Abstract
The densities, ρ, and viscosites, η, of mixtures of propanoic acid with equimolar mixtures of N,N-dimethyl formamide + methanol/ethanol/1-propanol, over the entire composition range of propanoic acid and including the pure liquids, have been measured at the temperatures T/K = 303.15, 313.15, and 323.15. From this experimental data, the excess molar volume, \( V_{\text{m}}^{\text{E}} \), deviation in viscosity, Δη, and excess Gibbs energy of activation of viscous flow, ΔG *E, have been determined at all three temperatures. The influence of temperature on these mixtures has been studied in terms of molecular interactions. The calculated deviation and excess parameters have been fitted to a Redlich–Kister type polynomial and the corresponding standard deviations were also evaluated. Negative values of \( V_{\text{m}}^{\text{E}} \) and positive values of Δη and ΔG *E are observed at all temperatures over the entire composition range in the mixtures studied. The observed negative and positive values of various excess and deviation parameters are attributed to the existence of strong interactions, like dipole–dipole interactions, H-bonding between the carbonyl group of acid molecules and hydroxyl group of alcohol groups, geometrical fitting of smaller molecules into the voids created by larger molecules in the liquid mixtures. The strength of these interactions in the mixtures was found to decrease with the rise in temperature and increase with an increase of chain length of the alcohols. The derived partial molar volumes and excess partial molar volumes also support the \( V_{\text{m}}^{\text{E}} \) results. The experimental viscosity data of all of these liquid mixtures have been correlated with four viscosity models, those of Grunberg and Nissan, Hind et al., Katti and Chaudhri, and Heric and Brewer. The Katti and Chaudhri model was found to be in good agreement with the experimental values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Knowledge of the temperature dependence of excess volumetric and transport properties of liquid mixtures provides valuable information on the nature of inter molecular interactions existing among the component molecules [1–3]. In continuation of our previous research, recently we reported ultrasonic and volumetric studies of the binary mixtures of N,N-dimethyl formamide with acrylic esters [4]. Alkanol molecules are polar and self-associated through hydrogen bonding of their hydroxyl groups [5]. Propanoic acid molecules are also polar. N,N-dimethyl formamide has a large dipole moment μ = 3.82 [6] and, in view of this, dipole–dipole interactions are expected to play an important role in molecular interactions present in the liquid mixtures. Alkanols are interesting simple examples of biological and industrial important amphiphilic materials [7]. Propanoic acid is a naturally occurring carboxylic acid and is used as an intermediate in the production of other chemicals, especially polymers, plastics, and cosmetics. Amides, relevant liquid systems for the study of molecular interactions, are among the most common solvents used in chemical reactions and in many industrial processes.
Some excess properties of binary liquid mixtures of propanoic acid with aniline, o-toluidine, o-anisidine, and o-chloroaniline were reported by Garcia et al. [8]. Solimo et al. [9] reported the thermodynamic properties of binary liquid acid–base mixtures. Viscosities and densities of binary mixtures of toluene with acetic acid and propanoic acid at several temperatures were reported by Rattan et al. [10]. As there are no temperature-dependent studies of similar liquid mixtures, particularly for the volumetric and transport properties of the present liquid mixtures, the present study of the liquid mixtures of propanoic acid with equimolar mixtures of N,N-dimethyl formamide and methanol/ethanol/1-propanol at three temperatures T/K = 303.15, 313.15, and 323.15 over the entire mole fraction range, x 1, of propanoic acid, is expected to reveal new and interesting results on molecular interactions.
In the present investigation, we report densities, ρ, and viscosities, η, of the liquid mixtures at three different temperatures. The excess and deviation properties such as excess molar volume, \( V_{\text{m}}^{\text{E}} \), deviation in viscosity, Δη, and excess
Gibbs energy of activation of viscous flow, ΔG *E, have been calculated using the experimental values of ρ and η. The variation of \( V_{\text{m}}^{\text{E}} \), Δη and ΔG *E with composition and temperature are discussed in terms of molecular interactions occurring among the molecules of these mixtures.
