Abstract
The curing behaviour of 3,4-epoxycyclohexylmethyl 3,4-epoxycyclohexane carboxylate was investigated by the dynamic differential scanning calorimetry (DSC) using phosphorus-containing poly(amide–imide)s (PAIs) having free amine groups, 4,4′-diaminodiphenylmethane (PM) and p-phenylenediamine (PA), in the ratio of 1:1. The PAIs were prepared by co-polymerization of diimide–diacid (DIDA) and phosphorus-containing triamines having phenylene moiety. l-Tryptophan and pyromellitic anhydride were used to synthesize DIDA. Triamines used in the synthesis PAIs were tris(3-aminophenyl) phosphine (TAP), tris(3-aminophenyl) phosphine oxide (TAPO) and bis(3-aminophenyl) aminotolyl phosphine (BAP). TAP-, TAPO- and BAP-containing PAIs were designated as PTAP, PTAPO and PBAP, respectively. These PAIs with free amine groups were characterized by FTIR, 1H NMR, 13C NMR spectroscopic techniques and elemental analysis. The mixture of PAIs and PM or/and PA in the ratios of 0:1, 1:0 and 0.5:0.5 was used for investigation. DSC was used to study the curing of epoxy by recording the DSC scans at heating rates of 10 °C min−1. Thermal stability of epoxy resin cured isothermally was evaluated by recording thermo gravimetric traces in nitrogen atmosphere at the heating rate of 20 °C min−1. All samples are highly stable, and the 10 % mass loss found was in the range of 335–520 °C. The percent char yield was highest in case of resin sample E/PM/PTAPO. The flame-retardant properties of cured epoxy resins were investigated by the limiting oxygen index test (LOI) and UL94 test. When phosphorus was incorporated in epoxy resin, the epoxy resin system met the UL94 V-0 classification and the LOI reached at 37.8, because of nitrogen–phosphorus synergistic effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins were first commercialized in 1946 and are widely used in many major industrial applications, especially where major technical advantages warrant their somewhat higher cost with respect to other thermosets. However, the application of epoxy resins is sometimes constrained owing to their high viscosity and especially their poor impact resistance [1]. The characteristics of high chemical and corrosion resistance, good mechanical and thermal properties, toughness, low shrinkage upon cure, high adhesion to various substrates, flexibility, good alkali resistance, good electrical properties and ability to be processed under a variety of conditions enable epoxy resins to be widely used in adhesive, fields of aerospace, building, automobile, laminating, surface coating and as structural insulating materials for electronic devices [2–8]. Nowadays, facile epoxy resins with special structures and properties are becoming academically and technologically more important, with the rapid development of ultrahigh technology, like the light-emitting diode encapsulation [9], special rubber and metal bonding [10] and wind turbine blades adhesive [11]. However, one of the major drawbacks of epoxy resin is its inherent flammability, which restricts its application in many fields in view of safety consideration [12]. Therefore, the issue of improving the flame retardancy of epoxy resin is very important. Epoxy resin can be rendered fire retardant either by incorporating fire-retardant additives or by copolymerization with reactive flame retardants [13–17]. Besides this, almost all properties of epoxy can be modified to meet a specific need. For example, despite the excellent dielectric properties of epoxy, silver-filled epoxies with good electrical conductivity are widely available—although epoxies are typically electrically insulating—and are considered promising alternatives for lead-containing solder alloy in the electronics industry.
