Abstract
2-Ethylhexyl nitrate (EHN) is an important nitro compound for chemical industry. However, EHN is an unstable material and frequently causes fire and explosion accidents. Therefore, it is necessary to understand the mechanism of its thermal decomposition. In this paper, EHN was investigated by differential scanning calorimeter under non-isothermal and isothermal conditions, and its thermal decomposition kinetics was calculated with Friedman method. A model for the decomposition reaction was established by Malek method and verified by an isothermal method. The results indicated that the values of activation energy (E) for EHN were (154 ± 3) kJ mol−1 under non-isothermal and (157 ± 7) kJ mol−1 under isothermal conditions. In addition, there was almost no difference in E at various extent of the reactant conversion, suggesting that a single-step reaction model is suitable to describe the decomposition reaction. In addition, the temperature of no return (T NR) and self-accelerating decomposition temperature were calculated under non-isothermal and isothermal conditions. Using Malek method, the decomposition model obtained is Avrami–Erofeev equation (n = 4/3). This model was verified by G(α), based on the results of isothermal experiment. The kinetic parameters under the isothermal conditions were in good agreement with those obtained under the non-isothermal conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitric ester is an important material widely used as plasticizer or energetic filler in detonators, propellants and explosives. There are one or more unstable functional groups (–O–NO2) in nitric ester. Yan et al. [1] studied the effect of molecular structure on thermal stability, decomposition kinetics and reaction models of ten nitric esters, all of them decompose under 250 °C. Hiskey et al. [2] believed that the chemical bond of –O–NO2 will break first for many nitric esters. In addition, many researchers worked on the synthesis and thermal stability of nitric ester in recent years [3–7].
2-Ethylhexyl nitrate (EHN) belongs to the family of nitric ester. It is used as cetane improver that can effectively increase the cetane number of diesel oil, improve the combustion performance of diesel oil, shorten the ignition time and lower the ignition point [8]. As a liquid propellant additive, EHN has numerous applications in defense industry [9]. Since the structure of EHN contains a functional group (–O–NO2) that is less stable, EHN has a lower decomposition temperature, and thus, it can readily cause fires and explosions. In 1982, an explosion accident occurred in America was triggered by the decomposition of EHN [10]. Therefore, if the decomposition kinetic parameters are established, it will provide useful data for the design of reactors and storage.
Pritchard [11] and Bornemann et al. [12] studied decomposition products of EHN and pointed out that the decomposition products are mainly formaldehyde, nitrogen dioxide (NO2) and some alkane radicals. Lu [13] performed a detailed study on the decomposition of EHN by using differential scanning calorimeter (DSC), and calculated kinetic parameters of the decomposition of EHN with Kissinger and Ozawa methods. Wang et al. [14] also calculated decomposition kinetics of EHN by using a microcalorimeter (C600), with the aid of AKTS software. Furthermore, Zeng et al. [15] calculated kinetic parameters of EHN decomposition under adiabatic conditions by using an accelerating rate calorimeter (ARC). In reference [16], the decomposition rules for EHN was investigated discussed as well.
Thermal decomposition characteristics of a substance at a certain constant temperature can be derived more accurately in isothermal tests as compared with non-isothermal tests [17]. Results of the isothermal tests are further used to evaluate the accuracy of the decomposition characteristics and reaction model for decomposition of the substance [18]. When the same substance is analyzed under both the non-isothermal and isothermal conditions, thermal decomposition characteristics of the substance can be revealed more thoroughly [19, 20]. Since there has been no literatures reporting the thermal decomposition kinetics of EHN at the isothermal conditions, this work was designed to investigate this.
A reaction model can well illustrate the decomposition rule of a substance, and is great importance in kinetic analysis. Wang et al. [14] calculated the decomposition kinetics by a model-free method. Lu suggested an integral form of the most probable reaction model using single heating rate program [15], and, according to the recommendation of International Confederation for Thermal Analysis and Calorimetry Societies (ICTAC) Kinetics Committee, this method should be avoided [21].