2 Experimental Details
Propanoic acid (PA), N,N-dimethyl formamide (DMF), methanol (MOH), ethanol (EOH) and 1-propanol (POH) used in the present study were AR grade products from LOBA Chemicals, India and were purified by standard methods described in the literature [11, 12]. The mass fraction purity of the liquids obtained are >0.995. Before use, the chemicals were stored over 0.4 nm molecular sieves for approximately 72 h to remove water and then degassed.
The mixtures were prepared by mass in air-tight bottles. The mass measurements were performed with a METTLER TOLEDO (Switzerland) ABB5-S/FACT digital balance with an accuracy ±0.01 mg. The uncertainty in the mole fractions is 1 × 10–4. Densities and viscosities of pure liquids and liquid mixtures have been determined using a 5 cm3 two-stem double-walled Parker and Parker type pyknometer [13] and an Ostwald viscometer that was calibrated as described by Naidu and Ravindra Prasad [14], using triply distilled water. The detailed experimental procedures adopted for the measurement of ρ and η are the same as in our earlier studies reported in the literature [4, 15, 16]. The reproducibility in the measured densities and viscosities are 3 in 105 parts and ±0.2 %, respectively. The densities and viscosities of the pure liquids used in this investigation, at T/K = 303.15, 313.15, and 323.15, are compiled in Table 1 together with the available literature data [10, 12, 17–26]. These results are found to be in good agreement with the reported literature data.
3 Results and Discussion
The experimentally measured values of ρ and η were used to calculate the excess and deviation properties \( V_{\text{m}}^{\text{E}} \), Δη, and ΔG *E using the following equations:
where V m and η are the molar volume and viscosity of the mixture. \( V_{1}^{ * } \) and \( \eta_{1}^{ * } \) are the molar volumes and viscosities of pure component 1 (propanoic acid) and \( V_{2}^{ * } \) and \( \eta_{2}^{ * } \) are the effective molar volume and viscosity of an equimolar mixture of component 2 (N,N-dimethyl formamide +, methanol, ethanol, 1-propanol), respectively, and x i represents the mole fraction of component ‘i’ in the mixture.
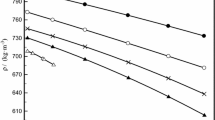
The experimental values of ρ and η and calculated values of \( V_{\text{m}}^{\text{E}} \), Δη, and ΔG *E are reported in Tables 2, 3 and 4 for the studied systems at different temperatures over the entire mole fraction, x 1, of PA. It was observed that the density varies monotonically with the concentration of propanoic acid at all temperatures, but the viscosity changes non-linearly showing a maximum at about x 1 ≈ 0.6 in the PA-rich region at all investigated temperatures in all of the systems. The observed maxima (peaks) were found to increase with increasing chain length of alcohols. Such deviations may be attributed to specific interactions arising from the formation of complexes among the mixing molecules.
The deviation/excess properties have been fitted to a Redlich–Kister type polynomial [27] equation:
where ΔY = Δη and Y E = \( V_{\text{m}}^{\text{E}} \) or ΔG *E. The A i are adjustable parameters of this function and are determined using the least-squares method. The corresponding standard deviations \( \sigma \left( {Y^{\text{E}} } \right) \) have been computed using the relation:
where ‘m’ is the total number of experimental points and ‘n’ is the number of coefficients in Eq. 4. The coefficients A i , and the standard deviations σ, of all of the liquid mixtures are presented in Table 5.
