Abstract
Having two active peroxide groups, 1,1-bis(tert-butylperoxy)cyclohexane (BTBPC) has a certain degree of thermal instability. It is usually used as an initiator in a chemical process, and therefore, careless operation could result in severe accidents. This study emphasized the runaway reactions of BTBPC 70 mass% (4.5–5.2 mg), the relevant thermokinetic parameters, and the thermal safety parameters. Differential scanning calorimetry was used to evaluate the above-mentioned thermokinetic parameters, using four low heating rates (0.5, 1, 2, and 4 °C min−1) combined with kinetic simulation method. The results indicated that apparent exothermic onset temperature (T o), apparent activation energy (E a), and heat of decomposition (ΔH d) were ca. 118 °C, 156 kJ mol−1, and 1,080 kJ kg−1, respectively. In view of process loss prevention, at the low heating rates of 0.5, 1, 2, and 4 °C min−1, storing BTBPC 70 mass% below 27.27 °C is a more reassuring approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to the low dissociation energy, the oxygen–oxygen in organic peroxides (OPs) easily breaks to form free radicals at relatively low temperatures [1–3]. As a result, they are widely used in the polymer industry. OPs are often used as an initiator to induce the RO∙ free radical in the initiation of a polymerization reaction [4]. They are also recognized for their thermal instability, as they are very sensitive to heating and mechanical shock. The thermal instability of OPs has contributed to many fires, explosions, and thermal runaway incidents worldwide as a result of heat accumulation, the addition of chemical contaminants, and mechanical shock, to name a few [5–7]. Incidents can occur during preparation, manufacturing process, storage, transport, or usage in industrial transformation [8]. If accidental contamination and thermal exposure are not prevented, runaway reactions leading to an accident could be triggered during any of these stages [9].
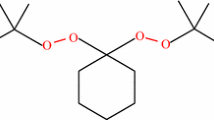
1,1-Bis(tert-butylperoxy)cyclohexane (BTBPC) has a pair of symmetrical oxygen–oxygen peroxide groups as illustrated in Fig. 1. The most important use of the symmetrical diperoxides is as initiators. They form free radicals that can initiate polymer growth from two different sites within the same fragment, which leads to a double-ended polymer. Higher molecular weight polymer can be produced at faster rates than using monofunctional initiators [10]. They are also used as a low temperature hardener agent for fiber reinforced polystyrene.
BTBPC is one of the most commonly used symmetrical bifunctional initiators for the commercial production of polystyrene (PS) and its copolymers. However, little attention has been devoted to this chemical, and its thermokinetic and thermal safety parameters have not been well defined. In 2011, the worldwide amount of PS production and demand was about 16.6 and 16.8 megatons, respectively. In Taiwan, these quantities were 680 and 120 kilotons; surplus PS is exported [11]. OPs will decompose quickly upon heating or under exposure to light; when the heat accumulated is excessive, the aftermath could be a fire or explosion accident [12, 13]. Due to the hazardous consequences of BTBPC decomposition and its extensive use in the industry, it is very important to know its thermokinetic and thermal safety parameters.
In this study, we measured the basic thermokinetic characteristics of BTBPC, and appraised the thermal experimental data of BTBPC from differential scanning calorimetry (DSC) using the calorimeter’s accompanying software. Recognized kinetic equations were applied to calculate apparent activation energy (E a) at four low heating rates (0.5, 1, 2, and 4 °C min−1). Finally, the thermal safety software (TSS) was applied for kinetic simulation. We attempted to obtain more precise parameters for BTBPC thermal decomposition properties using associated DSC software methods and comparing kinetic equations with mathematical calculations and simulations. Based on traditional curve-fitting method and chemical process reactor simulation, we could clarify the reaction type (nth order or autocatalytic reaction) of BTBPC and its thermal hazard properties. These properties include heat of decomposition (ΔH d), apparent activation energy (E a), rate constant (k), time to maximum heating rate under adiabatic conditions (TMRad), total energy release (TER), and time to conversion limit (TCL) [14–17]. These results are very useful to prevent fires, explosions, or any other runaway reactions under chemical processing, storage, and transportation.