Other than above references, there has been virtually no literatures discussing the reaction model for thermal decomposition of EHN, and thus, it is of great interest to establish a reaction model for decomposition of EHN. In this paper, the kinetic parameters of decomposition of EHN under two conditions were calculated by using the Friedman method, based on non-isothermal and isothermal DSC tests. Then the decomposition kinetic parameters of EHN were compared with other nitric esters. Moreover, the most probable reaction model for decomposition of EHN was obtained by the Malek method using experimental data of the non-isothermal DSC tests. In addition, the accuracy of the reaction model was evaluated by G(α) [an integral form of f(α)] based on results of the isothermal DSC tests.
Experimental
Material
EHN of 97 % purity was produced by Aladdin Reagent (Shanghai) Co., Ltd.
DSC-1 was produced by Mettler-Toledo. This equipment was calibrated with indium at low-temperature range and with zinc at high-temperature range; 30-μL capacity high-pressure stainless steel crucible with equipped with gold-plated seal was used. And a similar empty crucible was used as a reference. Both purge gas and dry gas were high-purity nitrogen, with flow rates of 100 and 200 mL min−1, respectively. In the non-isothermal experiments, the heating rates β were 0.5, 1, 2, 4 and 8 °C min−1, respectively, and the temperature ranges were 25–280 °C. In the isothermal experiments, the temperatures were 168, 172, 176 and 180 °C. The mass of samples used for DSC experiments was between 1.6 and 2.0 mg.
Experimental results and analysis
Experimental results
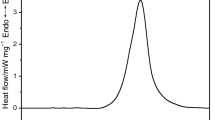
The results of the non-isothermal DSC and isothermal DSC tests of EHN are shown in Figs. 1 and 2 and Tables 1 and 2.
As can be seen from Fig. 1 and Table 1, the onset decomposition temperature (T onset) of EHN at a heating rate of 0.5 °C min−1 was 157.0 °C, indicating that EHN is very unlikely to decompose at a temperature substantially below this (e.g., room temperature). However, during decomposition, EHN releases substantial amount of heat (ΔH r) of (1867 ± 33) J g−1, i.e., severe consequences will be caused once a decomposition reaction of EHN occurs.
As shown in Fig. 2, the exothermic curves of EHN decomposition under the isothermal conditions descended gradually. And, according to Table 2, ΔH r of EHN decomposition at different temperatures was (1971 ± 32) J g−1, which was essentially comparable to that obtained in the non-isothermal DSC experiments. In order to understand the decomposition of EHN further, curves of conversion degree versus time under isothermal conditions are shown in Fig. 3. According to Fig. 3 and reference [21], it is reasonable to conclude that the EHN decomposition is a gradually decelerating process.
Decomposition kinetics
Kinetic parameters of EHN decomposition under non-isothermal and isothermal conditions were calculated by the Friedman method. The Friedman method is one of the most used methods for calculating kinetics, as recommended by ICTAC [21, 23]. As an isoconversional method, the Friedman method allows activation energy to be calculated without taking influences of reaction models into account of, and is a model-free method [22]. Moreover, the Friedman method is suitable for use in the calculation of kinetics under both non-isothermal and isothermal conditions [24, 25]. In the comparison of the calculation results under the non-isothermal and isothermal conditions, errors resulting from the calculation method are normally minimized. The derivation process is as follows.
A basic model for kinetics calculation is as follows:
where t is the reaction time, s; k is the reaction rate constant; T is the reaction temperature, K; α is the extent of conversion; and f(α) is the reaction model of the reaction process.
