Abstract
The isothermal pyrolysis characteristics of lump coal used in COREX was studied by the self-developed thermos-gravimetry analysis device varying the temperature from 900 to 1200 °C. The results indicated that pyrolysis temperature had a significant influence on the pyrolysis rate of the lump coal, but have a little influence on the weight loss ratio. The results obtained by nonlinear fitting of mechanism functions indicated that the pyrolysis process of lump coal satisfied the two-dimensional random nucleation and nuclei growth model. The pyrolysis kinetics equation is \( \frac{{{\text{d}}\alpha }}{{{\text{d}}t}} = - 1.03082\,\exp ( - \frac{46630}{8.314T})[(1 - \alpha )( - \ln (1 - \alpha ))^{0.5} ] \), and the pyrolysis activation energy is 46.63 kJ mol−1. In addition, the average activation energy calculated by iso-conversional method is 47.06 kJ mol−1, which proves that the kinetics equation determined by the master curve method is reliable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
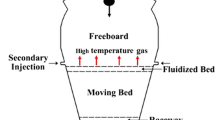
The COREX process is the new ironmaking technology which has realized industrialization with lump coal instead of coke to access to high-quality hot metal, so the dependence on coking coal resources is reduced. The industry practice of Baosteel in China and Arcelor Mittal in South Africa indicated that the quality of the lump coal had a great effect on COREX performance [1, 2]. Lump coal is charged into gasifier by the burden distributor, as shown in Fig. 1. The lump coal falls by gravity into the freeboard and goes through the fluidized bed and moving bed. At last, most the coal chars are burned in the raceway, and a few of them are reacted with slag and hot melt. The ascending gas transfers heat to the descending solid burden [3]. The recycled dusts and oxygen are injected through the dust burner. In order to ensure the cracking of volatile matters released from the lump coal, the dome temperature is kept at around 950–1100 °C. Thus, the pyrolysis process in upper space of gasifier is one of the main reasons for the performance degradation of lump coal. It is important to understand the pyrolysis process of lump coal at high temperature for improving the production efficiency of COREX process. The behavior of the lump coal in melter gasifier was investigated in the past few years. Gupta et al. [4] found that the heat consumption in the melter gasifier increased by 1.68 GJ h−1 as volatile matter of the lump coal increased by 1 %. Kim et al. investigated the pyrolysis and crack process of five Australian lump coals with different content of volatile matter. The study demonstrated that the swelling of lump coal had some relationship with its crack behavior, the lump coal with less swelling could be more easily cracked [5]. Mikin et al. studied the formation mechanism of different semi-coke from six lump coals at 200-800 °C as well as the relationship between the structure and its properties of semi-coke. It indicated that the strength of raw coal was between these of semi-cokes formed by coking coal and non-coking coal [6]. Campbell et al. investigated the agglomeration phenomenon of large coal (8 cm3) of three South Africa coals. It was found that time did affect lump coal agglomeration. The higher the vitrinite, liptinite and volatile matter contents of the coal, the greater tendency of the lump coal to agglomerate [7]. Coetee et al. studied on transient swelling behavior of large South African coal by X-ray computed tomography and mercury submersion measurement. The free swelling index of the pulverized coal did not correlate with the swelling and deformation of lump coal [8]. Fu et al. studied the transient swelling and shrinkage behavior of two types lump coal during pyrolysis by a digital camera. The volumetric behaviors of the raw and processed coal were different under the same conditions [9]. Sahoo et al. investigated the deterioration of weathered coal and its influence factors. The drop strength of lump coal was proposed to evaluate its cold crushing performance [10, 11].
Lump coal falling in the COREX melter gasifier [3]
Many other researchers have made significant contributions in coal pyrolysis field. Char formation mechanism [12, 13], char structure [14–16] and property [17, 18] were reported by many investigators. However, little information is available in the literature on the pyrolysis mechanism of large coal particle especially used in COREX gasifiers. Kim et al. [5] have investigated the influence factors of coal pyrolysis, but the reaction kinetics parameters were obtained based on the first-order reaction model, and no further discussion about the correctness of the reaction model was presented. Zhang et al. have investigated on the pyrolysis kinetic of lump coals and the optimal kinetic mechanical function. It was found that the three-dimensional diffusion was the primary restrictive link in the whole pyrolysis process of XLZ and DT lump coals. However, the research was focus on the pyrolysis process of multi-particles (about 50 g coals), and there was no detailed model validation and mechanism discussion [19].