Experimental methods
Hazardous sample
Seventy mass percent pure 1,1-bis(tert-butylperoxy)cyclohexane (BTBPC) in iso-paraffin hydrocarbon solvent, the mainly used initiator of PS in Taiwan’s chemical plants, was purchased directly from ACE Chemical Corp., Taiwan. It was stored in a refrigerator at 4 °C to maintain its stability and to keep it away from any unexpected heat exposure. It is a low volatility, yellowish liquid peroxyketal peroxide. Basic physical and chemical properties of pure BTBPC are listed in Table 1 [18].
Calorimetric measurements
A calorimetric temperature-programmed screening study in dynamic modes was completed using a microcalorimeter (Mettler TA8000 system) and an extra pure nitrogen purge. The differential scanning calorimetry, a DSC 821e instrument with a high pressure, gold-plated measuring test crucible (Mettler ME-00026732) [19] was used along with STARe software to obtain thermal curves. Crucibles work as containers for samples during thermal analytical measurements; a high pressure gold-plated steel crucible can withstand relatively high pressure about 15 MPa. The lid is pressed onto the crucible with a pressure of about a ton so that the seal tightens the crucible; a toggle press is used to seal the crucible. These crucibles can only be used for one measurement. The temperature and enthalpy for the DSC device were calibrated with indium and zinc as standards. The standards’ sample masses were selected to be about 6.0 mg with a heating rate of 4 °C min−1. Through these tests, we could validate that the apparent exothermic onset temperature and enthalpy change were located in the range of interest.
DSC is the prevailing thermoanalytical device that is used to detect the temperature difference between the sample and reference. First, the DSC has to be blank stabilized for at least half an hour, then the sample crucible is put onto the heating plate and started. After the test, the crucible mass is measured again, to verify there was no leakage during the experiment. To assess the sensitivity of thermal equilibrium [20], we used four low heating rates (β), including 0.5, 1, 2, and 4 °C min−1, in the dynamic mode of operation with nitrogen (50 mL min−1) as the purge gas. About 4.5–5.2 mg of the sample was used for acquiring the experimental data. The range of temperature rise for each test was to be from 30 to 300 °C.
Kinetic models
When a sample decomposes in the DSC, the reaction proceeds at a rate changing with temperature, and the type and location of the peak vary with the heating rate. Kissinger [21] proposes a possible method for calculating the apparent activation energy (E a), for a simple decomposition reaction regardless of reaction order, by making a number experiments with various heating rates (β) that generate corresponding maximum temperatures (T m), which are the temperatures where the DSC curve exhibits maximum enthalpy. The Kissinger kinetic equation is expressed in Eq. (1) [21].
where T m is the Kelvin temperature corresponding to the maximum enthalpy change in the DSC exotherm at a heating rate (β), R is the gas constant (R = 8.314 J mol−1 K−1), and A is the pre-exponential factor in the Arrhenius equation. We can obtain E a from the slope of the linear plot of ln(β/ \( T_{\text{m}}^{ 2} \)) against 1/T m. There are two sets of temperature data we can use T m to calculate E a obtained from the DSC test. DSC curve displays the true peak temperature (T p), which was detected from DSC. The other is extrapolated peak temperature (T ext), the intersection of the tangent drawn at the point of greatest slope on the right and left leading edge of the exothermic peak with each other, which was extrapolated from the curve of the reaction by STARe software.
Ozawa [22] and Ravi et al. [23] suggest a similar method to Kissinger, which relates logβ to the inverse of maximum temperature (T m). Identically, we can also compute both E a and A for the curing process of BTBPC. The Ozawa kinetic equation is shown in Eq. (2). Utilizing the slope of the ln(β) versus 1/T m, E a value can be similarly obtained too.
