. Soil samples were only sieved (<2 mm), but not ground to avoid possible changes in soil chemical or physical structure, which might influence TG results. This approach enabled a rapid and reliable determination of OC, and inorganic carbon (IC), nitrogen (N) and clay contents in a wide range of soil types. We suggested the use of thermal mass losses in 10 °C temperature steps (TML) instead of mass losses in large temperature areas such as MLI [29].
The dynamics of bound water release was found not only to correlate with the content of clay, but to be correlated also with CO2 evolved by biological degradation of SOM in samples rewetted to 65 % of field water holding capacity in laboratory incubation experiments [30–32]. We concluded that an increasing closeness of these interrelations in time may indicate that bound water is a possible factor involved in the regulation of microbiological stability of SOM [32]. These findings were not applicable to plant materials, composts, gardening muds or other similar soil like mixtures of geological parent materials containing organic carbon [28]. They imply new opportunities for a distinction between SOM and organic admixtures in soil samples. In contrast to SOM, organic admixtures are not formed by long-term biological regulation processes, which cause specific deviations from proportions between thermal mass losses found in soils untouched by human activity [33].
Here, we present the results of an extended data evaluation. The aim of the work was to reveal interdependencies between mass loss on ignition (MLI) and thermal mass losses in 10 °C temperature steps (TML), which were most closely connected with content of soil organic carbon, nitrogen, clay or other soil components. The second part is an application of these relationships for the assessment of soil organic matter content and soil biological transformation of easily biodegradable organic residues.
Materials and methods
Soil samples
Soil samples (n = 301) consisted of eight sample sets from regions with contrasting climate conditions, vegetation cover, and parent materials, including both natural, not disturbed by human activity sites and soils under different land uses. The list of soils with the general descriptions and references with detail information is reported in Table 1.
Sample preparation and analytical methods
Soil samples were gently air-dried, sieved to pass 2 mm and subsequently stored at 76 % relative humidity to insure comparable content of bound water in samples of different origins. The thermogravimetric experiments consisted of heating of around 1 g of a sample in a thermoscale (TGA/SDTA 851, Mettler-Toledo)) from 20 to 950 °C with 5 °C min−1 heating rate and a data acquisition rate of 1 reading per 4 s. As a purge gas was used flow of air 200 mL min−1, which was enriched by 76 % relative humidity (25 °C).
All samples were analyzed in duplicate by thermogravimetry (TG). First analyses were conducted in the same way as described above. The second analysis was carried out after the rewetting of the air-dried samples to around 65 % of field water holding capacity and incubating soils at 20 °C over 3 months under laboratory conditions with continuously measurement of biological CO2-respiration (details can be found in [30, 31]).
Sieved samples were further ground with an agate mortar and analyzed by dry combustion for carbon and nitrogen (LECO and ELEMENTAR) using standard methods.
Data treatment and analysis
Thermal mass losses (TMLs) for all calculations were obtained from TG curves as a mass loss in 10 °C intervals. This resulted in 93 TMLs from room temperature (ca. 30 °C) to 950 °C per analysis. In this work, a TML is referred to with its upper temperature limit of a 10 °C temperature increase interval. For example, TML110 describes mass losses between 100 and 110 °C. The abbreviation MLI is used for mass losses on ignition, which is represented here as the thermal mass losses between 110 and 550 °C, obtained from the same TG run as the TMLs. The lower limit of MLI determination of 110 °C is higher than the 105 °C according to DIN 18128. This change was the result of pre-experiments in which isothermal heating according to DIN 18128 was compared with continuously heating rate of 5 °C min−1 during thermogravimetric analyzes.
All correlation analyzes were based on linear, nonlinear, and exponential function models between MLI and TML between 30 and 950 °C. Because the application of nonlinear function models did not provide significantly higher coefficients of determination compared with linear function models, we present the data only from linear models.
Initially, all calculations were carried out separately for each of the eight sample sets listed above. Later the calculations were repeated with combined sample sets in the case of proven similar relationships.
Results
Initial correlation between MLI and TML revealed similar relationships for most sample sets, whereas the samples from Gezira region (North Africa) and from Antarctica were different. One example of the difference in sample sets is presented in Fig. 2 for the relationship between TML160 and MLI.
Data of soils from most regions showed a weak correlation between TML160 and MLI with a coefficient of determination (CD) R 2 = 0.64. The fitting of data of Antarctica soils (marked in Fig. 2 with “×”) resulted in higher slope of the regression with a similar R 2 = 0.68. A few samples from Antarctica fitted well to soils from other sources. Soils from Gezira region are represented as “+” in Fig. 2. The slope did not differ from Antarctica soils, but from all other sample sets. The regression for Gezira soils shows a higher R 2 = 0.90.