Generally, k can be determined according to the Arrhenius equation, and thus, Eq. (1) may be transformed into the following equation:
While under the non-isothermal conditions, Eq. (1) may be transformed into:
Taking logarithms of Eqs. (2) and (3) results in the following equations, respectively:
Equations (4) and (5) are expressions of the Friedman method under isothermal and non-isothermal conditions, respectively. Under the non-isothermal conditions, when α was constant, corresponding 1/T at different heating rates was varying. Therefore, linear fitting was performed on ln(βdα/dt) at different heating rates and 1/T, and the E value at this α could be obtained on the basis of the slope. Likewise, under the isothermal conditions, the time t to reach the same α under different temperatures was varying, i.e., ln(dα/dt) was varying. Therefore, linear fitting was performed on ln(dα/dt) at different temperatures and 1/T, and the E value at this α under the isothermal conditions could be obtained on the basis of the slope. The resulting intercept from the linear fitting was ln[Af(α)].
Because the α at the starting phase and the termination phase of heat release were greatly influenced by the selection of the baseline [18], in this paper, α used in the calculation of activation energy was 0.1–0.8, for both non-isothermal and isothermal conditions. The values of activation energy under non-isothermal and isothermal conditions calculated by the Friedman method are shown in Fig. 4.
Results of the calculation indicated that, under the non-isothermal conditions, EHN had a mean activation energy of (154 ± 3) kJ mol−1 at different α and had only small changes in the activation energy throughout the decomposition process (Fig. 4). Therefore, the EHN decomposition can be interpreted using a single-step reaction model under the non-isothermal condition. The mean value of activation energy of EHN decomposition under the isothermal conditions was (157 ± 7) kJ mol−1. With the continuous progress of conversion, the change in the activation energy was small (Fig. 4) as well, and it is reasonable to conclude that the decomposition under isothermal condition also obeyed a single-step reaction model.
The difference between the calculated values of activation energy under the non-isothermal and isothermal conditions was small, suggesting that the decomposition characteristics of EHN under the isothermal conditions followed the same mechanism as those under the non-isothermal conditions.
As mentioned earlier, some researchers have studied the thermal stability of nitric esters. In Table 3, the results for the decomposition of ten nitric esters reported by Yan et al. [1] were summarized and compared with EHN.
Ten nitric esters were nitroglycerine (NG), pentaerythritol tetranitrate (PETN), trimethylolethane trinitrate (TMETN), dipentaerythritol hexanitrate (DiPEHN), trimethylolpropane trinitrate (TMPTN), erythritol tetranitrate (ETN), xylitol pentanitrate (XPN), sorbitol hexanitrate (SHN), mannitol hexanitrate (MHN) and nitroisobutylglycerol trinitrate (NIBGT). T onset and T peak in Table 3 were determined by DSC under 10 °C min−1 using aluminum pan with a pin-holed cover, and the activation energies of these materials were calculated by Kissinger–Akahira–Sunose (KAS) equation based on thermogravimetry (TG) data.
Onset and peak temperature of EHN decomposition are 187.74 and 212.26 °C, respectively, and ΔH r is 1837.8 J g−1 based on experimental DSC data at 8 °C min−1. It is apparent that the thermal stability parameters of EHN are comparable to other nitric esters. This implies that the main pathway for EHN decomposition might be the same as the ten nitric esters. It is interesting that the decomposition heat is relatively high, close to that of PETN explosive. The implication is that when dealing EHN great care must be taken.
The SADT (self-accelerating decomposition temperature) is defined as the lowest ambient air temperature at which a self-reactive substance of specified stability undergoes an exothermic reaction in a specified commercial package in a period of 7 days or less. SADT values as packaged guide transportation and storage of hazardous materials.
In the literature [26, 27], there was the detailed step to derive the SADT. Firstly, the temperature of no return (T NR) should be calculated according to Eq. (6) [26]. Then SADT can be calculated according to Eq. (7) [27].