We also applied the kinetic simulation model to determine the thermokinetic parameters based on the experimental data of time (s), temperature (oC), and heat power (mW). All the experimental data were processed and evaluated by applying TSS, including TDPro and ForK software, which was developed by ChemInform Saint Petersburg (CISP) Ltd. [16, 24]. The method was thoroughly developed by Kossoy and Akhmetshin for the creation of a thermokinetic model and the algorithms that are adopted [25, 26]. If these experimental data were fitted, the thermokinetic parameters were extracted. Results were obtained using Kissinger’s method, Ozawa’s method, STARe software, and TSS software. Comparison of the results improves the understanding of the thermokinetic parameters and thermal decomposition reaction type of BTBPC.
Thermal safety parameters
OPs are well known, possessing thermal sensitivity hazards in chemical process, storage, and industrial transformation. Avoiding fires, explosions, and runaway reactions is the best engineering practice to prevent environmental pollution, human casualties, and property damages. Precisely determined thermal safety parameters can facilitate engineers in making the right and prudent strategic decisions, when an OP is on the verge of a runaway reaction. In this study, we investigated three important safety parameters, TMRad, TER, and TCL of BTBPC, according to the methodologies proposed by Townsend and Tou [27]. The results of these safety parameter studies provide a basis for preventing BYBPC runaway reactions.
TMRad is the time for a reaction to reach its maximum rate under adiabatic conditions starting from various temperatures. Dependency of this time on the defined initial temperature of BTBPC is assessed from the results of simulations of a reaction excursion. TMRad was proposed by Townsend and Tou, who derived two analytical expressions shown in Eqs. (3) and (4) [27].
where ΔT ad is the adiabatic temperature rise, C p is the specific heat capacity, and ΔH d is the heat of decomposition which we also identified as total energy release (TER). Since ΔH d is negative for an exothermic reaction, C p is positive; therefore, the value of ΔT ad is positive. The major limitation of this method is that the formulas are valid only for a single exothermic peak, nth order reactions. TMRad can be properly determined merely by applying a kinetic-based simulation. We can use the same method, kinetic-based simulation, to determine TER and have adopted it in the present study.
At a constant set temperature, the conversion of a reactant reaches a certain predefined value; the conversion limit. This time, called TCL, is a useful index for appraising the thermal stability of a substance. Taking long-term storage as an example, it can be chosen as the maximum permissible time during which the conversion of the product does not have any significant impact on the product quality. In this study, 10 % was set as the conversion limit value.
Results and discussion
Fundamental thermal analysis
A microcalorimeter (DSC), coupled with its built-in STARe software, was adopted to obtain thermal curves and other thermokinetic parameters from the BTBPC. Four low temperature-programmed heating rates (β) are used in this study, 0.5, 1, 2, and 4 °C min−1. Each sample weighed between 4.5 and 5.2 mg. Figure 2 demonstrates the four thermal curves at the various heating rates. These curves show very clearly that the thermal decomposition has only one single, strong, and smooth exothermic peak. Numeric results are provided in Table 2. Increasing β from 0.5 to 4 °C min−1 resulted in almost the same apparent onset temperature (T o, 123, 112, 116, and 122 °C). However, peak temperature (T p, 125, 132, 139, and 147 °C) and extrapolated peak temperature (T ext, 129, 133, 140, and 148 °C) obviously increased with β. Furthermore, apparent activation energy (E a, ca. 139, 141, 148, and 142 kJ mol−1), heat of decomposition (ΔH d, ca. 1074, 1123, 1090, and 1031 kJ kg−1), and the other thermokinetic parameters, such as pre-exponential factor (k o) and the order of reaction (n), all did not show a significant difference.
Kinetics parameter analysis
Kissinger [21] established a simple expression between the heating rates (β) as a function of E a and T m. By plotting the data of ln(β/\( T_{\text{m}}^{ 2} \)) versus the inverse of T m, we can obtain E a easily by Kissinger equation. Ozawa [16] suggests a similar method to estimate E a from the slope of logβ versus 1/T m. Kissinger and Ozawa equations have been shown as Eqs. (1) and (2) in the above section of experimental and methods. As defined earlier, T p and T ext are two different ways we received from the DSC. They could both deemed to be a T m. Table 3 summarizes these parameters for Kissinger and Ozawa equations.
