Abstract
The enthalpies of dissolution of dihydrazidinium bis(tetrazolyl)amine (DHBTA) in water and N-methyl pyrrolidone (NMP) were measured using a RD496-2000 Calvet microcalorimeter at 298.15 K under atmospheric pressure, respectively. Empirical formulae for the calculation of the enthalpy of dissolution (Δdiss H), relative partial molar enthalpy (Δdiss H partial) and relative apparent molar enthalpy (Δdiss H apparent) were obtained from the experimental data of the dissolution processes of DHBTA in water and NMP. Furthermore, the corresponding kinetic equations describing the two dissolution processes were dα/dt = 10−2.70(1 − α)0.82 for the dissolution of DHBTA in water, and dα/dt = 10−2.47(1 − α)0.66 for the dissolution of DHBTA in NMP, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High nitrogen heterocyclic compounds are one of the developing fields in energetic material research because of their positive enthalpy of formation, high thermal stability and low sensitivity to impact and friction. Moreover, the high nitrogen content is able to increase the density and to easily obtain an oxygen balance; the main combustion (decomposition) product is nitrogen. So, high nitrogen heterocyclic compounds are being considered as green energetic materials, which are used in solid propellants, explosives and civil combustible-gas generators [1–6].
Tetrazole has the highest nitrogen content in single heterocyclic compounds. Tetrazole is also the precursor to synthesize other high nitrogen heterocyclic compounds, such as 5-aminotetrazole, azotetrazole and N, N′-bis[1(2)H-tetrazol-5-yl]-amine (BTA). Tetrazole of BTA bears acidity, its isoelectronic character and symmetry (to a degree) of the anion, and by analogy, one might expect interaction of the tetrazolate anion with base containing nitrogen, such as ammonium salt and hydrazium salt. Dihydrazidinium bis(tetrazolyl)amine (DHBTA) has been prepared, and the structure of DHBTA is shown in Scheme 1. It is shown that DHBTA has high heat of formation, large gas production, good thermal stability and high reaction heat, and it has possible application as gas generant, low signature propellants, low-smoke or non-smoke pyrotechnics and high performance explosives [7–11].
In the present study, thermochemical properties of its solution have been studied for the first time. The aim of this work is to study the dissolution properties of DHBTA in water and N-methyl pyrrolidone (NMP). The kinetic equations of the two dissolution processes are obtained, respectively, which provide valuable information for its applications in the future.
Experimental
Materials
DHBTA used in the experiments was prepared and purified by Beijing Institute of Technology, and had a purity of more than 99.4 %. The sample was conserved under vacuum. NMP (ρ = 1.029 − 1.035 g cm−3) used as solvent was of analysis reagent grade, and their purities were higher than 99.5 %. The water used in the experiments was deionized with an electrical conductivity of 0.8 × 10−4 − 1.2 × 10−4 S m−1 and obtained by purification two times via a sub-boiling distillation device.
Equipment and conditions
A RD496-2000 Calvet Microcalorimeter (China Academy of Engineering Physics Mianyang CEAP Thermal Analysis Instrument Company) was used to measure the dissolution heat. The standard molar enthalpy of the dissolution of KCl (spectrum purity) in distilled water measured by a RD496-2000 Calvet microcalorimeter at 298.15 K was (17.234 ± 0.041) kJ mol−1, and the relative error was less than 0.04 % compared with the literature value (17.241 ± 0.018) kJ mol−1 [12]. This showed that the device for measuring the enthalpy used in this work was reliable. The enthalpies of dissolution were measured at (298.15 ± 0.005) K.
Results and discussion
Thermochemical behaviors of the dissolution of DHBTA in water and NMP
The proper molar sample of DHBTA was, respectively, dissolved in water and NMP at 298.15 K in order to form solution. The molar enthalpy of the dissolution (Δdiss H) was detected on a RD496-2000 Calvet microcalorimeter [13, 14]. Each process was repeated three times to ensure the precision of the data [15–17]. The dissolution of DHBTA in water and NMP was all endothermic processes. The thermochemical data obtained, Δdiss H, b (the molality of DHBTA), Δdiss H partial (the relative partial molar enthalpy of dissolution) and Δdiss H apparent (the relative apparent molar enthalpy of dissolution), are listed in Tables 1 and 2.