However, conventional epoxy resins are inefficient to satisfy the required properties in the field of advanced materials, which require specific properties such as high thermal and flame resistance. To meet some application requirements, many studies have been conducted on the curing reaction kinetics, flame retardancy and thermal stability of thermosetting epoxy resins employing various techniques, experimental procedures and data analysis methods [18–22]. Various types of curing agents, such as nitrogen-containing agents (amines and polyamides), oxygen-containing agents (anhydride), and sulphur-containing agents and halogen-based compounds have been reacted with epoxy resins to provide crosslinked adhesives. Traditionally, the incorporation of such compounds comprised an economical route for enhancing the flame retardancy of polymers without relinquishing product quality. Halogen-containing flame retardants are reported to be effective flame retardants for epoxy resins; therefore, their use is restricted due to their generating dense, toxic smoke and corrosive products generated during combustion [23]. However, regulatory concerns about the human and environmental contamination caused by the toxic dioxins and furans, which are evolved during the combustion of halogens, have pushed the market trend to halogen-free flame retardants. While being non-toxic and environmentally sound, halogen-free compounds, especially phosphorus-containing substances, require high levels of loading, leading to additional costs, processing difficulties and deterioration of polymer mechanical properties. Consequently, the objective of developing highly effective flame retardants has prompted a rapid progress during the last decade, extending research into novel technologies. In this study, an amino acid was used for the preparation of curing agents so that toxicity can be avoided at this stage at least. l-Tryptophan was chosen for this purpose as it contains an amine group for nucleophilic substitution to 3,4-epoxycyclohexylmethyl 3,4-epoxycyclohexane carboxylate (ECHM). The incorporation of organophosphorus functionality, either within the parent chain or as groups appended to it, has led to the production of inherently fire-retardant polymers with high thermal oxidative stability, enhanced solubility in organic solvents, improved miscibility and good adhesion to other compounds. Therefore, the phosphorus–nitrogen synergistic effect on flame retardancy is very interesting [14, 24–29]. The objective for selection of heterocyclic rings in the main chain or as pendent group of the synthetic polymer was to impart certain preferred properties to the polymer. Among different heterocyclic rings, the advantage of using a pyrrole nucleus is based on its high thermal stability and photoconductive property derived from the conjugated aromatic rings [30].
Recent studies demonstrate that epoxy resins cured with poly(amide–imide)s (PAIs) have superior physical properties compared to those with different amines and are widely used in the electronic industry as moulding and sealing compounds because they exhibit better heat resistance, higher modulus, lower tensile elongation and wider range of cure temperatures than amine-cured systems. In the present work, curing behaviour, resultant thermal stability and flame retardancy of ECHM were investigated using the mixture of PAIs and 4,4′-diaminodiphenylmethane (PM) or/and p-phenylenediamine (PA). Therefore, when phosphorus containing PAIs are incorporated into the network of thermoset polymers, they can increase the thermal property and flame retardancy of the polymers because of phosphorous–nitrogen flame-retardant synergy.
Experimental
Materials
ECHM, triphenyl phosphine, triphenylphosphine oxide, biphenyl tolyl phosphine, pyromellitic anhydride (PMDA), PM, PA were purchased from Sigma Aldrich and used as received. l-Tryptophan and glacial acetic acid were purchased from Merck and used without any purification. Pyridine (CDH) was purified by distillation under reduced pressure over calcium hydride. Triphenyl phosphite (TPP, Merck) was used as received. Anhydrous calcium chloride was dried under reduced pressure at 150 °C for 6 h prior to use. N-methyl-2-pyrrolidone (NMP, Sigma Aldrich) was dried over phosphorus pentoxide for at least 15 h and distilled under reduced pressure. Ethanol (Merck) and sodium acetate (Qualigens) were used as received. N,N′-dimethyl formamide (DMF, Qualigens) was dried by keeping it over calcium hydride for 72 h followed by distillation under reduced pressure.
Techniques
Differential scanning calorimetry (DSC) scans were obtained from samples of about 6–8 mg in nitrogen atmosphere at a heating rate of 10 °C min−1 using a TA 2100 thermal analyser having DSC 910 module. Thermal gravimetric analysis (TG) was performed with a Rheometric Scientific Module at a heating rate of 20 °C min−1 in nitrogen atmosphere. Flammability (reaction to a small flame) was investigated by limiting oxygen index (LOI) tests using flammability tester as per the standard ASTM D 2863-77 procedure. Flammability rating was also determined by UL94 vertical tests where the sample size of 125 × 12.5 × 3 mm was used. Specimens were mounted vertically. A flame was introduced to the specimen at an angle of 45° for 10 s.
Monomer synthesis
Synthesis of triamines
Tris(3-aminophenyl) phosphine (TAP, a), tris(3-aminophenyl) phosphine oxide (TAPO, b) and bis(3-aminophenyl) aminotolyl phosphine (BAP, c) were prepared by nitration of triphenyl phosphine or triphenyl phosphine oxide or diphenyl tolyl phosphine, respectively and then suggested to reduction with Pd/C and hydrazine hydrate according to the procedure reported in the literature [31] with some modifications as given below.