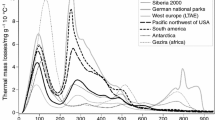
Figure 3 summarizes CDs of TML with MLI. Figure 3a provides background information with dynamics of mean mass losses from all samples. Marked TML shows temperature regions closely correlated with soil properties such as content of organic C (TML330 and TML350), nitrogen (TML330 and TML410) or clay (TML120 and TML530) contents [29]. In addition, Fig. 3 also shows the temperature areas that are related to the amount of CO2 released by biological decay of organic matter in laboratory incubation experiments with rewetted samples [30–32]. The capital letters refer to the major temperature areas with mass losses caused by following processes:
Dynamics of thermal mass losses in 10 °C temperature increase steps (TML) and correlations with mass losses on ignition (MLI). Upper part a Mean dynamics of thermal mass losses of all samples with marked the most important temperature intervals. Gray areas indicate TML correlating with soil properties and soil respiration in incubation experiments [28–32]. Lower part b Coefficients of determination of TML with MLI (110–550 °C) for sample sets Antarctic and Gezira separately and remaining sample sets together
-
A.
evaporation of bound water from SOM and organo-mineral complexes up to 200 °C,
-
B.
transformation of thermal labile organic matter between 200 and 450 °C,
-
C.
degradation of organic matter accumulated in dependency of the content of clay between 450 and 550 °C,
-
D.
decay of carbonates mostly above 550 °C, although the onset temperature can be modified by associated compounds or chemical composition [27, 28].
Figure 3b summarizes calculated coefficients of determination for all correlations of MLI (thermal mass losses 110–550 °C) with TML (thermal mass losses in 10 °C temperature increase steps between 30 and 950 °C) for all sample sets (bold solid line) except for samples from Gezira and Antarctica. These soil sample sets are presented separately due to the results given in Fig. 2. Coefficients of determination of these sample sets are illustrated with a thin solid line for Gezira region and a thin dotted line for samples from Antarctica.
As can be seen, the closeness of correlations of MLI with TML is changing with temperature. Below 50 °C, the bold line showed weak correlations with TML. For example, at 30 °C R 2 was <0.40. With increasing temperature, CD is increased. The R 2 reached values ~0.7 with a maximum at TML90 (R 2 = 0.74). Further temperature increase was accompanied by a decrease in correlation and then R 2 changed irregularly after that. Local maxima of R 2 were observed at TML210 (R 2 ≈ 0.89), TML330 (R 2 ≈ 0.93) and TML390 (R 2 ≈ 0.92). Therefore, up to 93 % of all variation of MLI can be explained with only one TML at 330 °C (TML330). As shown in Fig. 3a, this TML highly correlates with soil organic carbon. The samples from Gezira (thin black line) and Antarctica (dotted line) show different relationships. The maximum R 2 were located in different temperature regions than the other sample sets.
Introducing a second TML in these regression analyses increased CD. The application of power, exponential or other nonlinear function models did not lead to improvements in CD compared with a linear regression model, which was described by the formula:
where TML1 and TML2 were two independent thermal mass losses in 10 °C temperature intervals between 30 and 950 °C in mg g−1 sample each and a, b and c were fitted parameters.
The application of this function led to CDs >0.98 even for all samples sets together. The highest CDs between MLI and the two TMLs were observed for TML1 between 100 and 160 °C and TML2 between 280 and 360 °C.
Figure 4 presents the regression for the multiple linear regression model with the highest CD. According to this figure, the relationship of MLI with two TMLs results in an R 2 = 0.98 for all samples together (including Antarctica and Gezira) with the equation:
where MLI the thermal mass losses between 110 and 550 °C, TML330 thermal mass losses from 320 to 330 °C and TML140 thermal mass losses from 130 to 140 °C. All are given in mg g−1 sample. Using the onset temperature 105 °C (according to DIN 18128) instead of 110 °C for MLI calculation did not significantly lower the CD. Similar results were found if in Eq. (2) the TML330 was substituted either by TML410, or content of carbon or nitrogen determined by elemental analysis and/or TML140 were substituted either by TML530, or content of clay.
