Abstract
DA-6886 is a new 5-HT4 receptor agonist under development for the treatment of constipation-predominant irritable bowel syndrome. The objective of this work was to investigate the existence of polymorphs and pseudopolymorphs of DA-6886. Five crystal forms of DA-6886 have been isolated by recrystallization and characterized by differential scanning calorimetry (DSC), thermogravimetric (TG) analysis, and powder X-ray diffractometry (PXRD). From the DSC and TG data, it was confirmed that Form 2 is \( {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) methanol solvate, Form 3 is 1 methanol solvate, Form 4 is \( 1{\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) ethanol solvate, and Form 5 is \( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate. The PXRD patterns of five crystal forms were different, respectively. In the dissolution studies in pH 6.8 ± 0.05 buffer at 37 ± 0.5 °C, the solubility of Form 2 was the highest. And the dissolution rate at 5 min in water decreased in rank order: Form 2 > Form 4 > Form 1 > Form 3 > Form 5. After storage of 3 months at 2 °C, 24 % relative humidity, Form 1, Form 2, Form 3, and Form 4 were not transformed, but Form 5 (\( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate) was transformed to dihydrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceutical solids can exist in different crystal forms, such as crystalline, amorphous, or glass, and also in solvated or hydrated states [1, 2]. Polymorphism is defined as the ability of a substance to exist as two or more crystalline phases that have different arrangements and/or conformations of the molecules in the crystal lattice. Polymorphs share the same chemical composition but have different crystal structures. Because of their structural differences, polymorphs may have different physicochemical properties. For example, polymorphs can have different density, habit, melting properties, vapor pressure, solubility, dissolution rate, tableting, and mechanical properties [1–6].
Solvates are molecular complexes that have incorporated the crystallizing solvent molecule in their lattice. When the solvent incorporated in the solvate is water, it is called a hydrate. To distinguish solvates from polymorphs, which are not molecular compounds, the term pseudopolymorph is used [2, 3]. Identification of possible hydrate compounds is also important since their aqueous solubilities can be significantly less than their anhydrous forms [7–10]. Crystal form includes polymorphs, solvates, and amorphous forms as defined in the International Conference on Harmonization (ICH) Guideline Q6A [11].
Crystal form affects properties such as drug absorption, rate of dissolution, elimination rate, and stability in galenic preparations [12–17]. The successful utilization of a crystal form of significantly greater thermodynamic activity (i.e., solubility) than the stable modification may provide, in some instances, therapeutic blood levels from otherwise inactive drugs [18].
Companies have experienced market shortages because they have observed unpredicted changes in crystal form, which ultimately resulted in problematic quality release and stability testing of the finished dosage form [19–22]. A thorough understanding of the way in which solid-state properties influence solubility, stability, and other properties of the drug substance is critical in the development of profiling strategies and in the setting of criteria for developability assessment [23].
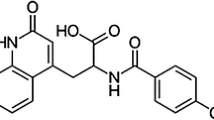
The compound DA-6886 (Fig. 1), N-((1-(3-(1,2,3-triazol-1-yl)propyl)piperidin-4-yl)methyl)-4-amino-5-chloro-2-methoxybenzamide hydrochloride, is the gastrointestinal prokinetic benzamide derivative and a new 5-HT4 receptor agonist being developed for the treatment of constipation-predominant irritable bowel syndrome (IBS-C) by DongA Pharmaceutical Co. Ltd., Korea. DA-6886 is a highly potent and selective 5-HT4 receptor agonist to accelerate colonic transit in mice, which might be therapeutic agent having a favorable safety profile in the treatment of gastrointestinal motor disorders such as IBS-C and chronic constipation [24].
In the case of a new drug substance, it is important that crystal form data should be generated prior to the initiation of pivotal clinical studies and primary stability batches. Thus, the thorough investigation of new solid states of a drug molecule is recognized as an essential and very important part of preformulation studies [25].
The aim of this study was to investigate the existence of polymorphs and pseudopolymorphs of DA-6886.
Experimental
Materials
DA-6886 was provided from DongA Pharmaceutical Co. Ltd., Korea. The purity of DA-6886 was not less than 99.0 %. All of the other chemicals were of reagent grade and were used without further purification.
Preparation of crystal forms
Form 1
Form 1 is the donated one and standard. Standard was always stored at 0–2 °C condition.