Triphenyl phosphine was nitrated by using conc. H2SO4 (30 mL) and conc. HNO3 (30 mL) at 0 °C. The nitrating mixture was cooled and then added into a solution of triphenylphosphine (10 g, 0.038 mol) in conc. H2SO4 taken at the temperature below 8 °C over a period of 3 h. The reaction mixture was poured into 1 L ice cold water under stirring to give gummy yellow precipitate. The precipitate was collected by filtration and washed with water until the filtrate was free from acid. The product was recrystallized from ethyl alcohol:acetic acid (2:1), and good yield of tris(3-nitrophenyl) phosphine was obtained. An ethanolic solution of tris(3-nitrophenyl) phosphine was reduced with Pd/C and hydrazine hydrate. Tris(3-nitrophenyl) phosphine and Pd/C (0.8 g) were dissolved in ethyl alcohol (125 mL) taken in a three-necked round bottom flask. Hydrazine hydrate (25 mL) was added drop wise inTO well-stirred mixture. The reaction was exothermic in nature, and (12 g, 0.030 mol) so the mixture was cooled initially. After dissolving the entire solid, a clear solution was obtained which was refluxed for 4 h with stirring. The hot solution was filtered and concentrated by removal of ethanol under reduced pressure. Upon cooling, white shiny crystals of (a) were obtained. These white crystals were recrystallized in methanol to give pure crystal of TAP (a) leaving behind impurities in solution. A similar procedure was used to prepare TAPO (b) and BAP.
Structures of both the synthesized phosphorus-containing amines, cycloaliphatic epoxy resin, l-tryptophan used and diamines are given in Scheme 1.
Synthesis of diimide–diacid (DIDA) (A)
Into a 100 mL round-bottom flask (5.45 g, 2.5 × 10−2 mol) containing PMDA l-tryptophan (10.21 g, 5.0 × 10−2 mol), a mixture of acetic acid and pyridine (50 mL, 3:2) and a stirring bar were placed. The mixture was stirred at room temperature for overnight and was refluxed for 4 h according to Scheme 2. The solvent was removed under reduced pressure, and the residue was dissolved in 100 mL cold water. Then the solution was decanted, and 7 mL of concentrated HCl was added. An orange colour precipitate was formed, filtered off and dried, to give (95.6 %) of compound.
Polymer synthesis
A mixture of DIDA (1 mmol) and phosphorus containing amines (1 mmol), CaCl2 (0.30 g), TPP (0.6 mL) and pyridine (1 mL) in NMP (5 mL) was stirred at 100 °C for 4 h in a 25 mL flask. The viscosity of the reaction solution increased after 1 h, and an additional volume of NMP was added to carry out the reaction in homogeneous medium. At the end of the reaction, the polymer solution was slowly added drop wise trickled into stirred methanol, to precipitate the product. The polymer was washed with hot water and methanol, filtered and dried in vacuum. All the other PAIs were synthesized by similar procedure. The syntheses of PAIs are shown in Scheme 2.
Characterization
The structural characterizations of the synthesized PAIs were done by FTIR, 1H NMR, 13C NMR, 31P NMR spectroscopic techniques and elemental analysis. IR spectra were recorded on Perkin Elmer RXI spectrophotometer. Spectra of solids were carried out using KBr pellets, and vibrational transition frequencies are reported in wavenumber (cm−l). 1H NMR, 13C NMR spectra were recorded on a BRUKER TOP-SPIN 300 MHz Spectrophotometer using dimethyl sulphoxide (DMSO)-d 6 as a solvent and tetramethyl silane as an internal reference at room temperature. Elemental analysis was carried out using GmbH VarioEL CHNS elemental analyser.