a Analytical (dots) and calculated (gray surface) data for thermal mass on ignition (MLI 110–550 °C) in dependency from thermal mass losses in two 10 °C temperature steps (TML) between 30 and 950 °C (N = 301). For better visibility of most data, the Y-axis is presented in logarithmic scale. b Comparing of MLI calculated from TML140 and TML330 with measured values of MLI, presented both in logarithmic scale
Figure 4b compares the measured MLI and MLI calculated from Eq. (2) in order to reveal the prediction accuracy and to inform about data distribution. Figure 5 shows measured and calculated MLI for samples from long-term agricultural experiments (Fig. 5a) together with the residuals of Eq. (2) as differences of calculated minus measured MLI (Fig. 5b). As can be seen in Fig. 5a, MLI varied with fertilization and with the experimental site. Considering the different contents of clay at every site, these results reports same challenges with assessment of SOM as shown in Fig. 1. In Fig. 5b, a higher number of negative than positive residuals indicated a prevalence of lower calculated MLI than measured ones. Non-fertilized and only mineral fertilized plots showed the lowest values at every site (calculated MLI < measured ones). In contrast, plots with organic fertilization or with combined (mineral and organic) fertilization resulted in higher and frequently positive deviations from Eq. (2) (calculated MLI > measured MLI).
Measured and calculated MLI (upper part) and residues of the interrelation between MLI to TML140 and TML330 for selected samples (lower part) of long-term agriculture experiments with comparable fertilization. The residues are presented as differences of calculated by equation [MLI = 10 × TML140 + 25 × TML330 − 2] minus measured MLI
In order to test whether easily biologically degradable organic residues were a possible cause for deviations from Eq. (2) in Fig. 5, Fig. 6 presents the residuals of the same equation as differences of calculated minus measured MLI for soils collected in Siberia in 2010 [31] at different sampling depths and before and after incubation. These samples were incubated to reduce the content of easily degradable organic residues by microbiological decay. As can be seen in Fig. 6, upper soil horizons show the highest values at each site (calculated MLI > measured MLI). With increasing sampling depth, the differences become lower. The difference between calculated and measured MLI was typically lower after incubation.
Selected results of thermogravimetric analyses of soil samples from Siberia (sampled in 2010, [31]) of different sampling depths before and after incubation under laboratory conditions. a Measured and calculated from thermal mass losses between 130 and 140 °C (TML140) and thermal mass losses between 320 and 330 °C (TML330) mass losses from 110 to 550 °C (mass losses on ignition, MLI) using equation: MLI = 10 × TML140 + 25 × TML330 − 2, b residues of the interrelation between MLI and TML represented here as differences of calculated via same equation MLI minus measured MLI
Discussion
Theoretical understanding of the relations
First, we would like to discuss the relationship between MLI and different TMLs (Fig. 2), which were most probably caused by distinguishing regional soil-forming conditions or prevalent SOC sequestration mechanisms. Samples from Antarctica were collected from very thin layers of mineral dust from melting glaciers under cold climate condition allowing only a very weak vegetation cover (less than 2 % of the surface in case of very few samples). In contrast to other regions, the organic matter was accumulated not from vegetation cover during ecosystem succession typical under moderate climatic conditions, but from residues of penguin colonies [37]. The soils in Gezira region were characterized by high clay content and peloturbation (mixing of layers due to clays shrinking and swelling [38]). Furthermore, the traditional fertilization of Gezira soils with wood ashes containing charcoal may have affected the results [33].
The distinguishing influence of regional specifics on our results was less important after the addition of a second TML in Fig. 4. As a result, the model represented by Eq. (2) seems to be universally and generally applicable for the determination of MLI from TML330 and TML120 across all sample sets used here.
Hoogsteen et al. [26] and Ghabbour et al. [25] referred to application risks for determination of SOM using MLI in clay-rich soils. As already mentioned, decomposition of SOM between 110 and 550 °C could be also accompanied by water release from soil minerals, mainly clays [39]. Accordingly, MLI in Eq. (2) is composed of mass loss of SOM degradation and water release mostly from clays (CW, “clay water”) released between 110 and 550 °C. This means, Eq. (2) can be extended by
This equation not only illustrates the challenges of direct determination of SOM from MLI due to CW or other unknown influences. It also implies a role of chemically bound clay water in processes of SOM accumulation. The relationship between SOM and CW implies that adsorption of water on clay might be a competitive process to SOM adsorption; similar observations were reported, for example, in the chemistry of zeolites [39–42]. However, these considerations are beyond the scope of this paper.