The variations of \( V_{\text{m}}^{\text{E}} \) for all of the systems, at different temperatures, are shown in Figs. 1, 2 and 3. Figure 4 shows the variation of Δη with mole fraction of PA at all temperatures for the (DMF + MOH) + PA system. The corresponding plot of Δη versus concentration at T/K = 313.15 is shown in Fig. 5. The variations of ΔG *E with mole fraction of PA for all of the systems at T/K = 323.15 are represented in Fig. 6. The observed negative \( V_{\text{m}}^{\text{E}} \) and positive Δη and ΔG *E values reveal that strong interactions exist among unlike molecules of the mixtures. The factors that are mainly responsible for the expansion of molar volume, i.e. positive values of \( V_{\text{m}}^{\text{E}} \), are the following. (i) Breaking of the structure of one or both of the components in a solution, i.e. the loss of dipolar association between the molecules (dispersion forces). (ii) The geometry of molecular structures which does not favor the fitting of molecules of one component into the voids created by the molecules of other component. (iii) Steric hindrance of the molecules. The negative values of \( V_{\text{m}}^{\text{E}} \) are due to strong specific interactions such as (iv) association of molecules through the formation of hydrogen bonds or association due to dipole–dipole interactions, or (v) the accommodation of molecules due to considerable differences in molar volumes. The variations of the excess molar volumes in the present investigation are negative over the entire mole fraction range [28]. The presence of strong interaction is due to the hydrogen bonding (O···H–O–) between the carbonyl group (–C=O) of amide molecules and the hydroxyl group (–OH) of alcohol molecules. When the third component PA is added to the equimolar mixture, the existing hydrogen bond between amide and alcohol groups is broken and a new hydrogen bond is formed between unlike molecules of acid and alcohol. Moreover, all of the components of the liquid mixtures studied are polar in nature having dipole moments of: methanol μ = 1.70 D [6], ethanol μ = 1.69 D [6], 1-propanol μ = 1.58 D [6], N,N-dimethyl formamide μ = 3.82 [6], and propanoic acid μ = 1.75 [6]. Therefore, dipole–dipole interactions are also present in the investigated liquid mixtures. In addition to these effects, physical interactions such as geometrical fitting of smaller molecules into the voids created by the larger molecules is also favorable for the present systems.
From Figs. 1, 2 and 3 it is observed that, as the temperature of the systems increases excess molar volumes become less negative, indicating a decrease of the interactions between the unlike molecules, but the interactions increase from MOH to POH. As the alkanol chain length increases, thereby decreasing the concentration of –OH groups in higher alkanols, it lowers the overall dipole moment, which causes weaker interactions. These are expected results. We have observed that the strength of interaction increases as we move from MOH to POH. This is due to the predominance of physical interactions over chemical interactions between the unlike molecules. In other words, geometrical fitting of smaller molecules into the voids created by the larger molecules among the molecular components of liquid mixtures. Similar studies were reported by Kumar et al. [29]. The strength of interactions in the mixtures follows the order (DMF + MOH) + PA < (DMF + EOH) + PA < (DMF + POH) + PA. The above discussion is also supported by the observed positive values of Δη and ΔG *E. Generally, negative values of Δη indicate the presence of dispersion forces or mutual loss of specific interactions among like molecules in the systems, due to weak intermolecular interactions, while positive values of the deviation in viscosity indicate the presence of strong interactions [30, 31]. The sign and magnitude of Δη depends on the combined effect of factors such as molecular size, shape, and intermolecular forces. From the above observations it is clear that, as the temperature increases, the interaction between unlike molecules decreases.