Based on the previous research, this article explores the optimal kinetic mechanical function of single-particle lump coal under high temperature condition by master curve method. The kinetic parameters of pyrolysis of single lump coal calculated by the optimal kinetic mechanical function were verified by iso-conversional method. In addition, the reaction mechanism of fast pyrolysis of lump coal under high temperature was discussed. The overall aim of this research is to investigate the pyrolysis mechanism of lump coal (20 × 20 × 20 mm) used in COREX under fast thermolysis conditions (900–1200 °C). The research will attempt to ascertain: (1) The influence of high temperature on lump coal pyrolysis behavior; (2) Determine the mechanism function of pyrolysis of lump coal under fast thermolysis conditions; (3) Verify the accuracy of this mechanism function, and determine apparent activation energy of lump coal during the pyrolysis.
Experimental
Raw materials
Owing to the Baosteel COREX-3000 has relocated in Xinjiang region in China, a new lump coal resource in local is the main fuel used in COREX. So a typical lump coal from Xinjiang province was chosen as a parent coal in this study. The coal was shaped into coal cake (20 × 20 × 20 mm) by manual grinding, as shown in Fig. 2. A symmetric cubic shape was used in order to keep the identical physical dimensions and ensure the same experimental condition. All the samples were dried in drying cases at 313 K for 10 h. The following analysis methods of coal properties were applied: (1) Maceral analysis (GB 8899-88); (2) Reflectance of vitrinite (GB/T 15591-1995); (3) Ultimate analysis (GB/T214-2007, GB/T476-2008); (4) Proximate analysis (GB/T212-2008).
Experimental
In order to simulate the pyrolysis process of lump coal under high temperature in COREX gasifier, new experiment equipment was invented. The self-developed thermo-gravimetry analysis device, which is consist of gas control system, heating furnace, electronic balance, temperature control system and computer acquisition system, are shown in Fig. 3. The heating furnace, the heating unit of which is MoSi2, can reach at 1600 °C at most. The weighting scope, accuracy class and sampling frequency of the electronic balance are 1 kg, 0.001 g and 6 times per second, respectively. The furnace was heated to experimental temperature and kept at special level for all the experiment process. The inert gases, which protected the coal from burning during the experiments, flowed from the bottom of the heating furnace to top at a rate of 2 L min−1 for 15 min. The crucible linked with the balance was kept at the constant temperature zone to ensure the accuracy of pyrolysis temperature. The electronic balance was located above the heating furnace. There was a hook used to hang the sample at the bottom of the electronic balance. The crucible was linked to the electronic balance through the molybdenum wire chain. Before the experiment, the electronic balance should be adjusted to ensure the crucible was located in the center of heating tub and avoided the collision with the tub wall. When the temperature in the heating furnace reached to the setting value, the crucible with lump coal was carefully put into the furnace and hung on the hook at the bottom of the electronic balance, and the experiment data were began to collect by computer at the same time. By the end of the experiment, the data were firstly saved, and then the crucible was quickly taken out and placed into the N2 container.
Repeatability of the experiment results
It is important to guarantee the stability of the experiment environment for accuracy of the results. Therefore, repeatability of the experiment data was investigated by preliminary experiments. Pyrolysis experiments of the lump coal (20 × 20 × 20 mm) were conducted at the same conditions for twice, and the repeatability of the results was analyzed. The pyrolysis convention ratio curves, namely the ratio of pyrolysis instantaneous mass loss to total weightlessness, which are obtained under the pyrolysis process of lump coal at 1000 and 1200 °C, respectively, are shown in Fig. 4. The two curves in each figure are overlapping together well, which indicates that the experiment results have good repeatability at the same conditions. The data obtained through the experiment are reliable.