With the help of the values of b and Δdiss H in Table 1, the empirical formula of enthalpy for the dissolution processes of DHBTA in water describing the b versus Δdiss H relation is obtained as:
The empirical formulae of relative apparent molar enthalpy (Δdiss H apparent), relative partial molar enthalpy (Δdiss H partial) and differential molar enthalpy (Δdif H 1,2) calculated by Eq. 1 are, respectively.
According to the values of b and Δdiss H in Table 2, the empirical formula of enthalpy for the dissolution processes of DHBTA in NMP describing the b versus Δdiss H relation is also obtained as:
The empirical formulae of relative apparent molar enthalpy (Δdiss H apparent), relative partial molar enthalpy (Δdiss H partial) and differential molar enthalpy (Δdif H 1,2) calculated by Eq. 5 are, respectively.
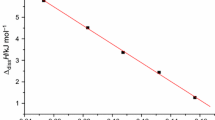
From Tables 1 and 2, we can see that the molality of the solution b can affect the values of Δdiss H, the calculated Δdiss H apparent and Δdiss H partial. In Table 1, the values of Δdiss H decrease with values of b increasing during the dissolution processes of DHBTA in water. One can also find that the relationships between Δdiss H and b 1/2 for the dissolution processes of DHBTA in water is linear equation from Fig. 1, and at the same time, the relationship between Δdiss H and b 1/2 for the dissolution processes of DHBTA in NMP is quadratic equation from Fig. 2.
The kinetics of dissolution process of DHBTA in water or NMP
Equations 9 and 10 are chosen as the model function describing the dissolution of DHBTA in water or NMP [18–20].
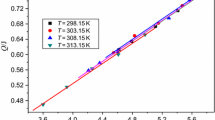
By putting the original data in Tables 3 and 4, −(dH/dt)i, (H/H 0)i, H ∞, i = 1, 2, …, L, into Eq. 10, the values of n and ln k are obtained and listed in Table 5, where n is the reaction order and k the reaction rate constant.
Substituting the values of n and k in Table 5 into Eq. 9, we can get
for dissolution process of DHBTA in water, and
for dissolution process of DHBTA in NMP.
Conclusions
The dissolution processes of DHBTA in water and NMP were investigated by RD496-2000 Calvet microcalorimeter at 298.15 K, respectively. The relationship between Δdiss H and b 1/2 of DHBTA dissolved in water is linear equation, and the relationship between Δdiss H and b 1/2 of DHBTA dissolved in NMP is quadratic equation.
The expressions describing values of Δdiss H, Δdiss H apparent, Δdiss H partial and Δdif H 1, 2 versus the molality (b) for DHBTA dissolved in water are Δdiss H = −126.68 − 1,348.48b + 819.75b 1/2, Δdiss H apparent = − 1,348.48b + 819.75b 1/2, Δdiss H partial = −2,696.96b + 1,229.63b 1/2, Δdif H 1,2 = − 1,348.48(b 2 − b 1) + 819.75 (b 1/22 − b 1/21 ). The expressions describing values of Δdiss H, Δdiss H apparent, Δdiss H partial and Δdif H 1,2 versus the molality (b) of DHBTA in NMP are Δdiss H = − 33.69 − 1,021.38b + 340.88b 1/2, Δdiss H apparent = − 1,021.38b + 340.88b 1/2, Δdiss H partial = − 2,042.75b + 511.31b 1/2, Δdif H 1,2 = − 1,021.38 (b 2 − b 1) + 340.88 (b 1/22 − b 1/21 ).
The kinetics equations of dissolution processes for DHBTA are dα/dt = 10−2.70(1 − α)0.82 in water, and dα/dt = 10−2.47(1 − α)0.66 in NMP.
References
Manfred F, Juan C, Thomas MK. BTA copper complexes. J Sci Chin Chem. 2005;44:8044–52.
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP. Advances in science and technology of modern energeic materials: an overview. J Hazard Mater. 2008;151:289–95.
Talawar MB, Sivabalan R, Mukundan T, Muthurajan H, Sikder AK, Gandhe BR, Rao AS. Environmentally compatible next generation green energetic materials(GEMs). J Hazard Mater. 2009;161:589–94.