According to Siewert [29], the TML330 in Eq. (2) can be substituted by SOC content and TML140 by the content of clay using equations SOC = 1.18 × TML330 − 0.05 and Clay = 4 × TML120 + 9.8.
Then, Eq. (2) can be rewritten as:
where all units are given in mg g−1 sample.
Transforming Eq. (4), SOC content can be determined not only from TML330 but as well from MLI using following Eq. (5):
where all units are given in %.
De Vos et al. [22] suggested the determination of SOC from MLI using relationship
Equation (6) was found valid for non-calcareous forests soils and less applicable for sandy soils with low SOC and clay content [22]. Formally, Eqs. (5) and (6) are similar and differ from each other only by coefficients. The different coefficients could be caused by the different soils used in Ref. [22] and in the current study and especially by carbonates in soils investigated in this work, because their decay starts in some cases below 550 °C [27, 43].
The differences in equation parameters may also result from our search for highest coefficients of determination, which suggests the possibility to substitute TML330 (SOC content) by TML around 410 °C (soil organic N content, [29]) giving Eqs. (7) and reduces slightly the CD.
Such modification can justify, among others, the functionality of nitrogen in organisms or the participation of proteinaceous components involved in water binding in soils [33]. We speculate that the decrease in CD for soil organic nitrogen in comparison with SOC may be connected with a changeable C/N ratio that depends on soil-forming processes and soil cultivation [44].
In addition, the interrelations between content of SOM, MLI and clay reflected in Eqs. (2), (5) or (6) provide the opportunity to calculate the content of clay in soils from the measurement of MLI and from SOC determined using element analyzes using Eq. (8)
Opportunities for practical application and validation
Such application, however, provides a connection to the already mentioned questions (Fig. 1) about a possible quality assessment of the easy measurable SOC. Usually, SOM is separated into at least two fractions [45]. Considering the majority of clay-associated SOC in Eq. (2) is stable, deviations are caused by a changing amount of labile, biologically easily degradable organic carbon. Such an idea is supported by the deviations shown in Fig. 5 by the dependency of results from fertilization and in the level of crop yield [46]. For example, organic fertilizers are known to provide highest yield and show in Fig. 5 the largest deviations between calculated and measured MLI. Similar results were obtained with the combination of organic and mineral fertilization. The deviations were less pronounced, however, for the application of only mineral fertilizers (NPK). Although mineral fertilization supports plant growth and thereby soil fertility, it can have adverse effect such as soil acidification, soil structure degradation and other undesired side effects. On the contrary, the plots, which were not fertilized, showed the lowest deviation from Eq. (2). These plots were reported to have lowest fertility [46] and thus the lowest content of organic residues.
Figure 6 provides a primary validation of the assumptions on the applicability of Eq. (2). The labile or fresh residues are quickly decomposed during soil incubation, which should lead to a decrease in the difference between calculated and measured MLI. Figure 6 reports results for both fresh soil samples (dark gray columns) and incubated ones (light gray columns). As a rule, the upper layers of natural soils are characteristic of a high amount of fresh organic residues, due to the absence of cultivation preventing fast microbiological decay and mixing with lower horizons. Thus, a high amount of residues implies larger differences between calculated and measured MLI, which was in line with the results in Fig. 6. This does not exclude negative values if the amount of residues is lower than the mean of investigated samples (e.g., due to low vegetation productivity at dry sites). In contrast, the supply of organic residues decreases with increasing sampling depth. Generally in Fig. 6, the differences of calculated minus measured MLI decrease with increasing depth, which supported the assumptions about the influence of a lower amount of labile SOC on calculated MLI.
Conclusions
Our results suggest lower importance of SOM content determination than in comparing relationships between organic carbon, clay, water and other components of soils, which are recognized as unique and very complex objects formed during long-term ecosystem succession. We assume the found relationships with TG analyses between components were enabled by equilibration of SOM by comparable humidity prior to analyses, which was facilitated by using only sieved, non-ground samples. A confirmation would imply a key role of bound water for understanding soil formation processes for both organic and inorganic soil compartments, including SOM accumulation, SOM/SOC ratios and other properties and processes. The heterogeneity of the sample sets used supported a distinction between regional specifics and detection of general or unifying for all soils regulations processes and features, which are applicable to a wide range of soils. The presented results require independent validation, which can initiate an open discussion about necessary investigations for practical land-use assessment.
References
Montgomery DR. Dirt: the erosion of civilizations. Berkeley: University of California Press; 2007.
Ehrlich PR, Ehrlich AH. Can a collapse of global civilization be avoided? Proc R Soc B. 2013;280:20122845.
