Abstract
Single crystals of the crystallosolvate [bicalutamide + DMSO] with 1:1 stoichiometry were grown, and their structures were solved by X-ray diffraction methods. Polymorphic modifications I and II, the amorphous state, and the DMSO crystallosolvate of bicalutamide were prepared and thermochemistry of fusion processes was studied by DSC technique. The temperature dependence of the saturated vapor pressure of polymorphic form I was obtained and the thermodynamic characteristics of the sublimation process including the crystal lattice energy were calculated. The solution enthalpies of the forms under consideration and the crystallosolvate were acquired by the solution calorimetry procedure. The phase transition enthalpies estimated for form I, form II, and the amorphous state followed the rank order: form I— > form II, form I— > amorphous state, and form II— > amorphous state. The crystal lattice energy of polymorphic form II was determined using the results of sublimation and solution calorimetric experiments. The difference between the crystal lattice energy of the crystallosolvate and unsolvated phases was observed. The dissolution kinetics of forms I, II, the amorphous state, and DMSO solvate in water were investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the key tasks in pharmaceutics is to solve the problem of poor drug solubility. Solubility is a very important property of drug substances, which determines both the optimal therapeutic doses and probable side effects. It is interesting to note that the analysis of drug compounds’ up-to-date databases shows that biological activity properties and solubility values are inversely proportional. In other words, the substances exhibiting high affinity to the receptors have very low solubility in aqueous solutions. Therefore, it is important to create well-soluble forms in order to strengthen drug compounds’ position on the market. There are a number of approaches to producing such forms. However, one of the most promising approaches is obtaining different polymorphic and solvatomorphic modifications [1], particularly co-crystals and crystallosolvates/crystallohydrates [2, 3]. Beside the application significance, polymorphism comprehensive study is also important fundamentally as it makes it possible to reveal the ratio between the molecular conformational flexibility and the number of potential polymorphic forms. It is also important that the molecule conformational flexibility is not a sufficient condition for polymorph formation, as in many cases nucleation and new phase growth processes are determined by the behavior of the molecular ensemble (both solute and solvent molecules), as well as by the kinetics of solvation shells rebuilding and defects relaxation in newly created crystals. Fundamental processes understanding, assisting a novel form creation, and the ability to rule trajectories of their evolution make it possible to obtain both solvated and unsolvated crystals with the required properties.
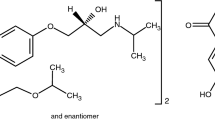
The object of our investigation is rather a well-known drug—bicalutamide (Scheme 1). Being a pharmaceutically active compound, bicalutamide possesses antiandrogenic activity and, therefore, is very useful for prostate cancer treatment. This drug is practically nonsoluble in water, but at the same time appears to have high membrane permeability. In this respect, according to the biopharmaceutical classification of substances (BCS) introduced by Amidon et al. [4], bicalutamide belongs to class II, including the compounds with such a combination of the biopharmaceutical properties as poor water solubility and high membrane permeability. So, being a typical representative of novel drugs, this compound has their basic features: conformational flexibility and very poor solubility. There are three additional reasons for the choosing of bicalutamide as the object of our investigation: Firstly, the crystal structures of two polymorphs, form I and form II, were described in the literature; secondly, the thermophysical and pharmacological properties of the above forms were also studied [5–8]; and thirdly, various methods of the preparation of both polymorphic form I and form II as well as bicalutamide co-crystals were characterized [7–9]. Moreover, it was shown that polymorph II could be derived from the bicalutamide amorphous state [7]. Vega et al. [6] tried to investigate the thermodynamic stability of the two bicalutamide modifications and to compare their properties with those of the amorphous state, both in solutions and solids. Német et al. [10] carefully studied the bicalutamide polymorph transitions caused by the amorphous state mechanical activation. Lindfors et al. [11] carried out the solubility studies of form I and the amorphous state in water by isothermal saturation and light scattering methods.