Results and discussion
The coal chemistry and petrology
The properties of the lump coal sample are listed in Table 1. Compared with the traditional lump coals used in the Baosteel COREX-3000 [19], the volatile content of HY lump coal is similar (34.45 %), which ensure the gas production in gasifier. The advantages of the HY lump coal are lower ash content, lower water content, lower sulfur content and higher fixed carbon content, which benefit to reduce the heat loss caused by the fuel composition.
From the maceral composition analysis, the vitrinite content is highest (62.93 %), followed by inertinite content (33.20 %), and the liptinite is rare in HY lump coal. The coal is ranked hight-volatile bituminous by mean-maximum vitrinite reflectance (0.60 %).
The influence of temperature on pyrolysis
Mass loss ratio curves of HY lump coal during pyrolysis at different temperatures, namely the ratio of pyrolysis instantaneous mass loss to initial sample mass, are shown in Fig. 5. It indicates that the pyrolysis curves move to the left and the slope of the curves increase when the pyrolysis temperature increases from 900 to 1200 °C. The inflection points of the curves move to the left as well, which indicates that the pyrolysis rate of the lump coal increases gradually with the pyrolysis temperature increase. Further, it can be also seen from Fig. 5 that the final mass loss ratio has almost no change as temperature increases, which is of the same order as the mass loss obtained by proximate analysis in laboratory (in Table 1). It indicates that pyrolysis temperature has a little influence on mass loss ratio in the range from 900 to 1200 °C. This conclusion is also consistent with the results of Kim’s experiments [5].
Pyrolysis conversion rate curves during pyrolysis of HY lump coal at different temperature are shown in Fig. 6. It can be found that the conversion rate curves move to the left as the pyrolysis temperature ranges from 900 to 1200 °C, and the peak is narrowed gradually, the peak value increases. This phenomenon indicated that the intensity of the pyrolysis process increases with the pyrolysis temperature increase. As the peak value moves to the left, the severely reaction moment occurs earlier. What is more, the time of maximum conversion rate and pyrolysis completion time can both be calculated. These calculated results are listed in Table 2. It is worth noting that the maximum pyrolysis rate is at the moment of 68 s at 1200 °C, and pyrolysis completes within 168 s. However, it should take 138 s to reach the maximum pyrolysis rate at 900 °C and all the pyrolysis process consumes 386 s. The maximum pyrolysis rate value under 1200 and 900 °C are 0.0105 and 0.0047 s−1, respectively. The results indicate that pyrolysis temperature has a great influence on pyrolysis rate of lump coal.
The relationship between conversion and conversion rate
The relationship between conversion and conversion rate at different pyrolysis temperatures are shown in Fig. 7. It indicates that conversion rate increases at the initial stage and then decreases as conversion increases. This is due to that the reaction rate depends on the reaction temperature before the peak value of the conversion rate. However, when the pyrolysis reaction approaches to a certain extent, surface area and activity center of lump coal are reduced. Meanwhile, the inhibit effect of surface area and activity center of lump coal are greater than the promotion effect of temperature. So the conversion rate decreases with the increase in conversion after the peak value [20, 21]. The peak becomes sharper as pyrolysis temperature increases, which indicates that there exists positive correlation between conversion rate and pyrolysis temperature. So the time of reaching same conversion is less, as the pyrolysis temperature increases.
Kinetic model
Method 1: determining the optimum mechanism function by the master curve method
The pyrolysis of coal has been studied by many researchers in the past few years [22–25]. Volatile matter separates out and the semi-coke forms due to the complex physical and chemical reaction during pyrolysis process of coal. The process of gas evolution and carbonizing may occur simultaneously during rapid pyrolysis at a high temperature level for the lump coal with large particle size used in COREX process, which can be regarded as solid-phase reaction. Pyrolysis process for lump coal in COREX is very complex due to the influence of mass and heat transfer, thus the macro-kinetics model is established in the present work. The pyrolysis mechanism for lump coal used in COREX is described as following formula:
The conversion rate of lump coal during isothermal pyrolysis is the function of pyrolysis time and temperature, and it can be expressed as:
where \( \frac{{{\text{d}}\alpha }}{{{\text{d}}t}} \) is conversion rate, s−1; k(T) is the rate constant, which is the function of temperature; f(α) is the differential form of pyrolysis mechanism function; t is pyrolysis time, s; α is pyrolysis conversion ratio.
k(T) is calculated by Arrhenius equation:
where A is the pre-exponential factor, which is positive to reaction rate, s−1, E is the activation energy, kJ mol−1; and R is standard molar gas constant, kJ mol−1 K−1.