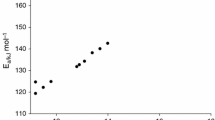
We took T ext as T m, and plotted the data as shown in Fig. 3. According to the slope of Eqs. (1) and (2), we acquired the activation energies: 141.68 kJ mol−1 (Kissinger’s method, R 2 = 0.98) and 141.23 kJ mol−1 (Ozawa’s method, R 2 = 0.98). These results are similar to those from the DSC built-in software STARe on BTBPC (see Table 2). But if we take T p as T m, the apparent activation energy is considerably reduced from 141.68 to 115.02 kJ mol−1 (Kissinger’s method, R 2 = 0.99) and 141.23 to 123.50 kJ mol−1 (Ozawa’s method, R 2 = 0.99). Moreover, these results are remarkably different from STARe software. Therefore, we believe that T ext is more suitable than T p as the real T m of this thermal decomposition reaction.
Kinetic model simulation
However, excluding the DSC built-in software STARe, we could not acquire any information but the apparent activation energy (E a) from Kissinger’s and Ozawa’s equations, such as n, k, and ΔH d. Therefore, we used TSS simultaneously, including TDPro and ForK, to simulate and compare the other thermokinetic parameters mentioned previously. According to all of these data, we could easily confirm those thermokinetic parameters of BTBPC.
There are two possible models of decomposition reaction that we could set in TSS for kinetic analysis: One is the nth order reaction, and the other is autocatalytic reaction [15, 24]. These two models are explained below.
If a single-stage reaction rate is dependent only on its reactant concentration, and not on its products, then we call it as an nth order reaction. The decomposition reaction is expressed below:
A → B reaction of nth order with respect to A
The reaction rate (r i) by this kinetic model may be expressed by Eq. (5):
where k o and E a denote the pre-exponential factor and apparent activation energy of the nth order reaction. R is the gas constant (R = 8.314 J mol−1 K−1), α is the degree of conversion, from 0 to 1, and n is the order of reaction.
If the reaction product itself is the catalyst for that reaction too, this chemical reaction is said to have undergone autocatalysis, or be an autocatalytic reaction. The decomposition reaction is expressed below:
A → B autocatalytic reaction
The reaction rate (r i) by this kinetic model is expressed by Eq. (6):
where k o, E a, R, and α were defined above, z is the autocatalytic constant, n 1, n 2 are the reaction orders for reactant A and product B, respectively [15].
We employed the coefficient of variation (CV), a statistical measurement of the dispersion of data points around the sample mean, to compare the degree of variation from one data series to another. CV represents the ratio of the standard deviation (SD) to the sample mean (\( \overline{X} \)). The lower the CV of the data set, the smaller differences they have. We compared the results of thermokinetic parameters of nth order with those for autocatalytic, which were evaluated from the simulation software, to estimate which was the more probable simulation model of the decomposition reaction of BTBPC and to estimate its thermokinetic parameters. As presented in Table 4, the heat of decomposition (ΔH d) has nothing to do with the type of kinetic model, because they have the same heat value and SD in nth order and autocatalytic reaction. Apart from this, the lower the CV value, the more probable a decomposition reaction is. Table 4 includes the thermokinetic parameters of nth order reaction, such as lnk o, E a, n, ΔH d, and k, and all have lower CV values than the autocatalytic parameters. This indicates the nth order parameters are more precise and closer to their mean value. However, the autocatalytic parameters including n 1, n 2, and z were random and scattered. Since the nth order reaction had smaller CV values than those for the autocatalytic reaction, we have strong evidence that the thermal decomposition reaction of BTBPC is nth order. These values of the thermokinetic parameters were almost the same as we obtained using the built-in software STARe of DSC, as demonstrated in Table 2.