One of the traditional approaches of obtaining polymorphic modification is growing them through crystallization from the saturated organic solutions under different conditions. This method uses the ability of solvation shells to fix the most thermodynamically stable molecular conformations in solution. After fixing such molecular conformations, create a nuclei of the critical size from which crystals grow. The solvents of different nature can facilitate the forming of various thermodynamically stable molecular conformations of the solutes and, as a consequence, “initiate” different trajectories of polymorphic modification crystallization. There are only several works on systematic investigation of the solvent contribution to the process of polymorphic crystallization (screening) [12–17]. A notable attempt to classify 218 organic solvents on the basis of 24 physicochemical descriptors by means of the principal component analysis was made by Allesø et al. [12]. Miller et al. [15] grew ritonavir polymorphs in 17 solvents, whereas Vrecer et al. [16] investigated the preparation procedure of piroxicam’s various forms in 9 solvents and Jarring et al. [17] analyzed the crystallization of formoterol fumarate in 9 solvents. The polymorphism of carbamazepine was studied by Hilfiker et al. [14] in 11 solvents, while Vrecer et al. [16] expanded the solvents’ list to 67. At the moment, the problem of how to formulate the main rules and algorithms of selecting the solvents for the preparation of different polymorphic forms is still to be solved.
Materials and methods
Compounds and solvents
Bicalutamide (C18H14F4N2O4S, MW 430.37, 99.8 %, racemate, (±)-N-[4-cyano-3-(trifluoromethyl)-phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide) was produced by Xiamen (Fine Chemical Import @ Export Co., LTD) and is polymorphic form I. Dimethylsulfoxide (DMSO, C2H6SO, MW 78.13, ARG) was purchased at Sigma Chemical Co. (USA), lot 129H0068.
Polymorphic form I crystals were prepared by the recrystallization from ethanolic solution. The amorphous state of bicalutamide was obtained by cooling the melt quickly to room temperature. The polycrystals of modification II were received by heating the polymorphic state to 120 °C and then cooling it quickly to room temperature. The bicalutamide DMSO solvate was grown by slow evaporation from saturated DMSO solution at room temperature.
Methods
Solubility determination
The isothermal saturation technique has been used in all the experiments at 25.0 ± 0.1 °C. The solid phase was removed by isothermal filtration (Acrodisc CR syringe filter, PTFE, 0.2 μm pore size) and centrifugation (Biofuge pico). The experimental results are the average values obtained in at least three replicated experiments. The drug molar solubilities were measured spectrophotometrically with an accuracy of 2–2.5 % by means of the protocol described earlier [18].
Sublimation experiments
Sublimation experiments have been carried out by the transpiration method described elsewhere [19]. In brief: A stream of an inert gas passes above the sample at the predetermined slow flow rate under a constant temperature in order to saturate the carrier gas with the vapor of the test substance. The vapor condenses at some point downstream, and the amount of the sublimate and its purity are determined. The vapor pressure over the sample can be calculated based on the amount of the sublimated sample and the volume of the inert gas used.
The equipment was calibrated using benzoic acid. The standard value of the sublimation enthalpy obtained here was \( \Updelta_{\text{sub}} H_{{}}^{0} \) = 90.5 ± 0.3 kJ mol−1. It is in good agreement with the value recommended by IUPAC of \( \Updelta_{\text{sub}} H_{{}}^{0} \) = 89.7 ± 0.5 kJ mol−1 [21]. Saturated vapor pressures were measured 5 times at each temperature with the standard deviation being within 3–5 %. Because the saturated vapor pressure of the investigated compounds is low, it may be assumed that the heat capacity change of the vapor with temperature is so small that it can be neglected. The experimentally determined vapor pressure data may be described in (lnP;1/T) co-ordinates in the following way:
The sublimation enthalpy value is calculated by the Clausius-Clapeyron equation:
whereas the sublimation entropy at a given temperature T was calculated from the following relation:
where \( \Updelta_{\text{sub}} G_{{}}^{\text{T}} \) = −RT·ln(p/p 0), as p 0 is the standard pressure of 105 Pa.