Curing studies
For curing studies, samples were prepared by mixing the required amount of ECHM with PAIs or PM or/ PA or mixture of PAIs/PM, or PAIs/PA at different molar ratios using minimum amount of low boiling solvent. After thorough mixing, the solvent was then evaporated by vacuum stripping, and the freshly prepared samples were used for recording DSC traces at a programmed heating rate from raising room temperature up to 300 °C. Epoxy resins cured with PAI–PTAP or PTAPO or PBAP and PM or PA designated as E/PTAP, E/PTAPO, E/BAP, E/PM and E/PA, respectively. Similarly samples cured using mixture PAIs/PM or/ PA were designated as E/PM/PTAP, E/PM/PTAPO, E/PM/PBAP, E/PA/PTAP, E/PA/PTAPO and E/PA/PBAP, respectively.
Thermal stability
Thermal stabilities of the epoxy resins cured isothermally by heating to 200 ± 20 °C for 3 h in an air oven in the presence of PAIs or PM or PA or a mixture of PAIs and diamines PM or/ PA in 0:1, 0.5:0.5 and 1:0 molar ratios were evaluated by recording TG/DTG traces. A heating rate of 20 °C min−1 and powdered samples (7 ± 4 mg) were used in each experiment.
Results and discussion
Structural characterization of DIDA
The PAIs synthesized in laboratory were insoluble in tetrahydrofuran, toluene and chloroform, but soluble in DMSO, NMP (N-methyl pyrrolidone), DMF (dimethyl formamide) and dimethyl acetamide. Table 1 shows the CHN analysis of DIDA and PAIs. The calculated values of CHN of DIDA and all PAIs agreed well with the experimentally determined values.
Figure 1 represents the FTIR spectrum of DIDA–AP. The FTIR spectrum of AP showed the characteristic absorption bands at 3,435 cm−1 (–OH, carboxylic acid), 1,770 cm−1 (imide C=O asymmetric stretching) and 1,730 cm−1 (imide C=O symmetric stretching and carboxylic acid C=O), confirming the presence of carboxylic groups and imide ring in the structure. As shown in Fig. 2, the 1H NMR spectrum of DIDA–AP presented signals in downfield region around 8.42–8.95 δ ppm due to Ar–H proton. A singlet was obtained at 11.1 δ ppm due to –COOH proton. Figure 3 shows the 13C NMR spectrum of DIDA–AP. In this spectrum, carbonyl carbon of imide group and carboxylic acid were observed at 165.8 and 177.3 δ ppm, respectively.
Polymer characterization
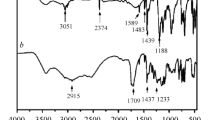
Structural features of all PAIs were characterized by FTIR, 1H NMR, 13C NMR and 31P NMR spectroscopic techniques. FTIR spectrum of polymer PTAPO exhibited characteristic absorption bands at 1,727 and 1776 cm−1 (typical of imide carbonyl symmetrical and asymmetrical stretching, respectively), 1,380 cm−1 (C–N stretching), 760 cm−1 (imide ring deformation) together with some absorption bands around 1,486 and 1,138 cm−1 due to P–Ar stretching and P–O stretching, respectively. A broad peak is obtained due to –NH2 group in the region of 3,360 cm−1. IR spectrum of PAI–PTAPO is shown in Fig. 4.
A representative 1H NMR spectrum of PAI–PTAPO is shown in Fig. 5, which is in good agreement with the polymer structure. In this spectrum, the existing aromatic protons resonate in the region with a range of 6.4–7.7 δ ppm. Among the aromatic protons, the protons adjacent to the imide ring appeared at the farthest downfield because of the resonance of imide ring. Characteristic signals are obtained at 4.4 and 7.9 δ ppm due to free amine group and –CONH of imide group, respectively. Two aromatic proton ‘a’ between imide rings appeared as singlet at 9.45 δ ppm. A sharp peak at 10.1 δ ppm is observed due to –NH proton present in pyrrole ring. Figure 6 represents 13C NMR spectrum of PAI–PTAPO. The spectrum showed signals for carbonyl carbons of amide and imide groups at 170.1 and 168.2 δ ppm, respectively. Carbons of benzene ring appeared between 113.8 and 144.4 δ ppm. The spectral data corresponding to other carbon atoms were in good agreement with the proposed structure of polymer.