Form 2
A suspension of Form 1 in methanol was heated to 50 °C for 10 min. The solution was filtered to remove most nuclei and then left undisturbed for 1 week at room temperature. The resulting solid was filtered and dried for 1 week in the desiccators to give Form 2.
Form 3
A suspension of Form 1 in methanol was heated to 50 °C for 10 min. The solution was filtered to remove most nuclei and then left undisturbed for 1 week at 4 °C. The resulting solid was filtered and dried for 1 week in the desiccators to give Form 3.
Form 4
A suspension of Form 1 in ethanol was heated to 50 °C for 10 min. The solution was filtered to remove most nuclei and then left undisturbed for 1 week at room temperature. The resulting solid was filtered and dried for 1 week in the desiccators to give Form 4.
Form 5
A suspension of Form 1 in water was heated to 50 °C for 10 min. The solution was filtered to remove most nuclei and then left undisturbed for 1 week at room temperature. The resulting solid was filtered and dried for 1 week in the desiccators to give Form 5.
Methods
Thermal analysis
Thermal analysis methods used in this study included differential scanning calorimetry (DSC) and thermogravimetric (TG) analysis. The DSC data were collected using a Mettler-Toledo DSC 1 STARe system (Mettler-Toledo AG, Schwerzenbach, Switzerland) within the temperature range of 30–350 °C at a heating rate of 10 °C min−1 using highly purified nitrogen gas, free of any other gases, in the surrounding atmosphere with a flow rate 30 mL min−1. The TG was carried out using a Mettler-Toledo TGA 1 STARe system (Mettler-Toledo AG, Schwerzenbach, Switzerland) within the temperature range of 30–350 °C at a heating rate of 10 °C min−1 using highly purified nitrogen gas, free of any other gases, in the surrounding atmosphere with a flow rate 30 mL min−1. Calibration for DSC was executed by the standard test method for temperature calibration of DSC (E967-08, 2014) and the standard practice for heat flow calibration of DSC (E968-02, 2014). Calibration for TG was executed by the standard test method for compositional analysis by thermogravimetry (E1131-08, 2014).
Powder X-ray diffraction
Powder X-ray diffraction patterns under ambient conditions were collected on D8 focus-Bruker AXS (Bruker AXS GmbH, Karlsruhe, Germany) diffractometer using graphite monochromatized CuKα radiation (λ = 1.54178 Å). The isothermal measurement conditions were: target, Cu; voltage, 30 kV; current, 10 mA. The PXRD patterns of the samples were compared with regard to peak position and relative intensity, peak shifting, and the presence of lack of peaks in certain angular regions.
Analytical method
The absorption maximum for DA-6886 was obtained at 290 nm. To construct a calibration curve for DA-6886, known amounts of the prepared samples were dissolved in water and the drug content was evaluated spectrophotometrically at 290 nm (Agilent 8453 UV–visible spectrophotometer, Santa Clara, USA). Six sets of aqueous standard solutions of DA-6886 were prepared twice a day for a 3-day period. The absorbance values of these solutions were measured at 290 nm. Analytical parameters such as linearity, precision, and accuracy were then evaluated. System suitability: standard deviation 0.005. Linearity was evaluated by correlation coefficient (R 2) value. R 2 = 0.999961. The limit of detection (LOD) and the limit of quantification (LOQ) were measured by calibration curves, respectively. LOD 0.05 µg mL−1, LOQ 0.14 µg mL−1. Precision was expressed as relative standard deviations (RSD), and the value of RSD within 1 % is acceptable. The accuracy (%) was 99.78 %.
Dissolution
The dissolution rate of DA-6886 crystal forms was measured according to the dissolution test (paddle method) of the Korean Pharmacopeia 8th Edition. A fixed amount (20 mg, 250–600 µm) of DA-6886 crystal forms was put into 900 mL of pH 6.8 ± 0.05 buffer equilibrated at 37 ± 0.5 °C and stirred at 90 rpm. At appropriate intervals, an aliquot (1 mL) was withdrawn with a syringe and filtered with 0.45-µm syringe filter. And then, it was analyzed spectrophotometrically at 290 nm.
Transformation
A certain amount (20 mg) of crystal forms was taken and placed in weighing dish. They were stored at 2 °C, 24 % relative humidity (RH). The transformation behavior of crystal forms was monitored by powder X-ray diffraction analysis, DSC and TG.