The partial molar volumes \( \overline{V}_{{{\text{m}},1}} \) of component 1 (propanoic acid) and \( \overline{V}_{{{\text{m}},2}} \) of component 2 (N,N-dimethyl formamide + methanol/ethanol/1-propanol) in the mixtures, over the entire composition range, were calculated by using the following relations [32]:
The derivatives \( \left( {\frac{{\partial V_{\text{m}}^{\text{E}} }}{{\partial x_{1} }}} \right)_{T,p} \) in Eqs. 6 and 7 were obtained by differentiating Eq. 4, which leads to the following equations for \( \overline{V}_{{{\text{m}},1}} \) and \( \overline{V}_{{{\text{m}},2}} \):
Here j is an integer taking the value of 4; using the above equations, \( \overline{V}_{\text{m,1}}^{\text{E}} \) and \( \overline{V}_{\text{m,2}}^{\text{E}} \) have been evaluated using:
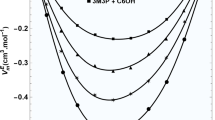
The values of \( \overline{V}_{{{\text{m}},1}} \) and \( \overline{V}_{{{\text{m}},2}} \) are reported in Table 6. The variations of excess partial molar volumes, \( \overline{V}_{\text{m,1}}^{\text{E}} \) and \( \overline{V}_{\text{m,2}}^{\text{E}} \), with mole fraction of PA are shown in Figs. 7, 8, 9. From the above table, it can be seen that the values of \( \overline{V}_{\text{m,1}} \) and \( \overline{V}_{{{\text{m}},2}} \) for both components in the mixtures are less than their respective molar volumes in the pure state. From Figs. 7, 8 and 9 it is seen that the excess partial molar volumes are almost always negative over the entire composition range. This suggest that a contraction of volume occurs in the binary mixtures, indicating the presence of strong interactions between unlike molecules of the mixtures as observed for \( V_{\text{m}}^{\text{E}} \).
Variation of the excess partial molar volumes \( \overline{V}_{\text{m,1}}^{\text{E}} \) (solid lines), \( \overline{V}_{\text{m,2}}^{\text{E}} \) (dashed lines) with the mole fraction of PA, x 1, of (DMF + MOH) + PA mixtures at T/K = 303.15 (filled circles), 313.15 (filled squares), and 323.15 (filled triangles)
Variation of the excess partial molar volumes \( \overline{V}_{\text{m,1}}^{\text{E}} \) (solid lines), \( \overline{V}_{\text{m,2}}^{\text{E}} \) (dashed lines) with the mole fraction of PA, x 1, of (DMF + EOH) + PA mixtures at T/K = 303.15 (filled circles), 313.15 (filled squares), and 323.15 (filled triangles)
Variation of the excess partial molar volumes \( \overline{V}_{\text{m,1}}^{\text{E}} \) (solid lines), \( \overline{V}_{\text{m,2}}^{\text{E}} \) (dashed lines) with the mole fraction of PA, x 1, of (DMF + POH) + PA mixtures at T/K = 303.15 (filled circles), 313.15 (filled squares), and 323.15 (filled triangles)
The partial molar volumes and excess partial molar volumes of the components at infinite dilution, \( \overline{V}_{{{\text{m}},1}}^{\infty } ,\;\overline{V}_{{{\text{m}},2}}^{\infty } ,\;\overline{V}_{{{\text{m}},1}}^{{{\text{E}},\infty }} \) and \( \overline{V}_{\text{m,2}}^{{{\text{E,}}\infty }} \), respectively, were obtained by putting x = 0 in Eq. 8 and x = 1 in Eq. 9:
The pertinent values of \( \overline{V}_{{{\text{m}},1}}^{\infty } \), \( \overline{V}_{{{\text{m}},2}}^{\infty } \), \( \overline{V}_{\text{m,1}}^{{{\text{E,}}\infty }} \) and \( \overline{V}_{\text{m,2}}^{{{\text{E,}}\infty }} \) are reported in Table 7. From this table it is seen that these values are negative, from which we conclude that strong interactions exist among the unlike molecules of the mixtures. The magnitude of the excess partial molar volumes at infinite dilution also follow the order (DMF + POH) + PA > (DMF + EOH) + PA > (DMF + MOH) + PA, which supports the trends of \( V_{\text{m}}^{\text{E}} \) values observed in these mixtures.
The dynamic viscosities of the binary liquid mixtures have been calculated using the following empirical relations. The Grunberg and Nissan [33] model is based on the Arrhenius equation for the viscosity of liquid mixtures,
where G 12 is an interaction parameter that is a function of components 1 and 2 as well as temperature. Hind and Ubbelohde [34] suggested an equation for the viscosity of binary liquid mixtures as
where H 12 is an interaction parameter that is attributed to unlike pair interactions. Katti and Chaudari [35] proposed the following equation
where W vis is an interaction term. Heric and Brewer [36] derived the relation
where Δ12 is the interaction term.