The values of thermokinetic parameters, such as lnk o, E a, n, and ΔH d, have no significant correlation with heating rates in the nth order. As listed in Table 4, the CV of all thermokinetic parameters was less than 5 %, except that for the rate constant (k) was about 45 %. It means that the derived rate constants were influenced by the heating rate. When the heating rate was higher, the rate constant became smaller. There was a negative correlation between rate constant and heating rate. The reaction rate became slower and T p of reaction became higher at high heating rate. However, its T o did not shift to a higher temperature (see Table 2).
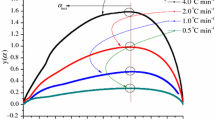
Figures 4 and 5 show the thermal curve of heat production and heat production rate versus time evaluated with kinetics-based simulation method. They clearly reveal that when the heating rates were raised from 0.5, 1, 2, to 4 °C min−1, the times at which the BTBPC decomposition and release energy were detected about 116, 64, 30, and 20 min, respectively. As expected, not only was the time shortened, but also the slope of these curves was sharper.
Thermal safety parameters analysis
TMRad, TER, and TCL are important thermal safety parameters during the process, storage, and transport of energetic materials. The hazard potential of BTBPC can be analyzed further by comparing the values of these parameters. Table 5 shows the dependencies of TMRad, TER, and TCL on temperature calculated on the basis of the kinetic simulation models. At 0.5 °C min−1 heating rate, the calculated TMRad could exceed 144,000 min (100 days) at the temperature equal to or lower than 27.27 °C. At 100 °C, TMRad dropped to 5.37 min. When the heating rates were 1, 2, and 4 °C min−1, the calculated TMRad could exceed 144,000 min (100 days) at higher temperature 34.55 °C. Moreover, at 100 °C, when the heating rates increased from 1, 2 to 4 °C min−1, the calculated TMR ad was also increased from 6.26, 7.54, to 9.85 min. As presented in Fig. 6, if temperature is higher than 34.55 °C, the β predicts a shorter TMRad. In contrast, in each heating rate alone, the higher temperature will cause TMRad to become shorter.
TER also has the same results as shown in Table 5. At 0.5 °C min−1 heating rate, BTBPC released the maximum energy at the temperature equal to or higher than 34.55 °C. However, when the heating rates are 1, 2, and 4 °C min−1, the maximum total energy release temperature will be all up to 41.82 °C. This is also presented in Fig. 7.
At the 10 % conversion limit value, TCL can be also listed in Table 5. Including the heating rates of 0.5, 1, 2, and 4 °C min−1, within the range 20–100 °C, BTBPC exceeds the upper limit, 3652.50 days (ca. 10 years), at the temperature equal to or lower than 27.27 °C. The same with TMRad parameter; if the temperature is higher than 34.55 °C, a lower β will predict a shorter TCL. However, because of the effect of apparent activation energy, no matter what the heating rate is, a higher temperature will cause TCL to become shorter. Figure 8 demonstrates that the lower heating rate will reveal the degree of reaction more completely.
Conclusions
Thermokinetic parameters and simulation-based reactive hazard analysis for this study were obtained from the kinetic model of BTBPC. The following conclusions were reached from the research conducted.
-
[1]
All the exothermal curves at four low heating rates of 0.5, 1, 2, and 4 °C min−1 had only one single strong and smooth exothermic peak under thermal decomposition.
-
[2]
When β was increased from 0.5 to 4 °C min−1, T o was about 118 °C, without significant change. T ext shifted from 129 to 148 °C, respectively. The calculated rate constant (k) became smaller.
-
[3]
We used T ext as T m in the Kissinger and Ozawa equations to estimate E a. Here, E a was ca. 142 kJ mol−1. It was better than employing T p as T m. This result was also closer to the result of built-in software of DSC; E a was 143 kJ mol−1. However, the calculated result from kinetic simulation method was about 10 % higher; E a was 156 kJ mol−1.
