Abstract
The presented study focuses on the use of thermogravimetric analysis (TG) for estimating organic (OC) and inorganic carbon (IC) contents in bulk soils of different soil types and agricultural practices. Total carbon (TC) was measured by dry combustion using a total organic carbon (TOC) analyzer. The OC content was obtained as the difference between the TC and IC measurements. Regression equations were developed for relations between the thermal mass losses over various temperature intervals (200–370, 105–550, 110–420, 180–450, 250–440, 250–650, and 200–575 °C) and the OC content to determine the optimal temperature interval for the estimation of OC. The results for IC contents were related to the thermal mass losses at temperatures ranging from 500 to 800 °C. The results demonstrated that the thermal mass losses between 180 and 450 °C were fairly well related to the OC content (R 2 = 0.63), whereas the root-mean-square error of cross-validation (RMSECV) was too high (0.70 % OC). The inclusion of the clay content in the multivariate predictive equation did not importantly lower the RMSECV. By contrast, the thermal mass losses within the interval from 500 to 800 °C were closely related to the IC content determined using the TOC analyzer (R 2 = 0.96), with an acceptable RMSECV of 0.26 %. These results indicate that TG can provide a reliable estimation of the IC content but only rough estimations of the OC content in bulk soils of different types, bedrocks, and land uses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The soil carbon pool is composed of soil organic carbon (SOC) and soil inorganic carbon (SIC). SOC is a measure of the total amount of organic compounds or carbon within a soil, independent of the soil’s origin or composition. SOC is of interest to soil scientists and researchers in related fields because of its importance to the principal physical, chemical, biological, and ecological functions of soil. SOC content is also a universal indicator of soil quality [1, 2]. Consequently, variations in the level of SOC can have serious implications for many environmental processes like soil fertility, erosion, and greenhouse gas fluxes [3]. The decline in SOC levels is recognized as a serious environmental threat [4]; however, the negative impacts of carbon sequestration on the ecosystem’s health have long been neglected [5].
SIC includes lithogenic IC which is derived from parent material and pedogenic IC which is formed through the dissolution and precipitation of carbonate parent material. SIC can lead to carbon sequestration [6]. Although it can dissolve (particularly under acidic conditions), it is far less prone to loss than SOC [7].
Various methods are available for determining the SOC, and the choice of assessment method critically affects the SOC estimation. The two basic approaches to quantifying the SOC are wet and dry combustion. The Walkley–Black dichromate oxidation method [8] was used as a standard method in most soil-testing laboratories throughout most of the twentieth century [9]. This method is limited by a variable recovery percentage reported to be from 59 to 88 %; however, this method is still used to measure SOC content because it is relatively rapid, simple, and inexpensive [10]. SOC is oxidized with potassium dichromate in the presence of concentrated sulfuric acid. The major drawback of this approach is the incomplete oxidation of the SOC, and thus a correction factor is required to adjust the SOC recovery. The quantities of SOC recovered vary with soil type, land usage, and even soil depth [11]. Since the method involves the use of concentrated sulfuric acid and Cr6+, which is the toxic form of Cr, it is problematic with respect to both the working environment and general environmental concerns.
Loss-on-ignition (LOI) is a rapid, relatively inexpensive approach and is the most widely used method of directly estimating soil organic matter (SOM). In this method, a dried sample is heated to its ashing temperature, and the mass loss is assumed to be organic matter. The temperature of ignition must be high enough to completely remove organic matter and minimize mass losses due to dehydroxylation of clay minerals and breakdown of carbonates [9, 12]. The LOI combustion temperatures given in the literature vary between 375 and 800 °C [13].
At present, it is becoming increasingly common to apply dry combustion using automated instruments like total organic carbon (TOC) analyzers; elemental carbon, hydrogen, and nitrogen (CHN) analyzers; and gas chromatographs [10, 14]. The soil carbon is oxidized at high temperatures, followed by detection of the evolved CO2. Complete carbon oxidation is achieved, and thus, this method is considered to be most reliable for acidic soils, in which the OC content corresponds to the total carbon [15]. When soils which contain IC are analyzed, the IC should be removed prior to combustion or a separate determination of IC must be performed. Automated dry combustion is used in many studies as a reference for calibrating the results of other methods, because of its accuracy and reliability [12].