In the process of international transportation, the standard for transportation is always 50 kg. So the overall heat transfer coefficient (U) is 5 W m−2 K−1 and the heat transfer area (S) is 1.4 m2. According to the literature [28], the specific heat capacity (C p) is 1.92 J m−2 K−1. Then based on the above experimental result and kinetic parameters under non-isothermal conditions and isothermal conditions, T NR can be calculated, and the result is 108 and 104 °C, respectively. Then the value of SADT is 100 °C under non-isothermal conditions and 97 °C under isothermal conditions, which were all similar to the experimental value in material safety data sheet (95 °C). The decomposition and safety parameters of EHN in this paper and in other literature [29] or material safety data sheet are listed in Table 4. According to this table, it can be believed that the values of different parameters are credible. In addition, it can be concluded that the EHN is stability and not easy to decompose in storage and transportation process under normal room temperature.
The most probable reaction model
When α was 0.1–0.8, the decomposition of EHN obeyed a single-step reaction model. Therefore, the reaction model for thermal decomposition of EHN could be obtained by using the Malek method in this paper.
The Malek method is very useful in determining the reaction models f(α) (a differential form) and G(α) (an integral form) from defining functions y(α) and Z(α) [30], and also it is one of the methods recommended by ICTAC Kinetics Committee [21]. Because when non-isothermal DSC data are applied with Malek method, there are hardly any hypotheses or approximate conditions. Therefore, the Malek method allows more accurate establishment of the reaction models. Either y(α) or Z(α) is obtained through deformation of Eq. (2), the derivation process of which is given in detail in references [21].
Where:
T and α in Eqs. (8) and (9) are the same as those described earlier. The resulting experimental values of y(α) are plotted as a function of α, and compared against theoretical y(α) master plots. A suitable model is identified as the best match between the experimental and theoretical y(α) master plots [21]. For practical reasons, the y(α) plots are normalized to vary from 0 to 1. The result is shown in Fig. 5.
However, for Z(α):
In the equations, x = E/RT, E is the activation energy calculated by the Friedman method; π(u) is a rational function, which can be obtained by the Luke approximate expression [21], and in this paper, π(u) was calculated by selecting a four-order function having an expression of :
In the equations, dα/dt is the relationship of the α versus time.
The Z(α)–α curve was plotted according to Eq. (8) using DSC data experimentally obtained at different heating rates. The Z(α)–α curves plotted according to Eq. (9) by using the commonly used reaction models were the standard curves. If the experimental curve overlaps or is even on a certain standard curve, f(α) corresponding to this standard curve is determined to be the most probable reaction model for decomposition of the substance [21]. The result is shown in Fig. 6.
Forty-one common mechanism models were screened herein. According to results of the y(α)–α and Z(α)–α curves, with the α in a range of 0.1–0.8, the 15th reaction model—Avrami–Erofeev equation (n = 4/3)—could satisfy both y(α) and Z(α) well, as shown in Figs. 5 and 6. The Avrami–Erofeev equation (n = 4/3) had an expression of:
Thus, we can conclude that the Avrami–Erofeev equation (n = 4/3) is the most probable reaction model for decomposition of EHN.
According to previous results, the value of activation energy calculated under the non-isothermal conditions was in good agreement with that obtained by isothermal method. Therefore, the reaction model obtained by using the non-isothermal data is also applicable under isothermal conditions.
The kinetic equation for the isothermal process can also be expressed as [18, 31]:
Under the isothermal conditions, because the activation energy has only minor changes throughout the decomposition process, it can be assumed that k(T) has a constant value and the selected G(α) and the reaction time t should have a linear relationship. Accordingly, using G(α), curves of G(α)–t were obtained at four temperatures with α in the range of 0.1–0.8, and linear fitting was performed on the four curves, with results as shown in Fig. 7.
Correlation coefficients of the fitting were all higher than 0.999 at four temperatures, indicating that the curve of G(α)–t had a definitive linear profile, and at the same time proving that the reaction model for decomposition at the isothermal conditions was well described by Eq. (14).
Substituting the reaction model into ln[Af(α)] obtained in the Friedman method, then the pre-exponential factors under the non-isothermal and isothermal conditions were obtained, logA = 14.4 ± 0.5 and logA = 14.7 ± 0.3, respectively.
