Abstract
Titanium dioxide (TiO2), especially in its anatase form, is an effective photocatalyst under ultraviolet (UV) light. The particle size of TiO2 is a critical factor to determine its photoactivity based on its quantum effectiveness under light irradiations. Thus, nanocrystalline TiO2 has been widely accepted to significantly enhance this effect. The sol–gel method is generally used to synthesize the anatase form of nanocrystalline TiO2. In this study, we expanded the synthesis method of TiO2 to high pressures under direct heating (hydrothermal method) and indirect heating (microwave-assisted method). It was found that pH value is one of the major factors to control nano-sizes of TiO2 particles, and the neutral condition in all methods is preferable for controlling the sizes of the prepared TiO2 particles. The microwave-assisted method further improves quality of synthesized nano-size TiO2 below 10 nm. These results have been confirmed by both the direct size measurement using TEM images and indirect determination using XRD peaks. The collected samples are further analyzed using UV–Vis spectroscopy to identify the particle size-dependent photoreactivity and to confirm the effectiveness of microwave-assisting under neutral conditions. DSC is also a powerful tool to identify the crystalline transition of TiO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium oxide (TiO2) was initially used as a cheap white pigment found in paints, plastics, paper, printing ink, chemical fiber, rubber, cosmetics, and many other industries [1, 2]. It is non-toxic and has great opacity, whiteness, and brightness of color. Its general crystallographic structures include anatase (tetragonal), rutile (tetragonal), and brookite (orthorhombic). Most importantly, its anatase form is photoactive under ultraviolet (UV) light [3]. Its strong oxidative potential of the positive holes can oxidize water to create hydroxyl radicals, making it a hydrolysis catalyst that performs well in sterilization, deodorization, and anti-fouling [4–6]. Based on similar mechanics, the anatase form of nano-size TiO2 is reported to be active in electricity production. This makes it a good candidate in dye-sensitized solar cells [7, 8].
The conventional approach for the synthesis of the nano-size TiO2 is the sol–gel method [9]. This study extends the synthesis approaches of the nano-size TiO2 further into the microwave hydrothermal method and the high-pressure hydrothermal method. The main advantages of the microwave hydrothermal processing of TiO2 include rapid and efficient energy input to maintain desired kinetics of crystallization. The convenience of using the microwave heating option was a function of the organic cation present in the precursor. The choices of the organic cations allow fine tuning of several critical properties of the TiO2 product, such as particle size and pore morphology [10, 11]. Potential organic cations include ammonium-based hydrocarbon substituents, such as diethylammonium, phenylammonium, and benzyltrimethylammonium, leading to the precipitation of nanocrystalline anatase powder with high specific surface area (up to 120 m2 g−1) in a short processing time (1–3 min). Other organic cations include alcohol and carboxylate. Functionalized under high-pressure conditions, particle size, morphology, and polymorph of TiO2 are expected to be finely controlled.
This work focused on the synthesis of nanocrystalline powders of high-activity TiO2 photocatalysts in either pure anatase or rutile/anatase mixture types of Titania with different morphologies through an environmentally friendly and novel process. A highly energy-efficient microwave-assisted hydrothermal method and a high-pressure hydrothermal method are employed to prepare TiO2 using the titanium isopropoxide (TTIP) [12–14]. For comparison purposes, the conventional sol–gel based TiO2 using titanium isopropoxide in water at the same synthesis temperatures and a commercially available AEROXIDE® P25 TiO2 (from Acros) were selected for the study. The influence of hydrothermal treatment conditions on the formation features, phase composition, particle size, and morphology of the products was investigated by X-ray diffraction and scanning electron microscopy. The photocatalytic activities of prepared samples were evaluated using ultraviolet–visible light spectroscopy (UV–Vis). Their phase transitions under elevated temperatures were evaluated using thermogravimetric/differential scanning calorimetry (TG/DSC).