In the above equations, η 1, η 2, M 1, and M 2 are the viscosities and molar masses of the pure components of PA and (DMF + MOH/EOH/POH), respectively. The theoretical values of viscosity of the liquid mixtures calculated using the above equations are compiled in Table 8. Table 9 presents the values of the interaction parameters along with the standard deviations, σ. The adjustable parameters G 12, H 12, W vis and Δ12 represent binary interactions. The variation of these parameters with composition follows the order (DMF + POH) + PA > (DMF + EOH) + PA > (DMF + MOH) + PA at constant temperature. Further, the interaction parameter values decrease with an increase in temperature for all of the systems studied. These results are in good agreement with the results derived from the excess properties. Prolongo et al. [37] reported positive values of interaction parameters corresponding to systems with negative excess molar volumes. This is consistent with our results. Among all of these models, the viscosity representations obtained from the Katti and Chaudhari model are in best agreement with the experimental viscosity data.
4 Conclusion
The densities and viscosities of liquid mixtures of propanoic acid with equimolar mixtures of N,N-dimethyl formamide and methanol/ethanol/1-propanol have been measured over the entire composition range at T/K = 303.15, 313.15, and 323.15. The parameters \( V_{\text{m}}^{\text{E}} ,\;\Updelta \eta ,\;\Updelta G^{{ * {\text{E}}}} ,\;\overline{V}_{{{\text{m}},1}} ,\;\overline{V}_{{{\text{m}},2}} ,\;\overline{V}_{\text{m,1}}^{\text{E}} \) and \( \overline{V}_{\text{m,2}}^{\text{E}} \) have been computed from the experimental results. The values of \( V_{\text{m}}^{\text{E}} \) are negative whereas Δη and ΔG *E are positive at all studied temperatures, indicating the presence of strong interactions such as hydrogen bonding (O···H–O–) and interactions between the carbonyl group (–C=O) of amide molecules and the hydroxyl group (–OH) of alcohol groups, and also intermolecular interactions between the carbonyl group (–C=O) of acid molecules and hydroxyl group (–OH) of alcohols, dipole–dipole interactions, and geometrical fitting of smaller molecules into the voids created by the larger. These deviations and excess properties have been fitted with a Redlich–Kister type polynomial. The strength of interactions follows the order (DMF + MOH) + PA < (DMF + EOH) + PA < (DMF + POH) + PA. The calculated values of the partial molar volumes have also been examined, indicating the existence of strong interactions among unlike molecules in the mixtures. The strength of interactions was also studied using the variation of these properties with temperature. The experimental viscosity values were compared with the viscosity values obtained from different empirical relations.
References
Giner, B., Martin, S., Artigas, H., Lopez, M.C., Lafuente, C.: Study of weak molecular interactions through thermodynamic mixing properties. J. Phys. Chem. B 30, 17683–17690 (2006)
Kinart, C.M., Kinart, W.J., Checinska-Majak, D., Cwiklinska, A.: Volumetric behaviour of binary liquid mixtures of 2-methoxyethanol with n-butylamine, sec-butylamine and tert-butylamine. J. Mol. Liq. 109, 19–22 (2004)
Iloukhani, H., Zoorasna, Z., Soleimani, R.: Excess molar volumes and speeds of sound of tetrahydrofuran with chloroethanes or chloroethenes at 298.15 K. Phys. Chem. Liq. 43, 391–401 (2005)