Conclusions
In summary, thermal decomposition kinetics of EHN were analyzed under non-isothermal and isothermal conditions and obtained the following conclusions:
-
1.
The decomposition of EHN under the isothermal conditions follows the decelerating rule. Under the isothermal conditions, the decomposition rate of EHN reaches a maximum value in a short period at the very beginning and then descends gradually.
-
2.
Values of activation energy of EHN decomposition calculated by the Friedman method were (154 ± 3) kJ mol−1 under the non-isothermal conditions and (157 ± 7) kJ mol−1 under the isothermal conditions. There was no significant change in values of activation energy in the progress of the decomposition process under both non-isothermal and isothermal conditions, and the decomposition process is well interpreted by a single-step reaction mechanism.
-
3.
The SADT and T NR of EHN were calculated under non-isothermal and isothermal conditions in this manuscript. The value of T NR is 108 and 104 °C, respectively. Then the value of SADT is 100 °C under non-isothermal conditions and 97 °C under isothermal conditions, which were all similar to the experimental value in material safety data sheet (95 °C). It can be concluded that the EHN is stability and not easy to decompose in storage and transportation process under normal room temperature.
-
4.
According to the Malek method, the reaction model for thermal decomposition of EHN is Avrami–Erofeev equation (n = 4/3), and at the same time, accuracy of this reaction model was further validated by G(α) based on results of the isothermal tests.
-
5.
Pre-exponential factors of EHN decomposition obtained under the non-isothermal and isothermal conditions are logA = 14.4 ± 0.5 and logA = 14.7 ± 0.3, respectively, according to the results calculated by using the obtained reaction mechanism models and the Friedman method.
-
6.
The kinetic parameters of EHN calculated under the non-isothermal conditions are in good accordance with those obtained under the isothermal conditions.
Abbreviations
- α :
-
Conversion rate
- β/°C min−1 :
-
Heating rates
- ΔH r/J g−1 :
-
Decomposition enthalpy
- T onset/°C:
-
Peak temperature of decomposition
- ΔH r/J g−1 :
-
Specific heat of material
- t/s:
-
Reaction time
- T/K:
-
Reaction temperature
- T NR/K:
-
The temperature of no return
- U/W m−2 K−1 :
-
Overall heat transfer coefficient
- R/J mol−1 K−1 :
-
Initial concentration of sample
- n :
-
Reaction order
- SADT/°C:
-
Self-accelerating decomposition temperature
- E/J mol−1 :
-
Activation energy
- A/s−1 :
-
Pre-exponential factor
- k/s−1 :
-
Reaction rate constant
- M/g :
-
The mass of package
- S/m2 :
-
Heat transfer area
References
Yan QL, Künzel M, Zeman S, Svoboda R, Bartošková M. The effect of molecular structure on thermal stability, decomposition kinetics and reaction models of nitric esters. Thermochim Acta. 2013;566:137–48.
Hiskey MA, Brower KR, Oxley JC. Thermal decomposition of nitrate esters. J Phys Chem. 1991;95(10):3955–60.
Zeng XL, Chen WH, Liu JC, Kan JL. A theoretical study of five nitrates: electronic structure and bond dissociation energies. J Mol Struct Theochem. 2007;810:47–51.
Oxley JC, Smith JL, Rogers E, Ye W, Allen AA, Timothy JH. Fuel combustion additives: a study of their thermal stabilities and decomposition pathways. Energy Fuels. 2000;14(6):1252–64.
Francisco MA, Krylowski J. Chemistry of organic nitrates: thermal chemistry of linear and branched organic nitrates. Ind Eng Chem Res. 2005;44(15):5439–46.
Chavez DE, Hiskey MA, Naud DL, Parrish D. Synthesis of an energetic nitrate ester. Angew Chem. 2008;120(43):8431–3.