Experimental
Preparation of titanium dioxide nanoparticle
Step 1: solution precursor
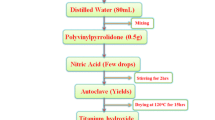
TTIP (titanium (IV) isopropoxide titanyl isopropyl, >98 % purity, from ACROS) and deionized water were mixed at a volume ratio of 0.5 of TTIP:H2O, and hydrochloric acid (trace metal grade, Fisher chemical) and ammonia hydroxide (trace metal grade, Fisher chemical) were added in the previous solution for pH adjustment (pH 2.0, 7.0, and 9.0) using a pH meter. At pH 7.0, phosphate buffer solution was added (phosphate-buffered solution NaHPO4–Na2HPO4, pH 7, from Ricca chemical). The pH value is an important factor affecting the particle size in the hydrothermal methods, and the pH value also changes during reactions. The buffer solution is used to slow down these changes and better maintain the pH. The prepared solution precursors, at different pH values, were uniformly mixed using a magnet stirrer (about 30 min) before the next step.
Step 2: regular sol–gel, hydrothermal, and microwave hydrothermal methods
The solution precursor is added into a 250 mL flask, which is in a water bath tank. The temperature is controlled at 80 °C, and flask is sealed to allow sol–gel reaction for 24 h. After washing using DI water, and being centrifuged (Adams Analytica centrifuge model N.J.07054) for 3 times and dried at 100 °C for 6 h, the TiO2 nanoparticle based on the sol–gel method is obtained. A batch of TiO2 nanoparticles is prepared based on different solution precursors at different pH values.
The aforementioned solution precursor (TTIP 50 mL + H2O 100 mL) was added into the Teflon-coated steel autoclave, heated to 200 °C, and pressurized to 100 psi initially using nitrogen and later 550 psi after the elevation of the desired temperature (200 °C). The stirrer spin rate setting was at 100 rpm, and reaction time was 1 or 2 h, respectively. After being cooled to room temperature, the pressure was relieved. The obtained sample was washed using DI water and centrifuged three times and dried at 100 °C for 6 h to obtain the TiO2 based on the hydrothermal method.
The solution precursor was added into a Teflon tank and placed in a microwave oven (Milestone ETHOS EZ). The temperature was set at 240 °C, the heating times were 10, 20, and 30 min, and the reaction time was 1 or 2 h. The sample was washed using DI water and centrifuged three times and dried at 100 °C for 6 h to obtain the TiO2 based on the hydrothermal method.
Sample characterization
All samples were ultrasonically (Bransonic ultrasonic model 2510R-DTH 100 W 42KHZ) prepared using ethanol before being characterized.
Ultraviolet–visible (UV–Vis) diffuse reflectance spectra were obtained using a Varian Cary 100 UV–Vis spectrophotometer (200–800 nm) equipped with the DRA-CA-30 diffuse reflectance accessory. In UV–Vis analysis, all samples are diluted by the 104 fold (10 mg solid TiO2 sample in 100 mL ethanol). P25 TiO2 is in mixed polymorphs, including anatase and rutile forms in a mass ratio of about 80:20.
The infrared spectra were recorded from 4,000 to 650 cm−1 (with a resolution of 2 cm−1 and 4 averaged scans) on a Perkin Elmer Spectrum One FTIR spectrometer with a UATR attachment using KBr pellets.
A confocal Raman system was self-assembled, based on several major components. They were a microscope (OLYMPUS model BX60M) to let light focus on tested samples, a He–Ne laser light source (MELLES GRIOT, model LHX1) at 632.8 nm and 30 mW of output power, a spectrometer (Newport, model MS260i™) with 1,200 line mm−1 grating to scatter the light source, a ICCD digital camera (Andor iStar ICCD Camera) connecting signal to computer, and other accessories such as data acquisition (Andor SOLIS).