Thermal analysis has a long history in the geosciences. Most reports regarding the thermal analyses of soils have focused on the characterization of the mineral component, whereas organic components have received less attention [16, 17]. In a previous study, the SOM within three agricultural soil samples acquired in Serbia was determined from the mass loss within the interval 250–650 °C on the thermogravimetric analysis (TG) curve and compared with the results obtained using the Kotzman and Tyurin methods [18]. Miyazawa et al. [19] evaluated a thermogravimetric method for the determination of SOM within several Brazilian soils based on mass losses within the interval 110–420 °C and compared it with the Walkley–Black method, reporting a high correlation between the results of the two methods and certain distinct advantages of the TG method. Salgado et al. [20] calculated SOM in soils acquired from Spanish forests affected by wildfires from the enthalpy of combustion obtained by differential scanning calorimetry (DSC) and by TG using the interval 200–575 °C. Linear correlations were found between the enthalpy of combustion of SOM measured by DSC and the organic matter content measured by TG. Siewert [21] correlated mass losses in Russian and German soils for each 10 °C temperature step from 30 to 1000 °C. A very good regression fit (R 2 = 0.98) between the SOC contents determined via dry combustion and the mass loss within the temperature interval from 340 to 350 °C was reported.
More recently, Plante et al. [16] published a review of the application of thermal analysis techniques in soil science, reporting two main exothermic DSC peaks attributed to the loss of organic matter in soils: The first one, at approximately 300–350 °C, was ascribed to the burning of carbohydrates and other aliphatic compounds, whereas a peak at 400–450 °C was ascribed to the loss of aromatic compounds. The effects of several experimental conditions have been studied for ensuring that reproducible and comparable results can be achieved when SOM quality is characterized using thermal techniques [17]. The possibility of using thermal analysis for characterization of SOM and interpretation of potential interferences from soil mineral matrix has been expanded by the application of an infrared analyzer based on CO2/H2O evolved gas analysis [22]. Duguy et al. [23] studied SOM contents in abandoned agricultural fields after wildfires from mass losses between 105 and 550 °C after demineralization with HF and also reported two peaks—one at approximately 300 °C and another at approximately 440 °C. Thermal analysis has also been reported to be a useful tool for studying the composting process [24, 25], characterizing humic acids in forest soils [26], investigating changes in the quality of clay-associated organic matter in terms of thermal properties [27], studying organic matter conversion in cattle manure [28], and studying the behavior of sewage sludge and biochar from sewage sludge pyrolysis after addition to agricultural soils [29]. Correlations between soil respiration and its thermal stability determined by TG analysis were recently studied and published by Siewert and Kučerík et al. [30–32]. Recently, it has also been reported that TG has a promising potential for distinguishing organic carbon of pedogenetic origin from carbon of other sources [33].
In several studies that have focused on the characterization of SOM in bulk soils, isolated soil fractions, and composts, various temperature ranges have been adopted and ascribed to the loss of organic matter. Most of the published studies have focused on SOM quantification based on one single soil type (e.g., forest soils, soils affected by wildfires, or agricultural soils from one location). Thermal mass losses have been correlated with the OC contents obtained by different wet oxidation methods which are not considered nowadays as a reference method for accurate determination of SOC. Although very good correlations have been reported, the uncommon use of TG for SOC determination, as well as different temperature ranges reported in the literature, suggests that some difficulties exist. Therefore, the objective of the presented work was to evaluate the capability of TG for simultaneous estimations of OC and IC in soils from various regions of Slovenia representing different land uses, bedrocks, and soil types. Regression equations were calculated between the OC determined via the combustion method using a TOC analyzer and the thermal mass loss, and the effects of different temperature ranges and clay contents were examined.
Experimental
Soil samples
A total of 46 samples were collected from soil depths of up to 30 cm at 28 locations throughout northeastern Slovenia with different soil types [34], bedrocks, and land uses (Table 1). Soil heterogeneity according to organic and inorganic carbon contents was the main reason for selecting those locations which are derived from different bedrocks, soil types, and land uses. The clay fraction was determined using the pipette method [35].
