Abstract
The enthalpies of dissolution of N-guanylurea dinitramide (GUDN) in dimethyl sulfoxide (DMSO) and N-methyl-2-pyrrolidone (NMP) were measured using an RD496-2000 Calvet microcalorimeter at 298.15 K under atmospheric pressure, respectively. Empirical formulae for the calculation of the enthalpy of dissolution (Δdiss H), relative partial molar enthalpy (Δdiss H partial), and relative apparent molar enthalpy (Δdiss H apparent) were obtained from the experimental data of the dissolution processes of GUDN in DMSO and NMP. Furthermore, the corresponding kinetic equations describing the two dissolution processes were dα/dt = 10−3.39(1 − α)0.70 for the dissolution of GUDN in DMSO, and dα/dt = 10−4.06(1 − α)1.11 for the dissolution of GUDN in NMP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
N-guanylurea dinitramide (GUDN) is a new high-energy and low sensitivity oxidizer, and it is a stable salt of dinitramide. In contrast to the more well-known dinitramide, ADN, it does not melt and it is significantly more thermally stable [1, 2]. GUDN has been demonstrated to be an extremely insensitive energetic molecule. It does not react in fall hammer and friction tests [3]. GUDN as a main ingredient has been used in insensitive gun propellants [4, 5]. It has also been used as a gas generation component in compositions for air bags due to its excellent burning characteristics [6–9]. It has been demonstrated that it also has a potential as an high explosive [10].
Quite a lot of properties of GUDN have been measured and studied, including density, detonation velocity, detonation heat, reactivity, thermal stability, compatibility, sensitivity, and so on [1, 11]. But the particular properties of its solution have rarely been reported. In the present study, an RD496-2000 Calvet microcalorimeter is used to measure the enthalpies of dissolution of GUDN in dimethyl sulfoxide (DMSO) and N-methyl pyrrolidone (NMP). The relationships for the measured enthalpies of dissolution and amounts of substance are studied, and the relative partial molar enthalpy (Δdiss H partial), and the relative apparent molar enthalpy (Δdiss H apparent) at 298.15 K are obtained. The kinetic equations of the two dissolution processes are also obtained, respectively, which will be useful for purification of GUDN in production, and can provide basic guidance for its applications.
Experimental
Materials
GUDN used in the experiment was prepared and purified by Xi’an Modern Chemistry Research Institute, and had a purity of more than 99.4 %. Both NMP (ρ = 1.029–1.035 g cm3) and DMSO (ρ = 1.098–1.102 g cm3) used as solvents were of analysis reagent grade, and their purities were higher than 99.5 %. Deionized water with an electrical conductivity of 0.8 × 10−4–1.2 × 10−4 S m−1 used in the experiments was obtained by purification two times via a sub-boiling distillation device.
Equipment and conditions
All the measurement experiments were performed on an RD496-2000 Calvet microcalorimeter (Mianyang CP Thermal Analysis Instrument Co., Ltd.). The standard molar enthalpy of the dissolution of KCl (spectrum purity) in distilled water measured by RD496-2000 Calvet microcalorimeter at 298.15 K was 17.234 ± 0.041 kJ mol−1, and the relative error was less than 0.04 % compared with the literature value 17.241 ± 0.018 kJ mol−1 [12]. This showed that the device for measuring the enthalpy used in this work was reliable. The enthalpies of dissolution were measured at 298.15 ± 0.005 K.
Results and discussion
Thermochemical behaviors of the dissolution of GUDN in DMSO and NMP
The proper molar sample of GUDN was dissolved in DMSO and NMP at 298.15 K to form solutions. The molar enthalpy of the dissolution (Δdiss H) was detected on an RD496-2000 Calvet microcalorimeter [13–16]. Each process was repeated three times to insure the precision of the data [17–19]. The dissolution of GUDN in DMSO was an endothermic process, but the dissolution in NMP was an exothermic process. The thermochemical data obtained, Δdiss H, b (the molality of GUDN), Δdiss H partial (the relative partial molar enthalpy of dissolution), and Δdiss H apparent (the relative apparent molar enthalpy of dissolution) were listed in Tables 1 and 2.