Murty D, Kirschbaum MUF, Mcmurtrie RE, Mcgilvray H. Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Global Change Biol. 2002;8:105–23.
Dinakaran J, Hanief M, Meena A, Rao KS. The chronological advancement of soil organic carbon sequestration research: a review. P Indian Acad Sci B. 2014;84:487–504.
Gao Y, Yu G, He N. Equilibration of the terrestrial water, nitrogen, and carbon cycles: advocating a health threshold for carbon storage. Ecol Eng. 2013;57:366–74.
Batlle-Aguilar J, Brovelli A, Porporato A, Barry DA. Modelling soil carbon and nitrogen cycles during land use change. A review. Agron Sustain Dev. 2011;31:251–74.
von Lutzow M, Kogel-Knabner I, Ludwig B, Matzner E, Flessa H, Ekschmitt K, Guggenberg G, Marschner B, Kalbitz K. Stabilization mechanisms of organic matter in four temperate soils: development and application of a conceptual model. J Plant Nutr Soil Sci. 2008;171(1):111–24.
Schmidt MWI, Torn MS, Abiven S, Dittma T, Guggenberger G, Janssens IA, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–55.
Liu M, Hu F, Chen X. A review on mechanisms of soil organic carbon stabilization. Acta Ecol Sin. 2007;27:2642–50.
Krull ES, Baldock JA, Skjemstad JO. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Funct Plant Biol. 2003;30:207–22.
Piccolo A. The supramolecular structure of humic substances: a novel understanding of humus chemistry and implications in soil science. Adv Agron. 2002;75:57–134.
Bronick CJ, Lal R. Soil structure and management: a review. Geoderma. 2005;124(1–2):3–22.
Kleber M, Sollins P, Sutton R. A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures of mineral surfaces. Biogeochemistry. 2007;85:9–24.
Six J, Bossuyt H, Degryze S, Denef K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Till Res. 2004;79(1):7–31.
Six J, Elliott ET, Paustian K. Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem. 2000;32(14):2099–103.
Gregorich EG, Kachanoski RG, Voroney RP. Carbon mineralization in soil size fractions after various amounts of aggregate disruption. J Soil Sci. 1989;40:649–59.
Rasmussen PE, Keith WT, Goulding JR, Brown PR, Grace H, Jansen H, et al. Long-term agroecosystem experiments: assessing agricultural sustainability and global change. Science. 1998;282:893–6.
Körschens M, Albert E, Barkusky D, Baumecker M, Behle-Schalk L, et al. Effect of mineral and organic fertilization on crop yield, nitrogen uptake, carbon and nitrogen balances, as well as soil organic carbon content and dynamics: results from 20 European long-term field experiments of the twenty-first century. Arch Agron Soil Sci. 2012;59:1–24.
Jandl R, Rodeghiero M, Martinez C, Cotrufo MF, Bampa F, van Wesemael B, et al. Current status, uncertainty and future needs in soil organic carbon monitoring. Sci Total Environ. 2014;468:376–83.
Gray JM, Humphreys GS, Deckers JA. Relationships in soil distribution as revealed by a global soil database. Geoderma. 2009;150(3–4):309–23.
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol. 2012;18:1781–96.
De Vos B, Vandecasteele B, Deckers J, Muys B. Capability of loss-on-ignition as a predictor of total organic carbon in non-calcareous forest soils. Commun Soil Sci Plan. 2005;36(19–20):2899–921.
Truong Xuan V, Heitkamp F, Jungkunst HF, Reimer A, Gerold G. Simultaneous measurement of soil organic and inorganic carbon: evaluation of a thermal gradient analysis. J Soil Sediment. 2013;13(7):1133–40.
Pribyl WD. A critical review of the conventional SOC to SOM conversion factor. Geoderma. 2010;156:75–83.
Ghabbour EA, Davies G, Cuozzo NP, Miller RO. Optimized conditions for determination of total soil organic matter in diverse samples by mass loss on ignition. J Plant Nutr Soil Sci. 2014;177(6):914–9.
Hoogsteen MJJ, Latinga EA, Bakker EJ, Groot JCJ, Tittonell PA. Estimating soil organic carbon trough loss on ignition: effects of ignition conditions and structural water loss. Eur J Soil Sci. 2015;66:320–8.
Pallasser R, Minasny B, McBratney AB. Soil carbon determination by thermogravimetrics. PeerJ. 2013;1:e6.
Siewert C. Investigation of the thermal and biological stability of soil organic matter. 1st ed. Aachen: Shaker; 2001.