Chemical analyses
The samples were air-dried, ball-milled for 8 min for homogenization, and analyzed for OC. The measurements of OC and IC were taken using a TOC analyzer with a SSM-5000A solid sample module (Shimatzu 5050A with SSM-5000A, Shimatzu, Kyoto, Japan). For the TC determination, sample quantities between 0.500 and 1.000 g were weighed into the sample boat and combusted at 900 °C in the presence of an oxidation catalyst. The evolved CO2 was carried to a non-dispersive infrared (NDIR) gas analyzer. The IC was measured at 200 °C after acidification of the sample with H3PO4 (85 %). The measurements were taken in accordance with ISO 10694 [36]. The OC content was obtained as the difference between the TC and IC measurements. Measurements were taken in triplicate for each sample.
Thermal analysis
The soil samples were air-dried and ball-milled as described for the carbon analysis. TG was performed using a METTLER TA 4000 system within the temperature range 30–800 °C in dynamic air with a flow rate of 100 mL min−1 and a heating rate of 10 °C min−1, using Al2O3 crucibles and sample masses of approximately 10 mg. The measurements were repeated at least three times for each sample. We evaluated the mass losses of bulk soils over various temperature ranges cited in published papers: 105–550 °C [23], 110–420 °C [19], 180–450 °C [17], 250–440 °C [21], 250–650 °C [18], and 200–575 °C [20]. For selected samples, additional TG measurements were taken after pretreatment with HCl in order to remove IC [23].
Statistical analysis
The organic and inorganic carbon contents obtained using the TOC analyzer (considered as a reference method) were used to develop regression equations using the predictor variables of thermal mass loss for different temperature intervals and clay contents, which varied between 4 and 42 % (Table 1). The dataset consisted of 46 samples for which averages of three analytical replicates of OC, IC, and thermal mass losses within various temperature intervals were used. Predictive models for the response variables OC and IC were fitted using the linear regression method. Univariate and multivariate models were developed. As a prediction variable, in the univariate cases, thermal mass losses of untreated soil samples over various temperature intervals were used, and in multivariate cases, the clay content was added as a predictor. Part of the analysis for response variable OC was carried out on 43 samples, because three samples were detected as outliers (Fig. 3). In the regression analysis with response variable IC and prediction variable thermal mass loss in the temperature interval 500–800 °C, 29 samples with inorganic carbon content more than 0.1 % were included. Part of the analysis for response variable IC was carried out on 26 samples because three samples were detected as outliers (Fig. 4). The goodness of fit was assessed using the R 2 parameter, and the model performance was evaluated in terms of the root-mean-square error of leave-one-out cross-validation (RMSECV) [37]. The analyses were performed in R Core Team [38] using the DAAG library [39] for cross-validation.
Results and discussion
The mean values of three independent measurements for the SOC contents for all the studied areas obtained using the elemental analyzer were between 0.38 and 26.1 %, whereas the SIC contents were between <0.1 and 7.07 % (Table 1).
Thermal analysis
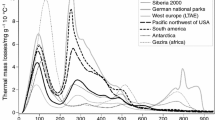
Figure 1 shows typical TG and derivative thermogravimetry (DTG) curves for three soil samples with different nature of soil organic matter and soil mineral matrix. Sample 3 represents an orchard soil with OC and IC contents of 0.91 and 2.96 %, sample 10 a pasture soil with OC and IC contents of 5.04 and 1.46 %, and sample 45 a forest soil with high OC (12.3 %) and very low IC contents (<0.1 %). The diagrams show the characteristic regions considered during the thermal analyses of the soil samples: In all samples, a mass loss within the temperature range up to 170 °C (DTG peaks between 50 and 55 °C) could be observed as a result of dehydration reactions. The decomposition of SOM occurred between 200 and 550 °C [16]. Two major stages were observed for samples with high OC (e.g., sample 45): The mass loss within the 200–320 °C range could be attributed to the combustion of carbohydrates and other aliphatic compounds, while in the second interval, 330–530 °C, thermal degradation of more stable aromatic structures occurred [17, 23, 26]. For samples with lower OC, only one broad step with a peak at 320 °C could be observed in this interval, whereas in soil samples with very low OC, the corresponding mass loss was barely visible. The mass loss above 600 °C could be attributed to carbonate thermal degradation, as reported in the literature [19, 26, 40]. The DTG curves for samples with higher IC displayed peaks at temperatures between 705 and 720 °C, whereas no comparable peaks were observed within this region for samples with very low IC.