The thermogravimetric data were collected on a TA Instrument Hi-Res TG 2950 Thermogravimetric Analyzer at a heating rate of 10 °C min−1 from room temperature to 1,000 °C in a nitrogen atmosphere. The thermocalorimetry data were collected on a TA Instrument 2029 modulated DSC Analyzer at a heating rate of 10°C min−1 from room temperature to 400 °C in a nitrogen atmosphere.
The samples were mounted on an aluminum stub using double-sided carbon tape, and were observed using a JEOL JSM 5400-LV scanning electron microscope (SEM–EDS). They were observed uncoated in low-vacuum mode (~20 kPa) using a backscatter electron detector. Elemental analysis was obtained using a KEVEX Quantum detector with a MOXTEK AP3.3 thin filmed window and an IXRF energy-dispersive spectrometer.
Transmission electron microscopy (TEM) images were collected from a JEOL model JEM-100CX. All samples were well prepared in a solution using ethanol. A drop of the prepared sample solution was added on formvar-coated grids on which some of the sample particles adhered after the excess solution was wiped away.
Results and discussion
The anatase form is not an equilibrium phase of TiO2, but it is kinetically stabilized. At temperatures between 550 and about 1,000 °C, the anatase form of TiO2 transforms to the equilibrium rutile phase. The temperature of this transformation strongly depends on the impurities or dopants present and morphology in the TiO2 samples.
X-ray diffraction (XRD)
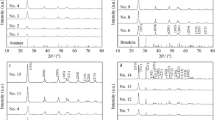
Figure 1 shows the XRD patterns of the prepared TiO2 samples in the wide angle ranges (20° < 2θ < 90°) of the incident X-ray. Peaks, which appear at 25.367°, 37.053°, 38.667°, 48.158°, 54.051°, 62.867°, 68.976°, and 75.303° are attributed to the 101, 004, 200, 211, 204, 220, and 215 crystalline structures of the anatase form of TiO2. All the prepared samples, including one commercial TiO2 sample, clearly present XRD peaks of all crystalline structures of the anatase form. The major peak appears at 25.367°, which could be also used for calculations of crystal grain sizes of samples, using Eq. 1:
where t is the average grain size, λ is a wavelength of X-ray (Cu Kα X-ray, λ = 0.1542 nm), B is the half width of the diffraction peak (full width at half maximum, FWHM), and θ is the incident angle (25.367°) of X-ray diffraction surface. The calculated grain sizes of the prepared samples are listed in Table 1. It could be found that the grain size of the commercial sample is close to 10 nm, and that based on the conventional sol–gel method is 30 nm. The hydrothermal and microwave-assisted methods produce even smaller grain sizes of the TiO2 particles of 9.17 and 8.17 nm, respectively. Generally, the size of particles smaller than 100 nm is defined as nanoparticles. Smaller particles have the strong quantum effect and are more efficient when used as a photocatalyst. Based on grain sizes of the prepared samples, TiO2 based on hydrothermal and microwave-assisted methods is more effective in becoming photocatalysts. This will be presented in UV–Vis tests.
SEM and TEM characterization
SEM images are shown in Fig. 2. We found larger blocks of the prepared TiO2 samples when the buffer was used to maintain the synthesis solution at pH 7. Smaller particles seemed to be obtained under acidic and alkali conditions. It is not clear if the size of blocks corresponded to actual sizes of the prepared TiO2. This may be caused by there being no special preparation procedure for SEM tests. Before TEM tests, the prepared samples were well dispersed in ethanol and sonicated for improvement of better images under TEM. The size histograms of the prepared TiO2 particles under a natural condition with buffers are shown in Fig. 3. TEM images clearly present nano-sizings of the prepared TiO2 samples. Based on statistical calculations, the average size of TiO2 based on microwave, hydrothermal, P25, and sol–gel methods are 5.72, 7.00, 8.30 and 13.71 nm, respectively. Sizes of TiO2 samples follow similar trends as those observed in XRD analysis, as shown in Table 1, despite some discrepancies to the actual values. All evidences of particle sizing demonstrate that the synthesis of TiO2 nanoparticles is successful, especially for methods under microwave assistance. Evidence from SEM and TEM also shows that a strong bonding effect is created when the particle size of TiO2 is smaller, which occurred in the neutral condition. This forms a larger agglomerate when no dispersion agent is used.