Considering the experimental reasons, sublimation data were obtained at elevated temperatures. However, in comparison with the effusion methods, the current temperatures are much lower so the extrapolation to room conditions becomes easier. In order to further improve the extrapolation of sublimation enthalpy to room conditions, we estimated the heat capacities (\( \Updelta_{\text{cr}} C_{\text{p}}^{298} \) value) of the crystals by means of the additive scheme proposed by Chickos and Acree [24]. Heat capacity was introduced as a correction for the recalculation of the sublimation enthalpy \( \Updelta H_{\text{sub}}^{\text{T}} \) value at 298 K (\( \Updelta H_{\text{sub}}^{298} \) value), according to the following equation:
Solution calorimetric experiments
An ampoule type isoperibolic calorimeter with a 50 cm3 titanium reaction vessel [20] was used to help measure solution and dilution enthalpies. The signal was transferred, and the dependences obtained were processed by the computer. The automated control scheme allowed the temperature to be maintained at the same level with accuracy over 6 × 10−4 K. The temperature and thermal sensitivities of the calorimeter measuring cell were 1 × 10−4 K and 1 × 10−3 J, respectively. The instrumental errors were 0.6–1 %. The accuracy of the weight measurements corresponded to ± 0.01 mg. Since the solution heat effects were low, a correction on the heat of breaking ampoule and evaporation of the solvent in the ampoule free volume (q) was made: q(293.15) = 0.034 J, q(303.15) = −0.018 J, q(318.15) = −0.059 J. The Calorimeter was calibrated using KCl (analysis, grade >99.5 %, from Merck) and water in a wide concentration interval with a number of measurements over 20. The obtained solution enthalpy standard value of 17240 ± 36 J mol−1 was very close to that of 17241 ± 18 J mol−1 [21], recommended by the IUPAC.
DSC experiments
The thermal analysis experiment was carried out using DSC 204 F1 Phoenix differential scanning heat flux calorimeter (NETZSCH, Germany) with a high sensitivity μ-sensor. A sample was heated at 10 K min−1 in argon and cooled by gaseous nitrogen. The DSC temperature calibration was performed against six high-purity substances: cyclohexane (99.96 %), mercury (99.99 + %), biphenyl (99.5 %), indium (99.999 %), tin (99.999 %), and bismuth (99.9995 %). The accuracy of the weighing procedure was ± 0.01 mg.
X-ray diffraction experiments
Single crystal X-ray measurements were carried out using a BRUKER P4 diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The intensity data have been received at 293 K by means of a ω−2θ scanning procedure. The crystal structures have been solved by direct methods and then have been refined through a full-matrix least-squares procedure. Bruker XSCANS [22] was applied for data collection, data reduction, and cell refinement. SHELXS-97 and SHELXL-97 software programs [23] were used to solve and to refine structures, respectively.
Results and discussion
Screening of polymorphic forms
The most popular method of crystalline polymorphic forms’ preparation is their crystallization from the saturated solutions consisting of individual solvents or their mixtures. As it was mentioned before, solvents play an important role in nucleation and growth processes of different polymorphic/solvatomorphic modifications. Nevertheless, the range of solvents applied to screening is often restricted by the solubility of the substances under investigation. Several solvents, methanol, ethanol, 2-propanol, acetonitrile, dimethylsulfoxide, and water, have been selected to perform the screening for the following reasons: (a) bicalutamide is well soluble in all above-mentioned organic solvents; (b) the solvents are well soluble in each other, and this fact allows us to prepare the mixtures in a wide concentration interval; (c) the organic solvents are well soluble in water, used as an “anti-solvent” (i.e., the solvent where the studied compound has a very low solubility value); (d) the current solvents are often used for the screening procedures.
The methods applied to grow bicalutamide crystals were: (a) evaporation from the saturated solution prepared on the basis of individual solvents or their mixtures at room temperature; (b) diffusion of “anti-solvent” into the saturated solution through the vapor phase. The DSC method was applied to identify the obtained phases.
Bicalutamide recrystallization from the individual solvents (with the exception of DMSO) led to the creation of form I. The DMSO crystallosolvate with melting point at 115.3 °C was received by method “a”. Then, the single crystals [bicalutamide + DMSO] (stoichiometry 1:1) were extracted from mother solution, dried up to constant mass, and analyzed by X-ray diffraction method (the crystal structure has been deposited at the Cambridge Crystallographic Data Centre and allocated the deposition number CCDC 879630). It should be mentioned that around 50 different combinations of solvents have been tested and, as a result, only bicalutamide polymorphic modification I has been obtained. Since bicalutamide form II has not been received from the solutions, the traditional method described by Vega et al. [6] has been used.