Siewert C. Rapid screening of soil properties using thermogravimetry. Soil Sci Soc Am J. 2004;68(5):1656–61.
Siewert C, Demyan M, Kucerik J. Interrelations between soil respiration and its thermal stability. J Therm Anal Calorim. 2012;110:413–9.
Kucerik J, Ctvrtnickova A, Siewert C. Practical application of thermogravimetry in soil science. Part 1: thermal and biological stability of soils from contrasting regions. J Therm Anal Calorim. 2013;113(3):1103–11.
Kucerik J, Siewert C. Practical application of thermogravimetry in soil science. Part 2: modeling and prediction of soil respiration using thermal mass losses. J Them Anal Calorim. 2014;116:563–70.
Siewert C, Kucerik J. Practical applications of thermogravimetry in soil science. Part 3: interrelations between soil components and unifying principles of pedogenesis. J Therm Anal Calorim. 2015;120:471–80.
Van Breemen N. Natural organic tendency. Nature. 2002;415:381–2.
Lal R, Lorenz K, Hüttl RF, Schneider BU, von Braun J. Terrestrial biosphere as a source and sink of atmospheric carbon dioxide. In: Lorenz K, Hüttl RF, Schneider BU, von Braun J, Lal R, editors. Recarbonization of the biosphere. Dordrecht: Springer; 2012.
Siewert C, Schaumann GE. Evolution und erdgeschichtliche Genese von Bodenbildungsprozessen. Natur- und Kulturlandschaft. 2002;5:76–81.
Bölter M, Siewert C, Kuhn D, editors. Patterns of soil microbes and soil organic matter characteristics in a periglacial environment at King George Island (Maritime Antarctic). Proceedings of the Symposium “Ecology of Antarctic Coastal Oasis”; 2001; Valtice, Czech Republic: Masaryk University Brno.
Elias EA, Alaily F, Siewert C. Characteristics of organic matter in selected soil profiles from the Gezira Vertisols determined by thermogravimetry. Int Agrophys. 2002;16:20–4.
Yariv S, Borisover M, Lapides I. Few introducing comments on the thermal analysis of organoclays. J Therm Anal Calorim. 2011;105(3):897–906.
Yariv S, Michaelian KH. Introduction to organo-clay complexes and interactions. In: Yariv S, Cross H, editors. Organo-clay complexes and interactions. New York: CRC Press, Marcel Decker Inc; 2002. p. 39–112.
Langier-Kuzniarova A. Thermal analysis of organo-clay complexes. In: Yariv S, Cross H, editors. Organo-clay complexes and interactions. New York: CRC Press, Marcel Decker Inc; 2002. p. 273–344.
Földvári M. Handbook of thermogravimetric system of minerals and its use in geological practice. Budapest: Geological Institute of Hungary; 2011.
Konen ME, Jacobs PM, Burras CL, Talaga BJ, Mason JA. Equations for predicting soil organic carbon using loss-on-ignition for north central US soils. Soil Sci Soc Am J. 2002;66(6):1878–81.
Fierer N, Grandy AS, Six J, Paul EA. Searching for unifying principles in soil ecology. Soil Biol Biochem. 2009;41(11):2249–56.
Bimueller C, Mueller CW, von Luetzow M, Kreyling O, Koelbl A, Haug S, et al. Decoupled carbon and nitrogen mineralization in soil particle size fractions of a forest topsoil. Soil Biol Biochem. 2014;78:263–73.
Koerschens M, Albert E, Baumecker M, Ellmer F, Grunert M, Hoffmann S, et al. Humus and climate change—results of 15 long-term experiments. Arch Agron Soil Sci. 2014;60(11):1485–517.
Acknowledgements
The authors are thankful for the support of the Deutsche Forschungsgemeinschaft (DFG, project Si 488/3-1).
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper is dedicated to the 80th birthday of Prof. Dr. sc. Martin Körschens, who encouraged Christian Siewert during his Ph.D. studies to search for methods to assess soil organic matter for demands of practical land use. Furthermore, Prof. Dr. Körschen’s example in maintaining long-term agricultural experiments in Germany during the last three decades has enabled and encouraged soil organic matter-related research.
Rights and permissions
About this article
Cite this article
Kucerik, J., Demyan, M.S. & Siewert, C. Practical application of thermogravimetry in soil science. J Therm Anal Calorim 123, 2441–2450 (2016). https://doi.org/10.1007/s10973-015-5141-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5141-8