With the help of the values of b and Δdiss H in Table 1, the empirical formula of enthalpy for the dissolution processes of GUDN in DMSO describing the b versus Δdiss H relation is obtained as:
The empirical formula of relative molar enthalpy and relative partial molar enthalpy calculated by Eq. (1) are, respectively,
According to the values of b and Δdiss H in Table 2, the empirical formula of enthalpy for the dissolution processes of GUDN in NMP describing the b versus Δdiss H relation is also obtained as:
The empirical formula of relative molar enthalpy and relative partial molar enthalpy calculated by Eq. (4) are, respectively,
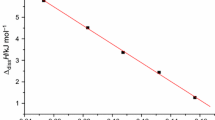
From Tables 1 and 2, we can see that the molality of the solution b can affect the values of Δdiss H, Δdiss H apparent and Δdiss H partial were calculated. We can also find the two curves for the dissolution processes of GUDN in DMSO or NMP are similar to each other, and the relationships between Δdiss H and b 1/2 are quadratic equation from Figs. 1 and 2.
The kinetics of dissolution process of GUDN in DMSO or NMP.
Equations (7) and (8) are chosen as the model functions [20–22] for describing the dissolution of GUDN in DMSO or NMP.
Combining Eqs. (7) and (8), yields
Substituting α = H/H ∞ into the Eq. (9), we get
In these equations, α is conversion degree, f(α) is the kinetic model function, H represents the enthalpy at time of t, i is any time during the process, H ∞ is the enthalpy of the whole process, k is the rate of GUDN in DMSO or NMP, n is the reaction order, and L is counting number.
The data needed for Eq. (10) are summarized in Tables 3 and 4.
Substituting the original data in Tables 3 and 4, −(dH/dt)i, (H/H ∞)i, H ∞, i = 1, 2,…, L, into the kinetic Eq. (8) yields the values of n and lnk that are listed in Table 5.
Substituting the values of n and k in Table 5 into Eq. (9), we can get
for dissolution process of GUDN in DMSO, and
for dissolution process of GUDN in NMP.
Conclusions
-
(1)
The dissolution process of GUDN in DMSO and NMP were investigated by RD496-2000 Calvet microcalorimeter at 298.15 K. The relationship between Δdiss H and b 1/2 of GUDN dissolved in DMSO and NMP are quadratic equation.
-
(2)
The expressions describing values of Δdiss H, Δdiss H apparent, and Δdiss H partial versus the molality (b) of GUDN in DMSO are Δdiss H = 17.00 − 232.47b 1/2 + 704.08b, Δdiss H apparent = −232.47b 1/2 + 704.08b, Δdiss H partial = −348.71b 1/2 + 1,408.16b. The expressions describing values of Δdiss H, Δdiss H apparent and Δdiss H partial versus the concentration (b) of GUDN in NMP are Δdiss H = 111.19 − 1,211.19b 1/2 + 3,485.31b, Δdiss H apparent = −1,211.19b 1/2 + 3,485.31b, Δdiss H partial = −1,816.79b 1/2 + 6,970.62b, respectively.
-
(3)
The kinetics equations of dissolution processes for GUDN are dα/dt = 10−3.39(1 − α)0.70 in DMSO, and dα/dt = 10−4.06(1 − α)1.11 in NMP.
References
Zhao FQ, Chen P, Yuan HA, Gao SL, Hu RZ, Shi QZ. Thermochemical properties and non-isothermal decomposition reaction kinetics of N-guanylurea dinitramide (GUDN). Chin J Chem. 2004;22:136–41.
Venkatachalam S, Santhosh G, Ninan Ninan K. An over view on the synthesis routes and properties of ammonium dinitramide (AND) and other dinitramide salts. Propellants Explos Pyrotech. 2004;29:178–87.
Bemm U. New energetic complexes. Scientific and Technical Aerospace Reports, Virginia, 2002.
Ostmark H, Bemm U, Bergman H. N-guanylurea-dinitramide: a new energetic material with low sensitivity for propellants and explosives applications. Thermochim Acta. 2002;384:253–9.