Sublimation of bicalutamide polymorphic form I
The temperature dependence of saturated vapor pressure and the thermodynamic functions of the sublimation and fusion processes are summarized in Table 1.
Thermochemistry of dissolution processes of bicalutamide polymorphic forms I and II, amorphous state, and DMSO crystallosolvate
In order to estimate the phase transition enthalpies of different bicalutamide polymorphic modifications, solution calorimetry procedure was applied. Such an experimental approach is based on obtaining the solution enthalpies and \( \Updelta_{\text{sol}} H_{{}}^{{\text{m}},298} \) values of various polymorphic forms in the same solvent under the identical experimental conditions. The nature of a solvent does not influence phase transition enthalpy. The main criteria of solvent choice are: (a) good solubility of the compounds under investigation and (b) a high value of the solubility heat effect. It should be noted that beside the two crystal polymorphic modifications, we have investigated the amorphous state of bicalutamide as well. The amorphous state was prepared by rapid cooling of the melt received by heating the initial form I from the temperature of 10 K above the melting point to room temperature at the strictly fixed cooling rate (20 K min−1). As a result, the homogeneous bicalutamide amorphous state has been obtained and the exothermic effects, corresponding to the crystallization of polymorphic phase II, were reproducible within the experimental errors. The monotropic properties of the studied polymorphic modifications have been proved by the DSC experiments.
DMSO has been selected as a solvent for the calorimetric experiments for the following reasons. Firstly, the solvent meets the above-mentioned requirements for obtaining polymorphic modifications; and secondly, the proposed design of the experiment makes it possible to estimate the difference between the crystal lattice energies of the crystallosolvate [bicalutamide + DMSO] and those of the unsolvated phase.
The solution enthalpy values of the two polymorphic forms of bicalutamide, amorphous state, and DMSO crystallosolvate in DMSO at 298 K are summarized in Table 2. It is interesting to note that the solubility process of the amorphous form is exothermal. It testifies that the solvation enthalpy of the drug molecule interaction with DMSO exceeds the enthalpy of the interaction between the bicalutamide molecules in the amorphous state.
In the general way, the phase transition enthalpy \( \Updelta_{\text{tr}} H^{298} \) can be described as the difference between the solubility enthalpies of various polymorphic forms in the same solvent. Thus, for the two monotropic polymorphic forms of bicalutamide:
and \( \Updelta_{\text{tr}} H^{298} ({\text{I}} \to {\text{II}}) \) = 5.5 ± 0.5 kJ mol−1.
The use of this approach makes it possible to estimate the phase enthalpies of the transition from forms I and II to the amorphous state: \( \Updelta_{\text{tr}} H^{298} ({\text{I}} \to A) \) = 24.1 ± 0.6 kJ mol−1; \( \Updelta_{\text{tr}} H^{298} ({\text{II}} \to A) \) = 18.6 ± 0.5 kJ mol−1.
It is interesting to compare the \( \Updelta_{\text{tr}} H^{298} ({\text{I}} \to {\text{II}}) \) value with the analogous one calculated from the fusion enthalpies: (\( \Updelta_{\text{fus}} H^{\text{Tm}} ({\text{I}}) \)=49.5 kJ mol−1; \( T_{\text{m}} \)(I) = 192.4 ± 0.2 °C; \( \Updelta_{\text{fus}} H^{\text{Tm}} ({\text{II}}) \) = 43.4 kJ mol−1; \( T_{\text{m}} \)(II) = 189.2 ± 0.2 °C);
To estimate the phase transition enthalpy at 298 K, we assumed that the difference between the heat capacities of two above-mentioned forms can be approximated by the difference between their fusion entropies:
In this case, \( \Updelta_{\text{tr}} H^{298} ({\text{I}} \to {\text{II}}) \) can be described as:
It is not difficult to see that the value calculated by the DSC experimental data is slightly higher than that received by the solution calorimetry measurements. However, the determination of accuracy of the two monotropic forms’ phase transition enthalpy obtained by the solution calorimetry experiments is higher than the analogous value acquired by the DSC technique.