-
[4]
According to the TSS and the built-in software STARe of DSC, the heat decomposition type of BTBPC is an nth order reaction, not an autocatalytic reaction.
-
[5]
When the heating rate was raised from 0.5 to 4 °C min−1, the time to detect decomposition was shortened from about 116 to 20 min. The degree of decomposition would also be more violent.
-
[6]
When the temperature is higher than 34.55 °C, bigger β predicts a longer TMRad, which means that both β and TMRad have a significant positive correlation. Moreover, when the temperature is lower than 27.27 °C, no matter what the heating rate is, TMRad will all exceed 100 days. Storing BTBPC below 27.27 °C is a more reassuring and safer approach if the material is not to be stored in excess of 100 days.
-
[7]
At the lower heating rate, the degree of decomposition reaction of BTBPC reaching to 10 % is predicted to be faster.
Abbreviations
- A :
-
Pre-exponential factor in the Arrhenius equation (s−1 M1−n)
- C p :
-
Specific heat capacity (J g−1 K−1)
- CV:
-
Coefficient of variation (dimensionless)
- E a :
-
Apparent activation energy of reaction (kJ mol−1)
- ΔH d :
-
Heat of decomposition (kJ kg−1)
- k :
-
Reaction rate constant (mol L−1 s−1)
- k o :
-
Pre-exponential factor of reaction (s−1 M1−n)
- m :
-
Mass of reactant (g)
- n :
-
Order of reaction (dimensionless)
- n i :
-
Reaction order of ith stage (dimensionless)
- r i :
-
Reaction rate of ith stage (g s−1)
- SD:
-
Standard deviation (dimensionless)
- ΔT ad :
-
Adiabatic temperature rise (°C)
- TCL:
-
Time to conversion limit (day)
- TER:
-
Total energy release (kJ kg−1)
- T ext :
-
Extrapolated peak temperature of thermal curve by software of DSC device (°C)
- TMR ad :
-
Time to maximum heating rate under adiabatic conditions (min, h, day)
- T m :
-
Temperature of DSC thermal curve exhibits maximum enthalpy (°C)
- T o :
-
Apparent exothermic onset temperature of decomposition reaction (°C)
- T p :
-
True peak temperature of thermal curve by DSC detector (°C)
- \( \overline{X} \) :
-
Sample mean (variable)
- Z :
-
Autocatalytic constant (variable)
- α :
-
Degree of conversion (dimensionless)
- Β :
-
Heating rate of DSC experiment (°C min−1)
References
Chen JR, Wu SH, Lin SY, Hou HY, Shu CM. Utilization of microcalorimetry for an assessment of the potential for a runaway decomposition of cumene hydroperoxide at low temperatures. J Therm Anal Calorim. 2008;93:127–33.
Chi JH, Wu SH, Shu CM. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal and isothermal calorimetric applications. J Hazard Mater. 2009;171:1145–9.
Chu YC, Tsai FC, Lin FR, Chiang TC, Shu CM. Evaluation unexpected energy released for three liquid organic peroxides. Energy Educ Sci Tech Pt A. 2013;30:977–82.
Lu KT, Chu Y, Chen T, Hu K. Investigation of the decomposition reaction and dust explosion characteristics of crystalline dicumyl peroxide. Process Saf Environ Prot. 2010;88:356–65.
Liu SH, Hou HY, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;556:226–32.
Hsueh KH, Chen WT, Chu YC, Tsai LC, Shu CM. Thermal reactive hazards of 1,1-bis(tert-butylperoxy)cyclohexane with nitric acid contaminants by DSC. J Therm Anal Calorim. 2012;109:1253–60.
Li AC, Tsai YT, Wu SH, Chiu CW, Shen SJ, Hsin R, Shu CM. Thermal runaway analysis for two organic peroxides with H2O and dry fire-extinguishing chemicals by DSC and VSP2. J Therm Anal Calorim. 2013;113:1611–8.