Latypov NV, Bergman J, Langlet A, Wellmar U, Bemm U. Synthesis and reactions of 1,1-diamino-2,2-dinitroethylene. Tetrahedron. 1998;54:11525–30.
Jiao BJ, Yan ZJ, Wang J, Jin X. Synthesis and characterization of calium/strontium complex with N, N′-bis[1(2)H-tetrozol-5-yl]-amine and phenanthroline. J Appl Chem Ind. 2009;38:1750–2.
Guo Y, Gao HX, Twamley B, Shreeve JM. Energetic nitrogen rich salts of N, N′-bis[1(2)H-tetrozol-5-yl]-amine. Adv Mater. 2007;19:2884–8.
Karaghiosoff K, Klapotke TM, Carles MS. Nitrogen-rich compounds in pyrotechnics: alkaline earth metal salts of 5,5′hydrazine-1,2-diylbis(1H-tetrazole). Eur J Inorg Chem. 2009;33:238-50.
Klapotke TM, Carles MS. Bistetrazoles: nitrogen-rich high-performing insensitive energetic compound. Chem Mater. 2008;20:3629–37.
Hiskey MA, Goldman N, Stine J. High-nitrogen energetic materials derived from azotetrazolate. J Energ Mater. 1998;16:119–27.
Tan BS, Huang M, Huang H, Long XP, Li JS, Nie F, Huang JL. Theoretical investigation of several 1,2,3,4-tetrazine-based high-energy compounds. Propellants Explos Pyrotech. 2013;38:372–5.
Mendenhall IV, Taylor RD. Gas generant compositions containing stabilizer. USP 5472535, 1995.
Kilday M. The enthalpy of solution of SRM 1655 (KCl) in H2O. J Res Natl Bur Stand. 1980;85:467–81.
Zhao FQ, Xue L, Xing XL, Hu RZ, Zhou ZM, Gao HX, Yi JH, Xu SY, Pei Q. Thermochemical properties and thermokinetic behavior of energetic triazole ionic salts. Sci China Chem. 2011;54:461–73.
Li N, Zhao FQ, Luo Y, Gao HX, Xiao LB, Hu RZ, Ju RH. Dissolution properties of N-guanylurea dinitramide (GUDN) in dimethyl sulfoxide and N-methyl pyrrolidone. J Therm Anal Calorim. 2014;115:869–73.
Li N, Zhao FQ, Gao HX, Hu RZ, Xiao LB, Yao EG, Huang XP, Chang P. Thermokinetics of the formation reactions of metal (Li, Na, Pb and Cu) salts of 3-nitro-1,2,4-triazol-5-one. Acta Phys Chim Sin. 2013;29:2101–6.
Xue L, Zhao FQ, Xing XL, Zhou ZM, Wang K, Gao HX, Yi JH, Xu SY, Hu RZ. Dissolution properties of 1,2,4-triazole nitrate in N-methyl pyrrolidone. J Chem Eng Data. 2011;56:259–62.
Xing XL, Xue L, Zhao FQ, Yi JH, Gao HX, Xu SY, Pei Q, Hao HX, Hu RZ. Dissolution properties of the CL-20 in ethyl acetate and acetone. J Therm Anal Calorim. 2010;99:703–7.
Xiao LB, Xing XL, Zhao FQ, Xu KZ, Yao EG, Tan Y, Hao HX. Dissolution properties of 2-(dinitromethylene)-5-methyl-1,3-diazacyclo-rentane in dimethyl sulfoxide and N-methyl pyrrolidone. Chem Res Chin Univ. 2012;28:743–6.
Gao HX, Zhao FQ, Hu RZ. Differential and integral isoconversional non-linear methods and their application to energetic materials. Chin J Chem. 2008;26:973–8.
Hu RZ, Gao SL, Zhao FQ, Zhang TL, Zhang JJ. Thermal Analysis Kinetics. 2nd ed. Beijing: Science; 2008.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (21173163).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, F.Q., Li, N., Yao, E.G. et al. Thermochemical properties of dihydrazidinium bis(tetrazolyl)amine in water and N-methyl pyrrolidone. J Therm Anal Calorim 120, 1853–1857 (2015). https://doi.org/10.1007/s10973-015-4517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4517-0