We can also compare the phase transition enthalpy \( \Updelta_{\text{tr}} H_{{}}^{298} ({\text{II}} \to A) \) (the crystallization of form II from the amorphous state) with the DSC data: \( \Updelta_{\text{tr}} H_{{}}^{\text{Tcr}} (A \to {\text{II}}) \) = −19.0 kJ mol−1. This value coincides (in absolute scale) with one obtained by solution calorimetry within the experimental error: \( \Updelta_{\text{tr}} H^{298} ({\text{II}} \to A) \) = 18.6 ± 0.5 kJ mol−1.
If the sublimation enthalpy of one of the modifications, \( \Updelta_{\text{sub}} H^{0.298} ({\text{I}}) \), is known, we can estimate the value \( \Updelta_{\text{sub}} H^{0.298} ({\text{II}}) \) of the other one as:
The difference between the crystal lattices of the crystallosolvate (S) and the unsolvated phase of bicalutamide (US) can be estimated by the following equations:
where guest is the guest molecule of the solvate (DMSO); the solvent is DMSO; n is the number of pure solvent molecules; m is the number of the solvent molecules in the solvation shell of bicalutamide in solution; l is the number of the solvent molecules in the solvation shell of the guest molecule in solution.
The crystal lattice energy of the crystallosolvate is 12.4 kJ mol−1 higher than that of the unsolvated bicalutamide (form I). This stabilization of the crystal lattice energy can be connected with the additional host–guest and guest–guest interactions of the crystallosolvate in contrast to host–host ones of pure bicalutamide. The relationship between the crystal lattice energies of the considered forms is presented in Scheme 2, where the lowest energy corresponds to the amorphous state, whereas the highest—to [bicalutamide + DMSO], for visualization.
It should be mentioned that polymorphic forms I and II are more stable than [bicalutamide + DMSO], which quickly loses solvent at room temperature. Thus, the crystallosolvate crystal lattice energy increase is accompanied by the entropic term decrease, leading to an essential growth in Gibbs energy. For example, the comparison of the fusion entropy alterations for the two polymorphic forms and the crystallosolvate gives the following results: forms I and II—106 and 93 J mol−1K−1, respectively; crystallosolvate—\( \Updelta_{\text{fus}} S_{{}}^{\text{T}} ({\text{bicalutamide}} + {\text{DMSO}}) = \Updelta_{\text{fus}} H_{{}}^{\text{T}} /T_{\text{fus}} \)= 75 J mol−1 K−1. With reference to \( \Updelta_{\text{fus}} S_{{}}^{\text{T}} \) values, the entropy changes under the phase transition of the crystallosolvate are 1.3–1.4 times lower than those of the unsolvated forms.
Thermodynamic and kinetic characteristics of solubility processes of bicalutamide forms I and II, amorphous and DMSO crystallosolvate
In order to compare thermodynamic stability of the two bicalutamide polymorphic forms, the solubility kinetic dependencies in water have been plotted. The prepared forms have been placed in vials with water, and the solubility values have been measured in a certain time interval. Every measurement has been conducted in at least three different vials to obtain the average value. The dispersion did not exceed 4 %. The results of the experiments are presented in Fig. 1.
It is evident that in 10 h, as usual, the solubility reaches the saturation (“plateau” on a curve) and this fact corresponds to the achievement of thermodynamic equilibrium between solute and bottom phase. Due to instability of form II, 110 h later, we observed the following transformation: (II → I). The transformation process goes rather slowly due to bicalutamide’s low solubility. The amorphous phase is unstable as well and transforms to the stable one (form I). The solubility kinetics of [bicalutamide + DMSO] in water is essentially different from the same process of the unsolvated form: 4.5 h later, the bicalutamide concentration in the aqueous solution reaches the maximal value, which leads to a gradual concentration decrease due to the slow destruction of the crystallosolvate and nucleation of bicalutamide form I. The maximal concentration, which was reached under [bicalutamide + DMSO] dissolution, was 3.3 times higher than that of bicalutamide form I during the same time. Figure 1 shows that the solubility curve of [bicalutamide + DMSO] intersects the analogous curves for the amorphous form and form II in 43 and 67 h, respectively. Thus, the slow kinetics of the crystallosolvate breaking gives an opportunity to keep the raised concentration of bicalutamide (in comparison with form I) in the aqueous solution for a long time.