Additional experiments performed using different heating rates (2, 5, and 20 °C min−1) offered no important advantages in comparison with the results obtained by using a heating rate of 10 °C min−1, which is in good agreement with published results. Although some studies have reported a slower heating rate of 5 °C min−1 [18, 21], most researchers have used the heating rate of 10 °C min−1, which represents the best compromise between the time required for analysis and the peak resolution to determine soil organic matter [16, 17, 20, 23]. The selected temperature range was from room temperature to 800 °C. The upper temperature limit of 700 °C reported in some studies was too low for some of the samples analyzed in this study because the mass loss between 700 and 800 °C remained considerable, whereas at >800 °C, mass losses were negligible for all investigated samples.
Figure 2 presents the TG curves for pasture soil sample 13 (2.94 % OC and 5.13 % IC) before and after carbonate removal with HCl. The mass loss within the region between 180 and 450 °C, which was used to calculate the OC using the TG method, did not change after carbonate removal, whereas the mass loss between 500 and 800 °C changed from 24.8 to 1.9 %; thus, this finding offers additional proof that the mass loss within this range can be primarily attributed to the decomposition of inorganic carbonates being removed from the samples during HCl treatment.
Thermal mass loss: soil OC relations
Various temperature ranges have been reported for the oxidation of the organic components present in soils. These temperature differences can be ascribed to differences in organic matter composition; degree of humification [17, 27]; the interaction of organic components with the mineral surface, which may have a significant impact on the stabilities of those components [41]; land use, which can alter the quality of clay-associated organic matter [27]; and experimental conditions, which can influence the resulting curves [17].
The mass losses within the temperature intervals of 200–370, 250–650, 110–420, 200–575, 250–440, 105–550, and 180–450 °C were 1.0–3.4, 3.0–14.4, 1.4–5.3, 2.3–5.6, 1.1–4.6, 2.3–11.0, and 1.5–5.3 %, respectively. The mass loss between 500 and 800 °C, which represents carbonate decomposition, ranged from 1.9 to 30.9 %.
The univariate regression analyses for response variables OC or IC and predictor variable the mass losses within temperature interval from 180 to 450 °C or from 500 to 800 °C, respectively, showed a good fit with R 2 values of 0.95 and 0.98. However, in regression models (Figs. 3, 4) with either of the two response variables (OC or IC), some samples with very high organic or inorganic carbon contents were detected as high leverage points (outliers) that highly influenced estimates and standard errors of linear regression coefficients and consecutively also R 2 values leading to unfair bias, especially in regression analysis with predictor OC. For that reason, they were excluded from the further analyses.
The relations between the OCs determined using the reference method and the mass loss values over different temperature intervals are described by the equations given in Table 2. The slopes (b) and intercepts (a) were found to be highly variable depending on the temperature interval, indicating the complicated nature of the decomposition processes. The intercept expresses the residual effects of factors other than organic compounds that contribute to the mass loss, e.g., hygroscopic and interlayer water loss, decomposition of carbonates, and dehydroxylation of clay minerals and metallic hydroxides [42, 43]. Within the region up to 200–250 °C, the thermal mass loss was mostly attributed to the loss of molecular water from salts and organic matter and to the dehydration of clay minerals, which lose most of their interlayer-bound water by 250 °C and a small remaining portion by 350 °C [18, 44], whereas carbonates begin to decompose above 500 °C [45].
The results suggest that the above-mentioned interfering effects inherent to the samples in temperature intervals with the upper limit lower up to 550 °C had no significant effect on the thermal mass losses, as none of the intercepts were significantly different from zero (p values were 0.508, 0.095, 0.401, 0.364, 0.571, respectively). However, these effects were particularly pronounced for intervals in which the upper temperature limit was >550 °C. The intercepts of the regression curves developed for the mass losses within the temperature intervals 200–575 and 250–650 °C were significantly different from zero (Table 2), revealing that other reactions were associated with the mass losses. We assumed that the decomposition of carbonates was the major contribution to the mass losses above 500 °C. Our assumption was supported by the results of multivariate regressions in which the clay content was included as a predictor in addition to the thermal mass losses which demonstrated that in our case, clay had no significant influence on the mass losses in temperature ranges with an upper limit above 440 °C (Table 3).