Differential scanning calorimetry analysis (DSC) and thermogravimetric analysis (TG)
DSC is a powerful tool to study desorption of the absorbed species on TiO2 and phase transition of TiO2 itself. The typical peaks in DSC curves of TiO2, corresponding to thermal activity, may include desorption of moisture at lower temperatures (50–150 °C) and organics at slightly higher temperatures (between 150 and 250 °C). An endothermic peak at 275 °C was attributed to the phase transition of TiO2 from the amorphous to the anatase form. Between temperatures starting at about 550 °C (550–750 °C), there should be a phase transition exothermic peak, where the anatase form of TiO2 transforms to the equilibrium rutile phase. According to quantum mechanics, the smaller particle, with large surface activity, may decrease the phase transition temperatures.
Figure 4 shows DSC curves of TiO2 particles based on the microwave-assisted method. It indicates that the peak temperatures at the anatase form transition are lower under neutral conditions than those at acidic and alkali conditions. As the heating rate increases (lower heating time elapse), a lower anatase transition temperature is expected. This is reversed under acidic conditions. The lower heat rate results in a lower anatase transition temperature. This is also the case when synthesis occurs under alkali conditions. Generally, the lower anatase transition temperatures correspond to the smaller particle size of the TiO2 nanoparticle. The summary of the anatase transition temperatures is listed in Table 2. It was found that sizes of TiO2 prepared under neutral conditions were smaller than those prepared under both the acidic and alkali conditions. Also, microwave heating is a better approach than normal heating in view of quality (size) of TiO2 nanoparticles because the microwave can effectively and directly deliver energy. It is interesting to find that there are phase transition peaks at about 275 °C, when the microwave-assisted method is applied. It is attributed to the transition of the amorphous form to the anatase form of TiO2.
TG curves of TiO2 from both the microwave-assisted method and the sol–gel method are similar. There is a mass loss peak at 100 °C corresponding to moisture vaporization; and a mass loss peak at about 245 °C. The latter one should correspond to adsorbed organics, whose mass loss process extends to 600 °C. There is no significant difference among mass loss amount of organics, which may be the result of equal adsorption capability among all TiO2 from different preparation methods (Figs. 5 and 6).
UV–Visible absorption spectrum
Figure 7 illustrates that P25 are photoactive in a range of UV spectrum (200–400 nm) and also Vis spectrum (400–650 nm), and more active in the UV spectrum than that in the Vis spectrum. The absorption peak appears at about 360–380 nm in the UV spectrum. The TiO2 based on the microwave-assisted method is also photoactive in both UV–Vis spectrum, but the peak moves into ultra-UV side, which is at 280–300 nm. The TiO2 prepared using the hydrothermal method shows a similar performance and peak at 300 nm. However, its photoactivity in the Vis spectrum totally disappears. The performance of the sol–gel based TiO2 is even worse. Its photoactivity moves into the ultra-UV spectrum and totally disappears in near-UV and Vis spectra. It seems that the sizes of the nanoparticle TiO2 are completely responsible for their photoactivities. The sol–gel based TiO2 has larger particle size, which is attributed to its lower photoactivity. Increased particle sizes indeed increase photoactivity, such as those TiO2 samples based on the hydrothermal method and the microwave-assisted method. Among them, the microwave method seems effective to decrease the particle size of the prepared TiO2, and consequently improves its photoreactivity. Some reports show good performance by TiO2 nanoparticles using the sol–gel method as a precursor, not the titanium isopropoxide in this study. Improvement of photoactivity of TiO2 based on microwave-assisted method using titanium tetrachloride as the precursor is expected.