The presented kinetic dependences allow estimating the solubility ratio of the studied polymorphic forms: \( X_{2}^{298} ({\text{II}})/X_{2}^{298} ({\text{I}}) = 2. 4 5 \). It should be mentioned that this value is in good agreement with the analogous value obtained by Vega et al. [7], corresponding to 2.4. It is interesting to note that the solubility value of modification I—1.38 × 10−7 which has been received in this study does not differ much from the value of 1.1 × 10−7 measured by Lindfors et al. [11] by the light scattering technique.
On the basis of the obtained experimental data, the description of the thermodynamic characteristics of the polymorphic modifications have been made:
So, while the bicalutamide phase is being transformed from form I to form II, the entropy increases by 11 J mol−1 K−1. The thermodynamic characteristics of the crystal polymorphic modifications’ phase transitions to the amorphous state can be estimated in a similar way: \( \Updelta_{\text{tr}} S^{298} ({\text{I}} \to A) \) = 73 ± 6 J mol−1 K−1; \( \Updelta_{\text{tr}} S^{298} ({\text{II}} \to A) \) = 62 ± 3 J mol−1 K−1.
Conclusions
The screening of the bicalutamide solutions in the individual organic solvents and their mixtures has been carried out to obtain novel polymorphic and solvatomorphic bicalutamide modifications by means of crystallization. The tests have resulted in creating a new, previously unknown DMSO crystallosolvate, the crystal structure of which has been solved by X-ray method. In all other cases, recrystallization led to creation of form I. The temperature dependence of saturated vapor pressure of polymorphic modification I has been obtained by the transpiration method and the sublimation thermodynamic characteristics have been calculated: \( \Updelta_{\text{sub}} G^{298} \) = 63.7 kJ mol−1; \( \Updelta_{\text{sub}} H^{298} \) = 124.7 ± 0.6 kJ mol−1; \( \Updelta_{\text{sub}} S^{298} \) = 205 ± 5 J mol−1 K−1. The phase transition enthalpies have been determined by the solution calorimetry technique at 298 K: \( \Updelta_{\text{tr}} H^{298} ({\text{I}} \to {\text{II}}) \) = 5.5 ± 0.5; \( \Updelta_{\text{tr}} H^{298} ({\text{I}} \to A) \) = 24.1 ± 0.6; \( \Updelta_{\text{tr}} H^{298} ({\text{II}} \to A) \) = 18.6 ± 0.6 kJ mol−1. The crystallosolvate crystal lattice energy exceeds that of form I by 12.4 kJ mol−1. The solubility kinetic curves of the two polymorphic forms and the amorphous state in water at 298 K have been studied. The following solubility ratios have been obtained: for the polymorphic forms—\( X_{2}^{298} ({\text{II}})/ \)/\( X_{2}^{298} ({\text{I}}) \) = 2.45; for the amorphous state and form I—\( X_{2}^{298} (A) \)/\( X_{2}^{298} ({\text{I}}) \) = 2.63. The maximal bicalutamide concentration reached during the [bicalutamide + DMSO] crystallosolvate dissolution is 3.3 times higher than that of form I. During the phase transition I → II, the entropy increases by 11 J mol−1 K−1, whereas for the polymorphic forms → the amorphous state transition, the results are: \( \Updelta_{\text{tr}} S^{298} ({\text{I}} \to A) \) = 73 ± 6 J mol−1 K−1; \( \Updelta_{\text{tr}} S^{298} ({\text{II}} \to A) \) = 62 ± 3 J mol−1 K−1.
References
Brittain HG. Polymorphism and Solvatomorphism. J Pharm Sci. 2007;96:705–28.