The predictive capabilities of the model for the temperature intervals 105–550, 250–650, and 200–575 °C were poor and not acceptable (Table 2) with average prediction errors (RMSECV) more than 1.0 % OC. For the other evaluated temperature intervals, the regression equation for estimating the OC from the thermal mass loss in the interval 200–370 °C exhibited the highest R 2 value, 0.70, and the lowest average prediction error (RMSECV), 0.64 %. The temperature range up to 320 °C is associated with the combustion of carbohydrates and other aliphatic compounds, whereas the thermal degradation of the more stable fraction occurs up to 530 °C [17, 23, 26]. The TG curves in Fig. 1 indicate that the degradations of those soil samples which were included in the regression analyses were not complete at 370 °C; the more stable organic compounds were oxidized up to 450 °C (Fig. 1). The temperature range 180–450 °C was selected for determining the thermal mass losses since it covered more of the range of organic compounds’ decomposition. The relation between the OC content measured using the TOC analyzer and the thermal mass loss (TG) within the interval 180–450 °C is illustrated in Fig. 3. Each data point represents an average value from three replicate measurements. The R 2 value was slightly lower (0.63) than the R 2 obtained for the interval 200–370 °C (0.70). The inclusion of the clay content in addition to thermal mass losses in bivariate regressions was significant in the temperature intervals 110–420 and 200–370 °C, but little improvement in the R 2 value was observed, whereas for all other studied intervals, the impact of the clay content was insignificant (Table 3). Some reasons for the weak relationship may be the fairly narrow range of SOC (0.38–5.04 %), the highly variable ratios between clay and SOC contents (1:1–110:1) indicating the different proportions between water fractions [46], sample compositions, and heterogeneities of soil organic compounds as soil samples derived from different soil types, bedrocks, land uses, and agricultural activity.
The relation between the mass loss within the temperature interval 500–800 °C and the IC content can be described using the following regression equation: IC/% = −0.47 + 0.25·TG/%. The results indicated a good fit (R 2 = 0.96) of the regression line between the mass loss and the IC content obtained using the elemental analyzer (Fig. 4) and an RMSECV of 0.26 %. Nevertheless, the significant negative intercept may suggest that the mass loss in this temperature interval can be partially attributed to the decomposition of soil components other than carbonates. The inclusion of clay content as a predictor in the multivariate regression revealed that clay had no significant effect on the thermal mass loss in this interval, and thus, the interfering effects were likely caused by the decomposition of organic carbon of high stability.
Conclusions
TG, which has been applied extensively in clay mineralogy, is problematic for the quantitative determination of the OC in bulk soil samples because the thermal mass losses are the result of various decomposition reactions in addition to the combustion of organic matter. Some of these interfering effects can be eliminated by choosing a temperature interval in which the lowest and highest temperatures are between 180 and 450 °C. Regression equations developed for intervals within this range possessed insignificant intercepts. In the temperature interval 180–450 °C, the regression model developed using the mass loss as the predictor and the OC determined using a TOC analyzer as the response variable exhibited a good fit; however, the RMSECV was too high (0.70 % OC) for reliable estimation of the OC in investigated soil samples. The inclusion of the clay content as a predictor in addition to the mass loss did not lower the RMSECV and, in our case, had a negligible effect. However, the thermal mass losses within the interval from 500 to 800 °C were closely correlated with the IC contents obtained using the TOC analyzer. The R 2 of 0.96 and the acceptable RMSECV of 0.26 % IC demonstrated that thermal analysis could provide reliable predictions of the IC content in soils containing at least 1 % IC. Thermogravimetry appears to be a suitable technique for the prediction of IC, but may be useful only when rough estimates of the OC in bulk soils of different soil types developed on different bedrocks, land uses, and agricultural activities are required.
References
Romig DE, Garlynd MJ, Harris RF, Mcsweeney K. How farmers assess soil health and quality. J Soil Water Conserv. 1995;50:229–36.
Stolbovoy V, Montanarella L, Filippi N, Jones A, Gallego J, Grassi G. Soil sampling protocol to certify the changes of organic matter carbon stock in mineral soil of European union, EUR 21576 EN/2. Luxembourg: Office for Official Publications of the European Communities; 2006. p. 56.