Charles D. New low-sensitivity modular charge propellant based on GUDN. In: Insensitive munitions and energetic materials technology symposium, Bristol, 2006.
Sjoberg P. Gas-generating material for gas-actuated car safety devices. WO 0040523, 2000.
Sjoberg P. Gas-generating material for gas-actuated car safety devices. USP 20040154711, 2004.
Persson S, Sjoqvist C. Composite gas-generating material for gas-actuated car safety. USP 6764562, 2004.
Persson S, Sjoqvist C. Composite gas-generating material for gas-actuated car safety devices. USP 20040231768, 2004.
Ostmark H, Helte A, Carlsson T. N-guanylurea-dinitramide (FOX-12): a new extremely insensitive energetic material for explosives applications. In: International detonation symposium, Virginia, 2006.
Santhosh G, Tien RPC, Ghee AH. Thermal decomposition kinetics of ammonium dinitramide–guanylurea dinitramide mixture analyzed by isoconversional methods. Thermochim Acta. 2008;480:43–8.
Marthada VK. The enthalpy of solution of SRM 1655 (KCl) in H2O. J Res Natl Bur Stand. 1980;85:467–81.
Xiao LB, Xing XL, Fan XZ, Zhao FQ, Zhou ZM, Huang HF, An T, Hao HX, Pei Q. Thermochemical properties of di(N,N-di(2,4,6-trinitrophenyl)amino)-ethylenediamine in dimethyl sulfoxide and N-methyl pyrrolidone. J Therm Anal Calorim. 2012;110:1431–6.
Xue L, Zhao FQ, Xing XL, Gao HX, Xu SY, Hu RZ. Dissolution properties of 1,3,3-trinitroazetidine (TNAZ) in ethyl acetate and N,N-dimethylformamide. Acta Phys Chim Sin. 2009;25:2413–6.
Xing XL, Xue L, Zhao FQ, Yi JH, Gao HX, Xu SY, Pei Q, Hao HX, Hu RZ. Dissolution properties of the CL-20 in ethyl acetate and acetone. J Therm Anal Calorim. 2010;99:703–7.
Xiao LB, Zhao FQ, Xing XL, Huang HF, Zhou ZM, An T, Pei Q, Tan Y. Dissolution properties of ammonium dipicrylamide in dimethyl sulfoxide and N-methyl pyrrolidone. Thermochim Acta. 2012;546:138–42.
Xing XL, Xue L, Zhao FQ, Gao HX, Hu RZ. Dissolution properties of 1,1-diamino-2,2-dinitroethylene (FOX-7) in dimethyl sulfoxide (DMSO). Thermochim Acta. 2009;32:53–7.
Xiao LB, Xing XL, Zhao FQ, Xu KZ, Yao EG, Tan Y, Hao HX. Dissolution properties of 2-(dinitromethylene)-5-methyl-1,3-diazacyclo-rentane in dimethyl sulfoxide and N-methyl pyrrolidone. Chem Res Chin Univ. 2012;28:743–6.
Gao HX, Zhao FQ, Hu RZ. Differential and integral isoconversional non-linear methods and their application to energetic materials. Chin J Chem. 2008;26:1973–8.
Xue L, Zhao FQ, Xing XL, Zhou ZM, Wang K, Xu SY, Gao HX, Yi JH, Hu RZ. Thermal behaviours of 3,4,5-triamino-1,2,4-triazole dinitramide. J Therm Anal Calorim. 2010;102:145–7.
Blaine RL, Kissinger HE. Homer Kissinger and the Kissinger equation. Thermochim Acta. 2012;540:1–6.
Hu RZ, Gao SL, Zhao FQ, Zhang TL, Zhang JJ. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant No. 21173163) and Technology Foundation of National Defense Key Laboratory of Propellant and Explosive Combustion in China (Grant No.9140C350307110C3506).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., Zhao, F.Q., Luo, Y. et al. Dissolution properties of N-guanylurea dinitramide (GUDN) in dimethyl sulfoxide and N-methyl pyrrolidone. J Therm Anal Calorim 115, 869–873 (2014). https://doi.org/10.1007/s10973-013-3303-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3303-0