Kondaiah, M., Sravana Kumar, D., Sreekanth, K., Krishna Rao, D.: Ultrasonic velocities, densities, and excess molar volumes of binary mixtures of N,N-dimethyl formamide with methyl acrylate, or ethyl acrylate, or butyl acrylate, or 2-ethyl hexyl acrylate at T = 308.15 K. J. Chem. Thermodyn. 43, 1844–1850 (2011)
Marcus, Y.: Introduction to Liquid State Chemistry. Wiley Interscience, New York (1977)
Reichardt, C.: Solvents Effects in Organic Chemistry, 3rd edn. Wiley, Weinheim (2003)
Eads, C.D.: Simple lattice model for solvation of non-polar molecules in hydrogen-bonded liquids. J. Phys. Chem. B 104, 6653–6661 (2000)
Garcia, B., Alcalde, R., Leal, J.M.: Excess properties for binary liquid mixtures of propanoic acid with aniline derivatives. Can. J. Chem. 69, 369–372 (1991)
Solimo, H.N., Riggio, R., Davolio, F., Katz, M.: Thermodynamic properties of binary liquid acid–base mixtures. Can. J. Chem. 53, 1258–1262 (1975)
Rattan, V.K., Seema, K., Tochigi, K.: Viscosities and densities of binary mixtures of toluene with acetic acid and propionic acid at (293.15, 303.15, 313.15, and 323.15) K. J. Chem. Eng. Data 47, 1182–1184 (2002)
Vogel, A.I.: Textbook of Organic Chemistry, 5th edn. Wiley, New York (1989)
Riddick, J.A., Burger, W.B., Sankano, T.K.: Techniques in Chemistry, 4th edn. Wiley, New York (1986)
Parker, H.C., Parker, E.W.: Densities of certain aqueous potassium chloride solutions as determined with a new pyknometer. J. Phys. Chem. 29, 130–137 (1925)
Naidu, P.S., Ravindra Prasad, K.: Ultrasonic velocity and allied parameters in solutions of cypermethrin with xylene and ethanol. Indian J. Pure Appl. Phys. 42, 512–515 (2004)
Sravana Kumar, D., Srikanth, K., Krishna Rao, D.: Molecular interactions in the mixtures of 2-chloroaniline with equimolar mixture of methanol and isopropanol/isobutanol. J. Mol. Liq. 136, 90–93 (2007)
Sreekanth, K., Sravana Kumar, D., Kondaiah, M., Krishna Rao, D.: Volumetric and viscometric study of molecular interactions in the mixtures of some secondary alcohols with equimolar mixture of ethanol and N,N-dimethylacetamide at 308.15 K. Phys. B 406, 854–858 (2011)
Nikam, P.S., Shirsat, L.N., Hasan, M.: Density and viscosity studies of binary mixtures of acetonitrile with methanol, ethanol, propan-1-ol, propan-2-ol, butan-1-ol, 2-methylpropan-1-ol, and 2-methylpropan-2-ol at (298.15, 303.15, 308.15, and 313.15) K. J. Chem. Eng. Data 43, 732–737 (1998)
Kharat, S.J., Nikam, P.S.: Density and viscosity studies of binary mixtures of aniline + benzene and ternary mixtures of (aniline + benzene + N,N-dimethyl formamide) at 298.15, 303.15, 308.15, and 313.15 K. J. Mol. Liq. 131–132, 81–86 (2007)
Pal, A., Singh, Y.P.: Excess molar volumes and apparent molar volumes of some amide + water systems at 303.15 and 308.15 K. J. Chem. Eng. Data 40, 818–822 (1995)
Joshi, S.S., Aminabhavi, T.M., Balundgi, R.H., Shukla, S.S.: Densities and viscosities of binary liquid mixtures of nitrobenzene with cyclohexane and N,N-dimethyl formamide. J. Chem. Eng. Data 35, 185–187 (1990)
Chan, G., Knapp, H.: Densities and excess molar volumes for sulfolane + ethyl benzene, sulfolane + 1-methylnaphthalene, water + N,N-dimethyl formamide, water + methanol, water + N-formyl morpholine, and water + N-methyl pyrrolidone. J. Chem. Eng. Data 40, 1001–1004 (1995)