Curing studies
Curing of epoxy resin depends on the structure and reactivity of the curing agent as well as on the stoichiometry. Curing of epoxy resin in the presence of amines of varying structure is widely reported in the literature [32, 33], but very few reports are available with PAIs having free amine group as curing agents. No literature reports are available on the use of phosphorus containing PAIs as curing agent. Figure 7 shows the DSC scans of ECHM in the presence of stoichiometric amounts of different PAIs and diamines at a heating rate of 10 °C min−1. In all the samples, a broad curing exotherm was observed upon curing with PAIs, diamines and with the mixture of PAIs/diamines. These results thus clearly showed that amine group of both PAIs and diamines act as co-curing agent for epoxy. DSC exotherms were characterized by noting the following temperatures:
-
T i = kick-off temperature, where the curing starts.
-
T onset = temperature where the first detectable heat is released. It was obtained by extrapolation of the steepest portion of curve.
-
T p = temperature of peak position of exotherm.
-
T f = temperature of end of curing exotherm obtained by extrapolation of the end set of the exotherm transition.
-
ΔH = heat of curing, calculated by measuring area under the exothermic transition.
The characteristic curing temperatures are summarized in Table 2.
All the characteristic curing temperatures, i.e. T i, T o, T p and T f showed the effect of phosphorus-containing PAIs, diamines and the mixture of PAIs/diamines. All these temperatures are the lowest in the presence of diamines PM or/ PA and the highest in case of PM/PTAPO mixture. The following trend was observed at the peak exotherm temperature.
Curing characteristics are dependent upon the nucleophilicity of amines. In the present work, PAIs have been derived from the DIDA and phosphorus-containing triamine TAP, TAPO and BAP. These PAIs thus differ by the presence of tolyl, phenyl and phenyl with P–O linkage in the amine moiety. It is well known that P–O is a strong electron-withdrawing group both by negative induction (−I) and negative mesomeric (−M) effects; thus, its nucleophilicity is considerably reduced. On the basis of nucleophilicity, one would have expected the highest curing temperature with P–O based PAI having free amine groups. However, in the present work, the highest curing temperature was observed with PTAPO and PM/PTAPO mixture having methylene linkage which has a positive induction effect and no mesomeric effect. These results are therefore difficult to explain on the basis of nucleophilicity. The other parameter which can affect the curing temperatures is diffusion. The highest curing temperature observed with PM/PTAPO in the present work could be restricted by diffusion. In case of all samples, a symmetrical exothermic peak was observed. The value of ΔH was in the range of 100.4–190.3 J g−1, which was the lowest for the sample E/PA and the highest for E/PM/PTAPO.
FTIR of cured E/PM/PTAPO
To understand the reaction mechanism of E/PM with PTAPO, FTIR spectra of E/PM/PTAPO were collected during various steps of curing process and are represented in Fig. 8, which shows the spectra of E/PM/PTAPO mixture at different temperatures. These spectra show that no peak change occurred from the room temperature to 200 °C, whereas after 250 °C was reached, a change in spectrum was observed. The dramatically reduced peak strength for the epoxide group at 924 cm−1 and amine group characteristic peak at 3,312 cm−1 suggested a ring-opening reaction between them.
Thermal stability
The relative thermal stabilities of the cured resins were evaluated by comparing the initial decomposition temperature (IDT), the temperature of maximum rate of mass loss (T max) and the percentage char at 800 °C. Figure 9 shows TG/DTG traces of epoxy resin samples i.e. E/PM, E/PA, E/PM/PTAPO and E/PA/PTAPO. The results of TG/DTG derivative traces in nitrogen atmosphere of epoxy cured isothermally (at 200 °C for 2 h) using PAIs/diamines are summarized in Table 3. All the samples were stable up to 239 °C and lost their masses above this temperature. The degradation temperature was dependent on the structure of network. Single-step degradation was observed in all samples, whereas sample E/PA shows two step decomposition. In case of all the samples, almost no mass loss was observed after 700 °C, whereas sample E/PM/PTAPO showed a mass loss of 11.2 in the temperature range of 700–800 °C. Char yield was the highest in case of cured resins having P–O and P–C linkages in the backbone. The trend in char yield was almost the same at 700 °C, but the char yield in case of E/PM/PTAPO was found to be 55 %. These results clearly show that flame-resistant cycloaliphatic epoxy resin can be obtained by using curing agents having combination of phosphorus and nitrogen as flame-retardant elements in their backbone.