Suppes GJ, Dasari MA. Synthesis and evaluation of alkyl nitrates from triglycerides as cetane improvers. Ind Eng Chem Res. 2003;42(21):5042–53.
Suppes GJ, Rui Y, Rome AC, Chen Z. Cetane-improver analysis and impact of activation energy on the relative performance of 2-ethylhexyl nitrate and tetraethylene glycol dinitrate. Ind Eng Chem Res. 1997;36(10):4397–404.
Oxley JC, Smith JL, Rogers E, Ye W. Heat-release behavior of fuel combustion additives. Energy Fuels. 2001;15(5):1194–9.
Aw C, Roper P, Fox S. Health hazard evaluation report no. HETA 82-285-1339. Plastifax Company Explosion Site, Gulfport, Mississippi. 1983.
Pritchard HO. Thermal decomposition of isooctyl nitrate. Combust Flame. 1989;75(3):415–6.
Bornemann H, Scheidt F, Sander W. Thermal decomposition of 2-ethylhexyl nitrate (2-EHN). Int J Chem Kinet. 2002;34(1):34–8.
Lu Y. Exploratory research on thermal sensitivity of four liquid energetic materials. Nanjing: Nanjing University of Science and Technology; 2008.
Wang B, Zhang DW, Jiang ML, Huang F. Study on thermal safety of 2-ethylhexyl nitrate. J Saf Technol. 2013;9(5):11–6.
Zeng XL, Chen WH, Wang FW. Experiment study on thermal stability of EHN. Chin Saf Sci J. 2009;19(8):67–72.
Yang T, Chen LP, Chen WH, Zhang CX, Gao HS, Lu GB, Zhou YS. Experimental method on rapid identification of autocatalysis in decomposition reactions. Acta Phys Chim Sin. 2014;30(7):1215–22.
Chervina S, Bodman GT. Mechanism and kinetics of decomposition from isothermal DSC data: development and application. Process Saf Prog. 1997;16(2):94–100.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008. p. 8–10.
Litwinienko G, Kasprzycka-Guttman T, Jarosz-Jarszewska M. Dynamic and isothermal DSC investigation of the kinetics of thermooxidative decomposition of some edible oils. J Therm Anal Calorim. 1995;45(4):741–50.
Bockhorn H, Hornung A, Hornung U. Mechanisms and kinetics of thermal decomposition of plastics from isothermal and dynamic measurements. J Anal Appl Pyrolysis. 1999;50(2):77–101.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C. 1964;6(1):183–195.
Vyazovkin S, Chrissafis K, Lorenzo MLD, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ. ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340:53–68.
Tiţa B, Marian E, Tiţa D, Vlase G, Doca N, Vlase T. Comparative kinetic study of decomposition of some diazepine derivatives under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2008;94(2):447–52.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37(1):1–30.
Fisher HG, Goetz DD. Determination of self-accelerating decomposition temperatures using the accelerating rate calorimeter. J Loss Prev Proc. 1991;4(5):305–16.
Yang T, Chen LP, Chen WH, Zhou YS, Gao HS, Zhong TT. Thermal stability of 2-ethylhexyl nitrate with acid. J Therm Anal Calorim. 2015;119(1):205–12.
Wang K, Liu D, Xu S, Cai G. Research on the thermal history’s influence on the thermal stability of EHN and NC. Thermochimica Acta. 2015;610:23–8.
Málek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69.
Tiţa D, Fuliaş A, Tiţa B. Thermal stability of ketoprofen. J Therm Anal Calorim. 2013;111(3):1979–85.
Acknowledgements
This investigation was financed by the National Natural Science Foundation of China (No. 51204099). The authors thank for this support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Gb., Yang, T., Chen, Lp. et al. Thermal decomposition kinetics of 2-ethylhexyl nitrate under non-isothermal and isothermal conditions. J Therm Anal Calorim 124, 471–478 (2016). https://doi.org/10.1007/s10973-015-5099-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5099-6