Pudipeddi M, Serajuddin ATM. Trends in solubility of polymorphs. J Pharm Sci. 2005;94:929–39.
Griesser UJ. The importance of solvates. In: Hilfiker R, editor. Polymorphism: in the pharmaceutical industry. Germany: Wiley; 2006. p. 211–33.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
Hu X-R, Gu J-M. N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenylsulfonyl)-2-hydroxy-2-methylpropionamide. Acta Cryst. 2005;E61:o3897–8.
Vega DR, Polla G, Martinez A, Mendioroz E, Reinoso M. Conformational polymorphism in bicalutamide. Int J Pharm. 2007;328:112–8.
Westheim JH. Bicalutamide forms, US 2004063782 (2004).
WO A1 Patent 2004/029201 A1 (April 8 2004).
WO A2 Patent 2004/074350 A2 (Sep 2, 2004).
Német Z, Sztatisz J, Demeter A. Polymorph transitions of bicalutamide: a remarkable example of mechanical activation. J Pharm Sci. 2008;97:3222–32.
Lindfors L, Forssen S, Skantze P, Skantze U, Zackrisson A, Olsson U. Amorphous drug nanosuspensions. 2. Experimental determination of bulk monomer concentrations. Langmuir. 2006;22:911–6.
Allesø M, Berg FVD, Cornett C, Jørgensen FS, Halling-Sørensen B, Diego HLD, Hovgaard L, Aaltonen J, Rantanen J. Solvent diversity in polymorph screening. J Pharm Sci. 2008;97:2145–59.
Florence AJ, Johnston A, Price SL, Nowell H, Kennedy AR, Shankland N. An automated parallel crystallisation search for predicted crystal structures and packing motifs of carbamazepine. J Pharm Sci. 2006;95:1918–30.
Hilfiker R, Berghausen J, Blatter F, Burkhard A, De Paul SM, Freiermuth B, Geoffroy A, Hofmeier U, Marcolli C, Siebenhaar B, Szelagiewicz M, Vit A, von Raumer M. Polymorphism—integrated approach from high-throughput screening to crystallization optimization. J Therm Anal Calorim. 2003;73:429–40.
Miller JM, Collman BM, Greene LR, Grant DJW, Blackburn AC. Identifying the stable polymorph early in the drug discovery—development process. Pharm Dev Technol. 2005;10:291–7.
Vrecer F, Vrbinc M, Meden A. Characterization of piroxicam crystal modifications. Int J Pharm. 2003;256:3–15.
Jarring K, Larsson T, Stensland B, Ymen I. Thermodynamic stability and crystal structures for polymorphs and solvates of formoterol fumarate. J Pharm Sci. 2006;95:1144–61.
Perlovich GL, Bauer-Brandl A. Solvation of drugs as a key for understanding partitioning and passive transport exemplified by NSAIDs. Curr Drug Deliv. 2004;1:213–26.
Zielenkiewicz W, Perlovich GL, Wszelaka-Rylik M. The vapour pressure and the enthalpy of sublimation: determination by inert gas flow method. J Therm Anal Calorim. 1999;57:225–34.
Kinchin AN, Kolker AM, Krestov GA. Calorimetric apparatus with thermostatic shell without liquid-based for measurements of solution heats at low temperatures. Russ J Phys Chem. 1986;60:782–3.
Cox JD, Pilcher G. Thermochemistry of organic and organometallic compounds. London: Academic Press; 1970.
Bruker. XSCANS. Bruker AXS, Inc. Madison, Wisconsin, USA, 1997.
Sheldrick GM. SHELXL97 and SHELXS97. Germany: University of Göttingen; 1997.
Chickos JS, Acree WE. Enthalpies of sublimation of organic and organometallic compounds, 1910–2001. J Phys Chem Ref Data. 2002;31:537–698.
Acknowledgements
This work was supported by the Federal Agency for Science and Innovations (N 02.740.11.0857)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perlovich, G.L., Blokhina, S.V., Manin, N.G. et al. Polymorphism and solvatomorphism of bicalutamide. J Therm Anal Calorim 111, 655–662 (2013). https://doi.org/10.1007/s10973-012-2540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2540-y