The pyrolysis conversion ratio is calculated by the following formula:
where m i is the initial mass of lump coal, g; m t is the instant mass of the sample during experiment, g; m ∞ is the sample mass after experiment, g.
The following formula can be got by Eqs. (2) and (3):
Equation (5) is regarded as the relationships between pyrolysis conversion rate and conversion ratio. The corresponding activation energy and the pre-exponential factor can be calculated from experimental data by employing nonlinear least-squares fitting method. The objective function can be expressed as follow:
where \( \left( {\frac{{{\text{d}}\alpha }}{{{\text{d}}t}}} \right)_{{{\text{exp,}}\,\text{i}}} \) is experiment data; \( \left( {\frac{{{\text{d}}\alpha }}{{{\text{d}}t}}} \right)_{{{\text{calc,}}\,\text {i}}} \) is value calculated by model; and N is the number of data. If theoretical curves calculated by model fit well with the experiment curves, it indicates that the mechanism function which the theoretical curve stands for is the most optimum one. The mechanism functions frequently used in solid-phase reaction are listed in Table 3 [26, 27].
Method 2: apparent activation energy calculated by iso-conversional method
Iso-conversional method is used frequently to calculate the activation energy in thermal analysis kinetics [28]. The following equation can be got by the integration of Eq. (2):
Take natural logarithm on both sides for Eq. (7):
where
is the integral form of the pyrolysis mechanism function.
Activation energy E can be obtained from the slop of the plot ln(t) to 1/T, and pre-exponential factor A can be obtained by the intercept of that line. It can be analyzed that the advantages of iso-conversional method for the calculation of the activation energy is not need to determine the mechanism, and it can also reflect the change of activation energy at different pyrolysis conversion.
The activation energy of pyrolysis process of lump coal in COREX is calculated using the two methods based on above mentioned in the present work.
Kinetic analysis
Determining the optimum mechanism function by mater curve method
The relationship between conversion rate and conversion are shown in Fig. 8. Fourteen kinetic control functions are obtained by taking the mechanism functions (listed in Table 3) into Eq. (5). Activation energy E and the corresponding pre-exponential factor A are calculated by nonlinear fitting based on the data in Fig. 8, and the results are shown in Table 4. “Negative value” of R 2 in Table 4 indicates that the fitting effect is very poor by the selected mechanism function; the symbol “–” in Table 4 indicates that the calculation cannot get convergence solution. Four different mechanism functions (A2, P3, R2 and R3) were selected for the model fitting calculation. Figure 8 was the experimental conversion rate curves of HY lump coal and those calculated with four models. From Table 4 and Fig. 8, it can be found that the imitative effect by two-dimensional random nucleation and nuclei growth model is the best, and the correlation index of which is 0.9839. The results obtained by fitting indicate that the pyrolysis mechanism function of HY lump coal is 2(1−α)[−ln(1−α)]1/2 (differential form)at the temperature range of 900 to 1200 °C.
The activation energy and pre-exponential factor calculated by two-dimensional random nucleation and nuclei growth mechanism function are 46.63 kJ mol−1 and 1.03 s−1, respectively. Control equation of pyrolysis of lump coal can be obtained by taking A and E into Eq. (5):
The conversion ratio at different temperature was taken into kinetic equations and having the integral for Eq. (10). Then the conversion curves through theoretical calculation can be obtained. As shown in Fig. 9, it is the comparison between theoretical curve and experimental curve of conversion for pyrolysis based on four models. From Fig. 9, it can be found that the theoretical calculation curves almost overlap with the experimental one by two-dimensional random nucleation and nuclei growth mechanism function, which verifies the accuracy of this model. As the pyrolysis reaction proceeds at high temperatures, the liquid phase within the lump coal will form, and then the generated liquid phase transforms into solid state. During this process, the solid particles are capable of acting as the nuclei and being wrapped by the liquid phase. Then the aggregation and growth of these small clusters will continue to occur. These behaviors satisfy the random nucleation and nuclei growth model. The schematic diagram of reaction mechanism of pyrolysis process is shown in Fig. 10.