Flame-retardant properties of epoxy thermosets
The flame-retardant properties of all samples have been examined by LOI and UL94 tests, and the data are summarized in Table 4. The LOI measures the minimum oxygen concentration (in an oxygen–nitrogen flowing mixture) required to support downward flame combustion which can be used as an indicator to evaluate the flame retardancy of polymers. It can be seen that LOI values significantly increase when phosphorus-containing PAIs were incorporated in epoxy network. This indicates that incorporation of phosphorus is very effective in improving the flame retardancy of epoxy resins. The vertical burning test (UL94) exhibits that the thermosets with phosphorus content can reach UL94 V-0 flammability rating. We can deduce that excellent charring interaction of phosphorus with phenyl moieties enhance the flame-retardant efficiency of the additives and that of nitrogen and phosphorus in backbone of PAIs curing agents, both combining to exert the synergistic flame retardant function of thermosets [34–36]. Moreover, the E/PAI and E/PAI/diamine systems were not dripping. The reason is that the phosphorus-containing PAI additives with outstanding charring property can promote materials to form stronger char layer. The char layer reduces the exothermicity caused by pyrolysis reactions and the thermal conductivity of the surface radiated from flame, and hence prevents the samples from dripping and improves the flame retardancy of the epoxy resin thermosets.
Conclusions
From the results of this study, it can be concluded that the thermal stability of cured epoxy was found to be dependent on the structure of network. Epoxy resin obtained with PM/PTAPO has much higher thermal stability. Composition of the mixture had a great influence on the curing and thermal behaviours. The E/PM/PTAPO thermosets with phosphorus content has the LOI value of 37.8 and reach UL94 V-0 flammability rating. Obviously, nitrogen–phosphorus synergistic flame-retardant system leads to higher flame-retardant efficiency to thermosets.
References
Zheng Y, Chonung K, Wang G, Wei P, Jiang P. Epoxy/nano-silica composites: curing kinetics, glass transition temperatures, dielectric, and thermal–mechanical performances. J Appl Polym Sci. 2009;111(2):917–27.
Meenakshi KS, Sudan EPJ, Kumar SA, Umapathy MJ. Development of dimethylsiloxane based tetraglycidyl epoxy nanocomposites for high performance, aerospace and advanced engineering applications. Prog Org Coat. 2012;74(1):19–24.
Kong J, Tang Y, Zhang X, Gu J. Synergic effect of acrylate liquid rubber and bisphenol A on toughness of epoxy resins. Polym Bull. 2008;60(2–3):229–36.
Barletta M, Vesco S, Trovalusci F. Effect of the substrate and interface on micro-scratch deformation of epoxy-polyester powder coatings. Prog Org Coat. 2012;74(4):712–8.
Toldy A, Szolnoki B, Marosi Gy. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications. Polym Degrad Stab. 2011;96(3):371–6.
Rosca ID, Hoa SV. Method for reducing contact resistivity of carbon nanotubes-containing epoxy adhesives for aerospace applications. Compos Sci Technol. 2011;71(2):95–100.
Azeez AA, Rhee KY, Park SJ, Hui D. Epoxy clay nanocomposites-processing, properties and applications: a review. Composites B. 2013;45(1):308–20.
Wang X, Hu Y, Song L, Xing W, Lu H, Lv P, Jie G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer. 2010;51(11):2435–45.
Chung PT, Yang CT, Wang SH, Chen CW, Chiang AST, Liu C-Y. ZrO2/epoxy nanocomposite for LED encapsulation. Mater Chem Phys. 2012;136(2–3):868–76.
Lee S-W, Park J-W, Park C-H, Lim D-H, Kim H-J, Song J-Y, Lee J-H. UV-curing and thermal stability of dual curable urethane epoxy adhesives for temporary bonding in 3D multi-chip package process. Int J Adhesion Adhesives. 2013;44:138–43.
Tseng Y-C, Kuo C-Y. Engineering and construction torsional responses of glass-fiber/epoxy composite blade shaft for a small wind turbine. Proced Eng. 2011;14:1996–2002.
Liu R, Wang X. Synthesis, characterization, thermal properties and flame retardancy of a novel non-flammable phosphazene-based epoxy resin. Polym Degrad Stab. 2009;94(4):617–24.