Results and discussion
The DSC and TG curves of Forms 1–5 are illustrated in Fig. 2. The DSC curve of Form 1 shows one endothermic peak at 248 °C. The DSC curve of Form 2 shows two endothermic peaks at 120 and 248 °C. Form 3 shows two endothermic peaks at 100 and 248 °C. The DSC curve of Form 4 shows two endothermic peaks at 91 and 248 °C. DSC curve of Form 5 shows three endothermic peaks at 92, 120, and 248 °C.
On TG curve of Form 2, the mass loss corresponding to the DSC endotherm at 90–120 °C was 2.13 %, which corresponds to the loss of 0.301 mol of methanol. The TG curve of Form 3 shows a single mass loss at 70–120 °C (6.7605 %) which corresponds to the loss of 1.0037 mol of methanol. The TG curve of Form 4 shows a single mass loss at 60–90 °C (8.1044 %) which corresponds to the loss of 1.26 mol of ethanol. On TG curve of Form 5, the mass loss corresponding to the DSC endotherm at 60–100 °C was 6.9414 %, which corresponds to the loss of 1.97 mol of H2O, the mass loss corresponding to the DSC endotherm at 140–150 °C was 3.9552 %, which corresponds to the loss of 1.04 mol of H2O, and the mass loss corresponding to the DSC endotherm at 170–180 °C was 2.8954 %, which corresponds to the loss of 0.73 mol of H2O. TG analysis represents a powerful adjunct to the other methods of thermal analysis, since a combination of either a DTA or a DSC study with a TG determination can be used in the assignment of observed thermal events [26].
The powder X-ray diffraction patterns of Forms 1–5 are illustrated in Fig. 3. The PXRD patterns of Forms 1–5 showed differences.
The DSC, TG, and PXRD results confirmed the existence of five crystal forms of DA-6886.
The dissolution patterns of five crystal forms of DA-6886 are illustrated in Fig. 4 and Table 1. In the dissolution studies in pH 6.8 ± 0.05 buffer equilibrated at 37 ± 0.5 °C, the solubility of Form 2 was the highest. And the dissolution rate at 5 min in water decreased in rank order: Form 2 > Form 4 > Form 1 > Form 3 > Form 5. The dissolution rate of anhydrate was higher than that of \( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate (Form 5). It has been noted from the earliest dissolution work [27] that for many substances, the dissolution rate of an anhydrous phase usually exceeds that of any corresponding hydrate phase. These observations were explained by thermodynamics, where it was reasoned that the drug in the hydrates possessed a lower activity and would be in a more stable state relative to their anhydrous forms [18]. This general rule was found to hold for anhydrate/\( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate of DA-6886. The dissolution rate of solvates (Form 2, Form 3, and Form 4) was higher than that of hydrate (Form 5). Also in our report on DA-6034 [5], the dissolution rate of solvates was higher than that of hydrate. Dissolution rate of solvates (Form 2, Form 3, and Form 4) at 5 min exceeds that of anhydrate (Form 1), as in the case of urapidil [28], glibenclamide [29], and sulindac [30]. These trends would imply that a nonaqueous solvate phase could be considered as being a high-energy form of the solid with respect to dissolution in water [18].