Verifying mechanism function by iso-conversional method
Rapid pyrolysis for HY lump coal in a high temperature level mainly occurs before conversion ratio reaching 0.95, thus the conversion ratio value are taken in the range from 0.1 to 0.95 during calculation. The reaction time corresponding to different conversion ratio at different temperature is calculated according to the data obtained from rapid pyrolysis experiment, and then ln(t) is got, as shown in Table 5. Activation energy at different conversion ratio and average activation energy for the whole process of pyrolysis can be determined by data fitting, and the results are listed in Table 6. The relations between ln(t) and 1/T can be found in Fig. 11. The hollow label represents experiment data and straight line represents the fitting one. It can be seen in Table 6 that the correlation coefficients R 2 for activation energy calculation at different conversion ratio are all greater than 0.99, which indicates good correlation and high credibility for the results.
The average activation energy of HY lump coal calculated according to iso-conversional method is 47.06 kJ mol−1, which agrees with the result calculated by random nucleation and nuclei growth model. From the calculation results, it is possible to verify that the mechanism function determined by curve-fitting method is accurate. It can be seen from Table 6 and Fig. 12 that the activation energy at different conversion ratio ranges from 45 to 48 kJ mol−1 and has small changes.
Conclusions
-
1.
The pyrolysis rate increases as temperature increases in the range from 900 to 1200 °C, while the maximum mass loss ratio, which is almost the same order for all the temperature, have a little relation with pyrolysis temperature.
-
2.
Two-dimensional random nucleation and nuclei growth model is well satisfied the pyrolysis process of lump coal according to the nonlinear fitting results, and the kinetic equation of pyrolysis is:
$$ \frac{{{\text{d}}\alpha }}{{{\text{d}}t}} = - 1.03082\,\exp \left( { - \frac{46630}{8.314T}} \right) \quad \times\,\left[ {\left( {1 - \alpha } \right)\left( { - \ln (1 - \alpha )} \right)^{0.5} } \right] $$(11)The pyrolysis activation energy of HY lump coal calculated based on this kinetic equation is 46.63 kJ mol−1. The results calculated by the theoretical kinetic equation coincide well with the experiment, which indicates that this mechanism function clearly describes the relationship between conversion ratio and time for isothermal rapid pyrolysis of lump coal at 900–1200 °C.
-
3.
The average pyrolysis activation energy of HY lump coal calculated according to iso-conversional method is 47.06 kJ mol−1. The value agrees well with the result calculated by random nucleation and nuclei growth model. This result validates the correctness of the pyrolysis kinetic equation of HY lump coal. The pyrolysis activation energies of HY lump coal at different conversion ratio have small changes (ranging from 45 to 48 kJ mol−1).
References
Prachethan Kumar P, Rao YS, Chidambaran K, et al. Influence of coal size on the performance of Corex process. World Iron Steel. 2010;04:13–7.
Xu W, Guo Y, Wang C. Analysis of the factors affecting the fuel rate in the COREX process and improvement measures. Baosteel Technol Res. 2011;5(1):45–50.
Liu X, Pan G, Wang G, Wen Z. Mathematical model of lump coal falling in the freeboard zone of the COREX melter gasifier. Energy Fuel. 2011;25:5729–35.
Gupta SK. Corex process utilisation of noncoking coal from India: prospects and problems. J Mines Metals Fuels. 2002;50(3):300–5.
Kim B, Gupta S, Lee S, et al. Devolatilization and cracking characteristics of australian lumpy coals. Energy Fuels. 2008;22(1):514–22.
Minkina M, Oliveira FLG, Zymla V. Coal lump devolatilization and the resulting char structure and properties. Fuel Process Technol. 2010;91(5):476–85.
Campbell QP, Bunt JR, de Waal F. Investigation of lump coal agglomeration in a non-pressurized reactor. J Anal Appl Pyrol. 2010;89(2):271–7.