Stolbovoy V, Filippi N, Gallego J, Montanarella L, Piazzi M, Putrella F. Validation of the EU sampling protocol to detect the changes of organic carbon stock in mineral soils in the Piemonte Region (Italy). EUR 22339 EN, Office for Official Publications of the European Communities, Luxembourg; 2006. p 41.
Huber S, Syed B, Fredenschuß A, Ernstsen V, Loveland P. Proposal for European soil monitoring and assessment framework. European Environment Agency, Technical Report 61, Copenhagen; 2001. p. 58.
Gao J, Yu G, He N. Equilibration of the terrestrial water, nitrogen, and carbon cycles: advocating a health threshold for carbon storage. Ecol Eng. 2013;57:366–74.
Sahrawat KL. Importance of inorganic carbon in sequestering carbon in soils of the dry regions. Curr Sci. 2003;84:864–5.
Victoria R, Banwart SA, Black H, Ingram H, Joosten H, Milne E, Noellemeyer E. The benefits of soil carbon: managing soils for multiple economic, societal and environmental benefits. In: Goverse T, editor. UNEP year book 2012: emerging issues in our global environment. UNEP, Nairobi; 2012. p. 19–33.
Walkley A, Black TA. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38.
Pribyl DW. A critical review of the convencional SOC to SOM conversion factor. Geoderma. 2010;156:75–83.
Chatterjee A, Lal R, Wielopolski L, Martin MZ, Ebinger MH. Evaluation of different soil carbon determination methods. Crit Rev Plant Sci. 2009;28:164–78.
Díaz-Zorita M. Soil organic carbon recovery by the Walkley–Black method in typic hapludoll. Commun Soil Sci Plant Anal. 1999;30:739–45.
Sleutel S, De Neve S, Singier B, Hofman G. Quantification of organic carbon in soils: a comparison of methodologies and assessment of the carbon content of organic matter. Commun Soil Sci Plant Anal. 2007;38:2647–57.
Bisutti I, Hilke I, Raessler M. Determination of total organic carbon—an overview of current methods. Trends Anal Chem. 2004;23:716–26.
Krishan G, Srivastav SK, Kumar S, Saha SK, Dadhwal VK. Quantifying the underestimation of soil organic carbon by the Walkley and Black technique—examples from Himalayan and Central Indian soils. Curr Sci. 2009;96:1133–6.
Chaćon N, Dezzeo N, Fölster H, Mogollón P. Comparison between colorimetric and titration methods for organic carbon determination in acidic soils. Commun Soil Sci Plant Anal. 2002;33:203–11.
Plante AF, Fernández JM, Leifeld J. Application of thermal analysis techniques in soil science. Geoderma. 2009;153:1–10.
Fernández JM, Plante AF, Leifeld J, Rasmussen C. Methodological considerations for using thermal analysis in the characterization of soil organic matter. J Therm Anal Calorim. 2011;104:389–98.
Gaál F, Szöllősy I, Arnold M, Paulik F. Determination of the organic matter, metal carbonate and mobile water in soils. J Therm Anal. 1994;42:1007–16.
Miyazawa M, Pavan MA, De Oliveira EL, Ionashiro M, Silva AK. Gravimetric determination of soil organic matter. Braz Arch Biol Technol. 2000;43:475–8.
Salgado J, Mato MM, Vázquez-Galiñanes A, Paz-Andrade MI, Carballas T. Comparison of two calorimetric methods to determine the loss of organic matter in Galician soils (NW Spain) due to forest wildfires. Thermochim Acta. 2004;410:141–8.
Siewert C. Rapid screening of soil properties using thermogravimetry. Soil Sci Soc Am J. 2004;68:1656–61.
Fernández JM, Peltre C, Craine JM, Plante AF. Improved characterization of soil organic matter by thermal analysis using CO2/H2O evolved gas analysis. Environ Sci Technol. 2012;46:8921–7.
Duguy B, Rovira P. Differential thermogravimetry and differential scanning calorimetry of soil organic matter in mineral horizons: effect of wildfires and land use. Org Geochem. 2010;41:742–52.
Dell’Abate MT, Benedetti A, Sequi P. Thermal methods of organic matter maturation monitoring during a composting process. J Therm Anal Calorim. 2000;61:389–96.