Yeh PY, Shu CM, Duh YS. Thermal hazard analysis of methyl ethyl ketone peroxide. Ind Eng Chem Res. 2003;42:1–5.
Chang RH, Shu CM, Duh YS, Jehng JM. Calorimetric studies on the thermal hazard of methyl ethyl ketone peroxide with incompatible substances. J Hazard Mater. 2007;141:762–8.
Kee JK, Kyu YC, Alexander JC. Dynamics of a continuous stirred tank reactor for styrene polymerization initiated by a binary initiator mixture. II: Effect of viscosity dependent heat transfer coefficient. Polym Eng Sci. 1992;32:494–505.
Liu ZZ. Plastic materials—PS market’s present situation. IBP Technology Co., Ltd. http://www.ibuyplastic.com/tech_center/tech_paper/tech_detailcontent_print.phtml?id=576.
Hsu JM, Su MS, Huang CY, Duh YS. Calorimetric studies and lessons on fires and explosions of a chemical plant producing CHP and DCPO. J Hazard Mater. 2012;217–218:19–28.
Tseng JM, Lin CP, Huang ST, Hsu J. Kinetic and safety parameters analysis for 1,1,-di-(tert-butylperoxy)-3,3,5-trimethylcyclohexane in isothermal and non-isothermal conditions. J Hazard Mater. 2011;192:1427–36.
Lin CP, Tseng JM. Green technology for improving process manufacturing design and storage. Chem Eng J. 2012;180:284–92.
Lin CP, Chang CP, Chou YC, Chu YC, Shu CM. Modeling solid thermal explosion containment on reactor HNIW and HMX. J Hazard Mater. 2010;176:549–58.
Kandelbauer A, Wuzella G, Mahendran A, Taudes I, Widsten P. Model-free kinetic analysis of melamine-formaldehyde resin cure. Chem Eng J. 2009;152:556–65.
Kossoy AA, Benin AI, Akhmetshin YG. An advanced approach to reactivity rating. J Hazard Mater. 2005;118:9–17.
ChemicalBook, Inc., Chemcial Product Information, http://www.chemicalbook.com/ProductChemicalPropertiesCB7451603_EN.htm#MSDSA.
Toledo Mettler. STARe Software with Solaris Operating System. Lund: Operating Instructions; 2004.
Lega D, Antonini A, Ciccioli A, Brutti S, Lenzuni P. Low scan rate DSC study of the monoclinic-tetragonal transition in zirconia. Thermochim Acta. 2011;524:18–22.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Ravi P, Gore GM, Sikder AK, Tewari SP. Thermal decomposition kinetics of 1-methyl-3,4,5-trinitropyrazole. Thermochim Acta. 2012;528:53–7.
ChemInform Saint-Petersburg (CISP) Ltd. Thermal Safety Software (TSS). http://www.cisp.spb.ru/.
Kossoy AA, Akhmetshin Y. Identification of kinetic models for the assessment of reaction hazards. Process Saf Prog. 2007;26:209–20.
Kossoy AA, Sheinman I. Evaluating thermal explosion hazard by using kinetics-based simulation approach. Process Saf Environ Prot. 2004;82:421–30.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Shangyu Shaofeng Chemical Co., Ltd., http://www.zjsysf.com/pages/pro26.html.
Acknowledgements
The authors wish to express their gratitude to Dr. Arcady A. Kossoy of ChemInform Saint Petersburg (CISP), Ltd., St. Petersburg, Russia, and Mr. Anthony M. Janeshek of Dow Chemical Co., Freeport, Texas, USA, for providing technical assistance. Furthermore, the authors are indebted to Mr. Chia-Yuan Wen for his help on lab tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsueh, KH., Chen, WC., Liu, SH. et al. Thermal parameters study of 1,1-bis(tert-butylperoxy)cyclohexane at low heating rates with differential scanning calorimetry. J Therm Anal Calorim 118, 1675–1683 (2014). https://doi.org/10.1007/s10973-014-4045-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4045-3