Five crystal forms were stored under the condition of 2 °C, 24 % RH. After storage of 3 months at 2 °C, 24 % RH, Form 1, Form 2, Form 3, and Form 4 showed no change in DSC, TG, and PXRD patterns (not shown). DSC and TG curves of stored sample of Form 5 are illustrated in Fig. 5. On TG curve of stored sample of Form 5, the mass loss corresponding to the DSC endotherm at 80–110 °C was 7.2769 %, which corresponds to the loss of 1.93 mol of H2O. Upon dehydration crystal hydrates can transform to crystalline less hydrated forms [31]. The powder X-ray diffraction pattern of stored sample of Form 5 is illustrated in Fig. 6. The stored sample of Form 5 shows same PXRD pattern as Form 5. When solvents are employed in the purification of new drug substances by recrystallization, it is observed that the isolated crystals include solvent molecules, either entrapped within empty spaces in the lattice or interacting via hydrogen bonding or van der Waals force with molecules constituting the crystal lattice [32]. The term “desolvated solvates” has been applied to compounds that were originally crystallized as solvates but from which the solvent has been removed [33]. Frequently, these “desolvated solvates” retain the crystal structure of the original solvate form and exhibit relatively small changes in lattice parameters. Based on crystal lattice studies, three cases have been distinguished following desolvation of solvates: (a) The residue is amorphous or poorly crystalline, (b) the residue recrystallizes with a different crystal lattice, and (c) the crystal lattice of the residue is nearly identical to that of the original hydrate [32]. It is thought that Form 5 (\( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate) corresponds to case (c). Form 1, Form 2, Form 3, and Form 4 were not transformed at 2 °C, 24 % RH, and it maintained its crystal structure, and it is confirmed that Form 1, Form 2, Form 3, and Form 4 are stable at 2 °C, 24 % RH. Depending on the nature of molecular packing arrangements, it may happen that the inclusion of solvent is necessary to build a stable crystal structure. When solvent molecules increase the strength of the crystal lattice, they can affect the stability of the compound to solid-state decomposition [32].
The dissolution rate at 5 min of stored sample of Form 5 (dihydrate) was higher than that of \( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate (Form 5), as the report of Giron et al. [31]. This confirms the usual observation that increasing degrees of hydration result in slower dissolution rates [18].
Conclusions
Five crystal forms of DA-6886 were prepared by recrystallization from different solvents. The crystal forms were characterized by DSC, TG, and PXRD. In the dissolution studies in pH 6.8 ± 0.05 buffer equilibrated at 37 ± 0.5 °C, the solubility of Form 2 was the highest. And the dissolution rate at 5 min in water decreased in rank order: Form 2 > Form 4 > Form 1 > Form 3 > Form 5. After storage of 3 months at 2 °C, 24 % RH, Form 1, Form 2, Form 3, and Form 4 were not transformed, but Form 5 (\( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate) was transformed to dihydrate.
References
Chadha R, Arora P, Garg M, Bhandari S, Jain DS. Thermoanalytical and spectroscopic studies on different crystal forms of nevirapine. J Therm Anal Calorim. 2013;111:2133–42.
Perlovich GL, Blokhina SV, Manin NG, Volkova TV, Tkachev VV. Polymorphism and solvatomorphism of bicalutamide. J Therm Anal Calorim. 2013;111:655–62.
Haleblian J. Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J Pharm Sci. 1975;64:1269–88.
Haleblian J, McCrone W. Pharmaceutical applications of polymorphism. J Pharm Sci. 1969;58:911–29.
Shin JY, Sohn YT. Solid state of a new flavonoid derivative DA-6034. J Therm Anal Calorim. 2014;115:2457–61.
Drebushchak VA, Drebushchak TN, Boldyreva EV. New interpretation of heat effects in polymorphic transitions. J Therm Anal Calorim. 2013;113:419–24.
Maria TMR, Castro RAE, Silva MR, Ramos ML, Justino LLG, Burrows HD, Canotilho J, Eusebio MES. Polymorphism and melt crystallisation of racemic betaxolol, a β-adrenergic antagonist drug. J Therm Anal Calorim. 2013;111:2171–8.
Kuhnert-Brandstätter M, Burger A. Untersuchungen zum Aufloesungsverhalten polymorpher, pseudopolymorpher and amorpher Phasen von Arzneimitteln. Pharm Ind. 1972;34:187–90.
Liu W, Dang L, Wei H. Thermal, phase transition, and thermal kinetics studies of carbamazepine. J Therm Anal Calorim. 2013;111:1999–2004.
Szterner P, Legendre B, Sghaier M. Thermodynamic properties of polymorphic forms of theophylline. Part I: DSC, TG, X-ray study. J Therm Anal Calorim. 2010;99:325–35.
FDA. Q6A international conference on harmonization; guidance on Q6A specifications: test procedures and acceptance criteria for new drug substances and new drug products: chemical substances, Federal Register. vol 65. 2000. p. 83041–63.
Seo HO, Sohn YT. Crystal transformation of a flavonoid derivative DA-6034. J Therm Anal Calorim. 2015;120:749–57.
Zhang GGZ, Law D, Scmitt EA, Qiu Y. Phases transformation considerations during process development and manufacture of solid oral dosage forms. Adv Drug Deliv Rev. 2004;56:371–90.