Pietro M, Paola C. Thermal analysis for the evaluation of the organic matter evolution during municipal soild waste aerobic composting process. Thermochim Acta. 2004;413:209–14.
Montecchio D, Francioso O, Carletti P, Pizzeghello D, Chersich S, Previtali F, Nardi S. Thermal analysis (TG-DTA) and drift spectroscopy applied to investigate the evolution of humic acids in forest soil at different vegetation stages. J Therm Anal Calorim. 2006;83:393–9.
Plante AF, Pernes M, Chenu C. Changes in clay-associated organic matter quality in a C depletion sequence as measured by differential thermal analyses. Geoderma. 2005;129:186–99.
Otero M, Lobato A, Cuetos MJ, Sánchez ME, Gómez X. Digestion of cattle manure: thermogravimetric kinetic analysis for the evaluation of organic matter conversion. Bioresour Technol. 2011;102:3404–10.
Gazcó G, Paz-Ferreiro J, Méndez A. Thermal analysis of soil amended with sewage sludge and biochar form sewage sludge pyrolysis. J Therm Anal Calorim. 2012;108:769–75.
Siewert C, Deyman MS, Kučerík J. Interrelations between soil respiration and its thermal stability. J Therm Anal Calorim. 2012;110:413–9.
Kučerík J, Čvrtníčkova A, Siewert C. Practical applications of thermogravimetry in soil science, Part 1. Thermal and biological stability of soils from contrasting regions. J Therm Anal Calorim. 2013;113:1103–11.
Kučerík J, Siewert C. Practical applications of thermogravimetry in soil science, Part 2. Modelling and prediction of soil respiration using thermal mass losses. J Therm Anal Calorim. 2014;116:563–70.
Siewert C, Kučerík J. Practical applications of thermogravimetry in soil science. J Therm Anal Calorim. 2014;. doi:10.1007/s10973-014-4256-7.
World reference base for soil resources: a framework for international classification, correlation and communication. Report no. 103. Food and Agriculture Organisation of the United Nations (FAO), Rome; 2006.
ISO 11277. Soil quality—determination of particle size distribution in mineral soil material—method by sieving and sedimentation. International Organisation for Standardization, Geneva; 1998.
ISO10694. Soil quality—determination of organic and total carbon after dry combustion (elementary analysis). International Organisation for Standardization, Geneva; 1995.
Kohavi R. A study of gross validation and bootstrap for estimation and model selection. In: Processing of the fourteenth international joint conference on artificial intelligence 1995;2(12):1137–43.
R Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014.
Maindonald J, Braun WJ. Data analysis and graphics data and functions. R package version 1.16.; 2013.
Atanasov O, Rustschev D. Thermal analysis of peat and peat soils. Thermochim Acta. 1985;90:373–7.
Dlapa P, Simkovic I, Doerr SH, Kanka R, Mataix-Solera J. Application of thermal analysis to elucidate water-repellency changes in heated soils. Soil Sci Soc Am J. 2008;72:1–10.
Grewal KS, Buchan GD, Sherlock RR. A comparison of three methods of organic carbon determination in some New Zealand soils. J Soil Sci. 1991;42:251–7.
De Vos B, Vandecasteele B, Deckers J, Muys B. Capability of loss-on-ignition as a predictor of total organic carbon in non-calcareous forest soils. Commun Soil Sci Plant Anal. 2005;36:2899–921.
Schulte EE, Hopkins BG. Estimation of soil organic matter by weight loss-on-ignition. In: Magdoff FR, editor. Soil organic matter: analysis and interpretation. Madison, WI: Soil Science Society of America Inc.; 1996.
Rosell RA, Gasparoni JC. Assessment methods for soil carbon. Florida: Lewis Publishers; 2001.
Wang Y, Lu S, Ren T, Li B. Bound water content of air-dry soils measured by thermal analysis. Soil Sci Soc Am J. 2011;75(2):481–7.
Acknowledgements
The authors gratefully acknowledge the assistance of Mr. Oriol Saura Puig and Ms. Tjaša Ahej with the thermogravimetric analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kristl, M., Muršec, M., Šuštar, V. et al. Application of thermogravimetric analysis for the evaluation of organic and inorganic carbon contents in agricultural soils. J Therm Anal Calorim 123, 2139–2147 (2016). https://doi.org/10.1007/s10973-015-4844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4844-1