Coetzee S, Neomagus HWJP, Bunt JR, et al. The transient swelling behaviour of large (−20 + 16 mm) South African coal particles during low-temperature devolatilisation. Fuel. 2014;136:79–88.
Fu Z, Guo Z, Yuan Z, et al. Swelling and shrinkage behavior of raw and processed coals during pyrolysis. Fuel. 2007;86(3):418–25.
Sahoo R, Roach D. Degradation behaviour of weathered coal during handling for the COREX process of iron making. Powder Technol. 2005;152(1–3):1–8.
Sahoo R, Roach D. Quantification of the lump coal breakage during handling operation at the gladstone port. Chem Eng Process. 2005;44(7):797–804.
Lu L. Coal char reactivity and structural evolution during combustion-factors influencing blast furnace pulverized coal injection operation. Fuel Energy Abstr. 2002;43(4):283.
Lu L, Sahajwalla V, Harris D. Characteristics of chars prepared from various pulverized coals at different temperatures using Drop-tube furnace. Energy Fuels. 2000;14(4):869–76.
Li K, Khanna R, Zhang J, et al. The evolution of structural order, microstructure and mineral matter of metallurgical coke in a blast furnace: a review. Fuel. 2014;133:194–215.
Yu J, Lucas JA, Wall TF. Formation of the structure of chars during devolatilization of pulverized coal and its thermoproperties: a review. Prog Energy Combust Sci. 2007;33(2):135–70.
Cui X, Zhang X, Yang M, et al. Study on the structure and reactivity of COREX coal. J Therm Anal Calorim. 2013;113(2):693–701.
Huo W, Zhou Z, Chen X, et al. Study on CO2 gasification reactivity and physical characteristics of biomass, petroleum coke and coal chars. Bioresour Technol. 2014;159:143–9.
Feng B, Bhatia SK, Barry JC. Structural ordering of coal char during heat treatment and its impact on reactivity. Carbon. 2002;40(4):481–96.
Zhang S, Zhu F, Bai C, et al. High temperature pyrolysis behaviour and kinetics of lump coal in COREX melter gasifier. Ironmak Steelmak. 2014;41(3):219–28.
Sekine Y, Ishikawa K, Kikuchi E, et al. Reactivity and structural change of coal char during steam gasification. Fuel. 2006;85:122–6.
Wang G, Zhang J, Hou X, et al. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char. Bioresour Technol. 2015;177:66–73.
Zhong S, Baitalow F, Nikrityuk P, et al. The effect of particle size on the strength parameters of German brown coal and its chars. Fuel. 2014;125:200–5.
Zhang C, Jiang X, Wei L, et al. Research on pyrolysis characteristics and kinetics of super fine and conventional pulverized coal. Energy Convers Manag. 2007;48(3):797–802.
Wang J, Du J, Chang L, et al. Study on the structure and pyrolysis characteristics of Chinese western coals. Fuel Process Technol. 2010;91(4):430–3.
Gong X, Guo Z, Wang Z. Variation of char structure during anthracite pyrolysis catalyzed by Fe. Energy Fuels. 2009;23(9):4547–52.
Vlaev LT, Markovska IG, Lyubchev LA. Non-isothermal kinetics of pyrolysis of rice husk. Thermochim Acta. 2003;406(1–2):1–7.
Zou S, Wu Y, Wu M, et al. Characteristics and dynamics of pyrolysis process microalgae. J Combust Sci Technol. 2007;04:330–4.
Ren S, Zhang J. Thermogravimetric analysis of anthracite and waste plastics by iso-conversional method. Thermochim Acta. 2013;561:36–40.
Acknowledgements
Authors acknowledge the financial support for this work provided by national basic research Program of China (973 Program) (No. 2012CB720401) and national key technology R&D Program of China (No. 2011BAC01B02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, R., Zhang, J., Wang, G. et al. Isothermal kinetic analysis on fast pyrolysis of lump coal used in COREX process. J Therm Anal Calorim 123, 773–783 (2016). https://doi.org/10.1007/s10973-015-4972-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4972-7