Otsuka M. Effects of environmental temperature and compression energy on polymorphic transformation during tableting. Drug Dev Ind Pharm. 1993;19:2241–69.
Griesser UJ, Weigand D, Rollinger JM, Haddow M, Gstrein E. The crystal polymorphs of metazachlor. J Therm Anal Calorim. 2004;77:511–22.
Dichi E, Legendre B, Sghaier M. Physico-chemical characterization of a new polymorph of caffeine. J Therm Anal Calorim. 2014;115:1551–61.
Petit S, Mallet F, Petit MN, Coquerel G. Role of structural and macrocrystalline factors in the desolvation behavior of cortisone acetate solvates. J Therm Anal Calorim. 2007;90:39–47.
Brittain HG, Grant DJW. Effects of polymorphism and solid-state solvation on solubility and dissolution rate. In: Brittain HG, editor. Polymorphism in pharmaceutical solids. New York: Marcel Dekker; 1999. p. 279–330.
de Oliveira GGG, Ferraz HG, Severino P, Souto E. Analysis of phase transition and dehydration process of nevirapine. J Therm Anal Calorim. 2012;108:53–7.
Terada K, Kurobe H, Ito M, Yoshihashi Y, Yonemochi E, Fujii K, Uekusa H. Polymorphic and pseudopolymorphic transformation behavior of acyclovir based on thermodynamics and crystallography. J Therm Anal Calorim. 2013;113:1261–7.
Nicolai B, Espeau P, Ceolin R, Perrin MA, Zaske L, Giovanni J, Leveiller F. Polymorph formation from solvate desolvation. J Therm Anal Calorim. 2007;90:337–9.
Gana I, Ceolin R, Rietveld IB. Bicalutamide polymorphs I and II. J Therm Anal Calorim. 2013;112:223–8.
Huang LF, Tong WQ. Impact of solid state properties on developability assessment of drug candidates. Adv Drug Deliv Rev. 2004;56:321–34.
Lee MJ, Cho KH, Park HM, Sung HJ, Choi SH, Im WB. Pharmacological profile of DA-6886, a novel 5-HT4 receptor agonist to accelerate colonic motor activity in mice. Eur J Pharmacol. 2014;735:115–22.
Rodriguez-Spong B, Price CP, Jayasankar A, Matzger AJ, Rodriguez-Hornedo N. General principles of pharmaceutical solid polymorphism: a supramolecular perspective. Adv Drug Deliv Rev. 2004;56:241–74.
Brittain HG. Methods for the characterization of polymorphs and solvates. In: Brittain HG, editor. Polymorphism in pharmaceutical solids. New York: Marcel Dekker; 1999. p. 227–78.
Shefter E, Higuchi T. Dissolution behavior of crystalline solvated and nonsolvated forms of some pharmaceuticals. J Pharm Sci. 1963;52:781–91.
Botha SA, Caira MR, Guillory JK, Lötter AP. Physical characterization of the methanol solvate of urapidil. J Pharm Sci. 1989;78:28–34.
Suleiman MS, Najib NM. Isolation and physicochemical characterization of solid forms of glibenclamide. Int J Pharm. 1989;50:103–9.
Tros de Ilarduya MC, Martin C, Goni MM, Martinez-Oharriz MC. Dissolution rate of polymorphs and two new pseudopolymorphs of sulindac. Drug Dev Ind Pharm. 1997;23:1087–93.
Giron D, Goldbronn Ch, Mutz M, Pfeiffer S, Piechon Ph, Schwab Ph. Solid state characterization of pharmaceutical hydrates. J Therm Anal Calorim. 2002;68:453–65.
Guillory JK. Generation of polymorphs. In: Brittain HG, editor. Polymorphism in pharmaceutical solids. New York: Marcel Dekker; 1999. p. 183–226.
Byrn S, Pfeiffer R, Ganey M, Hoiberg C, Poochikian G. Pharmaceutical solids: a strategic approach to regulatory considerations. Pharm Res. 1995;12:945–54.
Acknowledgements
This study was supported by the research grant from Duksung Women’s University (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tak, SR., Sohn, YT. Crystal forms of a new 5-HT4 receptor agonist DA-6886. J Therm Anal Calorim 123, 2477–2483 (2016). https://doi.org/10.1007/s10973-015-4897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4897-1