Bhuiyan, M.M.H., Uddin, M.H.: Excess molar volumes and excess viscosities for mixtures of N,N-dimethyl formamide with methanol, ethanol and 2-propanol at different temperatures. J. Mol. Liq. 138, 139–146 (2008)
Bhuiyan, M.M.H., Ferdaush, J., Uddin, M.H.: Densities and viscosities of binary mixtures of dimethyl sulfoxide + aliphatic lower alkanols (C) at temperatures from T = 303.15 K to T = 323.15 K. J. Chem. Thermodyn. 39, 675–683 (2007)
Akhter, S., Bhuiyan, M.M.H., Uddin, M.S., Sultana, B., Nessa, M., Saleh, M.A.: Viscosity of aqueous solutions of some alcohols. Phys. Chem. Liq. 37, 215–227 (1999)
Marcheselli, L., Marchetti, A., Tagliazucchi, M., Tassi, L., Tosi, G.: N,N-dimethyl formamide–2-methoxyethanol solvent system. Densities and excess molar volumes at various temperatures. J. Chem. Soc. Faraday Trans. 88, 3159–3163 (1992)
Saleh, M.A., Akhtar, S., Nessa, M., Uddin, M.S., Bhuiyan, M.M.H.: Excess molar volumes of aqueous solutions of 1-propanol, 2-propanol, allyl alcohol and propyl alcohol. Phys. Chem. Liq. 36, 53–65 (1998)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Reddy, K.C., Subramanyam, S.V., Bhimasenachar, J.: Thermodynamics of binary liquid mixtures containing cyclohexane Part 1. J. Phys. Soc. Jpn. 19, 559–566 (1964)
Kumar, H., Kumar, B., Kumar, A., Angmo, T., Yadav, S.: Densities and excess molar volumes of cyclopentane (1) + 1-alkanol (2) systems at (298.15 and 308.15) K. J. Chem. Eng. Data 54, 165–167 (2009)
Rafiqul Islam, M., Quadri, S.K.: Ultrasonic velocity and viscosity of binary liquid mixtures. Thermochim. Acta 115, 335–344 (1987)
Tewari, K., Patra, C., Chakravortty, V.: Molecular interactions study on binary liquid mixtures of dimethyl sulphoxide with benzene, carbon tetrachloride and toluene from the excess properties of ultrasonic velocity, density and viscosity. Acoustics Lett. 19, 53–59 (1995)
Wang, H., Liu, W., Huang, J.: Densities and volumetric properties of a (xylene + dimethyl sulfoxide) at temperature from (293.15 to 353.15) K. J. Chem. Thermodyn. 36, 743–752 (2004)
Gurnberg, L., Nissan, A.H.: Mixture law for viscosity. Nature 164, 799–800 (1949)
Hind, R.K., McLaughlin, E., Ubbelohde, A.R.: Structure and viscosity of liquids camphor + pyrene mixtures. Trans. Faraday Soc. 56, 328–330 (1960)
Katti, P.K., Chaudhari, M.M.: Viscosities of binary mixtures of benzyl acetate with dioxane, aniline and m-cresol. J. Chem. Eng. Data 9, 442–443 (1964)
Heric, E.L., Brewer, J.C.: Viscosity of some binary liquid non electrolytic mixtures. J. Chem. Eng. Data 12, 574–583 (1967)
Prolongo, M.G., Masegosa, R.M., Hernandez-Fuentes, I., Horta, A.: Viscosities and excess volumes of binary mixtures formed by the liquids acetonitrile, pentyl acetate, 1-chlorobutane, and carbon tetrachloride at 25°C. J. Phys. Chem. 89, 2163–2167 (1984)
Acknowledgments
The authors are thankful to the University Grants Commission (U.G.C), New Delhi, Government of India, for providing financial support through an infrastructure Grant under the DRS-SAP program (Letter No. F4-1/2006/(BSR)/7-2/2007(BSR) dated 23-12-2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondaiah, M., Sreekanth, K., Sravana Kumar, D. et al. Volumetric and Viscometric Properties of Propanoic Acid in Equimolar Mixtures of N,N-dimethyl Formamide + Alkanols at T/K = 303.15, 313.15, and 323.15. J Solution Chem 42, 494–515 (2013). https://doi.org/10.1007/s10953-012-9898-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9898-0