Conclusions
-
(1)
The pH value is one of the major factors in controlling the nano-sizes of TiO2 particles; also, the neutral condition in all methods is preferable to control sizes of the prepared TiO2 particles.
-
(2)
DSC is a powerful tool in identifying the crystalline transition of TiO2 and a useful tool to quickly determine the particle size of TiO2.
-
(3)
Nano-size of TiO2 has been confirmed by both the direct size measurement using TEM images, and indirect determination using XRD peaks.
-
(4)
The collected samples were further analyzed using UV–Vis spectroscopy to identify the particle size-dependent photoreactivity. The microwave-assisted method under neutral conditions could better control size of TiO2 below 10 nm, and thus confirm the effectiveness of photoreactivity using the microwave assisting under neutral condition.
References
Reyes-Coronado D, Rodr´ıguez-Gattorno G, Espinosa-Pesqueira ME, Cab C, de Coss R, de Coss R, Oskam G. Phasepure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology. 2008;19:145605.
Cui Y, Cao Y, Pan WP. Preparation of copper-based oxygen carrier supported by titanium dioxide. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3131-2.
Zhou WY, Tang SQ, Wan L, Li DY, Zhang SY. Influence of preparation conditions on Nano TiO2 crystal phase structure. Chin J Rare Matals. 2004;28(4):631.
Ozuomba JO, Ekpunobi AJ. Natural dyes adsorbed on nanocrystalline TiO2 for photovoltaic applications. Pelagia Research Library. Der Chemica Sinica. 2013;4(3):137–43.
Munusamy S, Aparna R, Prasad R. Photocatalytic effect of TiO2 and the effect of dopants on degradation of brilliant green. Sustain Chem Process. 2013;1(1):1–8.
Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C. 2000;1:1–21.
Jeng MJ, Wung YL, Chang LB, Chow L. Particle size effects of TiO2 layers on the solar efficiency of dye-sensitized solar cells. Int J Photoenergy. 2013. doi:10.1155/2013/563897.
Guldin S, Hüttner S, Kolle M, Welland ME, Buschbaum PM, Friend RH, Steiner U, Tétreault N. Dye-sensitized solar cell based on a three-dimensional photonic crystal. Nano Lett. 2010;10(7):2303–9.
Ismagilov ZR, Tsykoza LT, Shikina NV, Zarytova VF, Zinoviev V, Zagrebelnyi SN. Synthesis and stabilization of nano-sized titanium dioxide. Russ Chem Rev. 2009;78(9):873.
Vijayalakshmi R, Rajendran V. Synthesis and characterization of nano-TiO2 via different methods. Applied Science Research. Arch Appl Sci Res. 2012;4(2):1183–90.
Ibrahim NB, Yusrianto E, Ibrahim NBZ. Effect of different TiO2 preparation techniques on the performance of the dielectric bolometer Ba06Sr0.4TiO3 as a distance sensor. Sains Malaysiana. 2012;41(8):1029–35.
Diebold U. The surface science of titanium dioxide. Surf Sci Rep. 2003;48(5):53–229.
Liu G, Wang LZ, Yang HG, Cheng HM, Lu QG. Titania-based photocatalysts-crystal growth, doping and heterostructuring. J Mater Chem. 2010;20:831–43.
Frosch T, Hormann U, Kubiak P, Kucerova G, Pfanzelt M, Weiss CK, Behm RJ, Husing N, Kaiser U, Landfester K, Wohlfahrt-Mehrens M. High surface area crystalline titanium dioxide: potential and limits in electrochemical energy storage and catalysis. Chem Soc Rev. 2012;41:5313–60.
Acknowledgements
This study is under support of the ICSET of WKU for an Internship program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, CW., Cao, Y., Li, H. et al. Synthesis of TiO2 based on hydrothermal methods using elevated pressures and microwave conditions. J Therm Anal Calorim 116, 1241–1248 (2014). https://doi.org/10.1007/s10973-014-3740-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3740-4