Katsoulis C, Kandola BK, Myler P, Kandare E. Post-fire flexural performance of epoxy-nanocomposite matrix glass fibre composites containing conventional flame retardants. Composites A. 2012;43(8):1389–99.
Ye J, Liang G, Gu A, Zhang Z, Han J, Yuan L. Novel phosphorus-containing hyperbranched polysiloxane and its high performance flame retardant cyanate ester resins. Polym Degrad Stab. 2013;98(2):597–608.
Zhang W, Li X, Jiang Y, Yang R. Investigations of epoxy resins flame-retarded by phenyl silsesquioxanes of cage and ladder structures. Polym Degrad Stab. 2013;98(1):246–54.
Perret B, Schartel B, Stob K, Ciesielski M, Diederichs J, Doring M, Kramer J, Altstadt V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur Polym J. 2011;47(5):1081–9.
Qian L-J, Ye L-J, Xu G-Z, Liu J, Guo J-Q. The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups. Polym Degrad Stab. 2011;96(6):1118–24.
Xing W, Jie G, Song L, Wang X, Lv X, Hu Y. Flame retardancy and thermal properties of epoxy acrylate resin/alpha-zirconium phosphate nanocomposites used for UV-curing flame retardant films. Mater Chem Phys. 2011;125(1–2):196–201.
Haurie L, Lacasta AM, Ciudad A, Realinho V, Velasco JI. Addition of flame retardants in epoxy mortars: thermal and mechanical characterization. Constr Build Mater. 2013;42:266–70.
Yoo MJ, Kim SH, Park SD, Lee WS, Sun J-W, Choi J-H, Nahm S. Investigation of curing kinetics of various cycloaliphatic epoxy resins using dynamic thermal analysis. Eur Polym J. 2010;46(5):1158–62.
Wan J, Li B-G, Fan H, Bu Z-Y, Xu C-J. Nonisothermal reaction, thermal stability and dynamic mechanical properties of epoxy system with novel nonlinear multifunctional polyamine hardener. Thermochim Acta. 2010;511(1–2):51–8.
Ghaffari M, Ehsani M, Khonakdar HA, Assche GV, Terryn H. The kinetic analysis of isothermal curing reaction of an epoxy resin–glassflake nanocomposite. Thermochim Acta. 2012;549:81–6.
Wang J-S, Liu Y, Zhao H-B, Liu J, Wang D-Y, Song Y-P, Wang Y-Z. Metal compound-enhanced flame retardancy of intumescent epoxy resins containing ammonium polyphosphate. Polym Degrad Stab. 2009;94(4):625–31.
Gao F, Tong L, Fang Z. Effect of a novel phosphorus–nitrogen containing intumescent flame retardant on the fire retardancy and the thermal behaviour of poly(butylenes terephthalate). Polym Degrad Stab. 2006;91(6):1295–9.
Chen S, Zheng Q-K, Ye G-D, Zheng G-K. Fire-retardant properties of the viscose rayon containing alkoxycyclotriphosphazene. J Appl Polym Sci. 2006;102(1):698–702.
Sun D, Yao Y. Synthesis of three novel phosphorus-containing flame retardants and their application in epoxy resins. Polym Degrad Stab. 2011;96(10):1720–4.
Chen X, Gu A, Liang G, Yuan L, Zhuo D, Hu J-T. Novel low phosphorus-content bismaleimide resin system with outstanding flame retardancy and low dielectric loss. Polym Degrad Stab. 2012;97(5):698–706.
Xiong X, Chen P, Ren R, Lu F, Yu Q. Cure mechanism and thermal properties of the phthalide-containing bismaleimide/epoxy system. Thermochim Acta. 2013;559:52–8.
Zhang W, Li X, Yang R. Blowing-out effect in epoxy composites flame retarded by DOPO–POSS and its correlation with amide curing agents. Polym Degrad Stab. 2012;97(8):1314–24.
Kaleemullah M, Khan SU, Kim J-K. Effect of surfactant treatment on thermal stability and mechanical properties of CNT/polybenzoxazine nanocomposites. Compos Sci Technol. 2012;72(16):1968–76.
Khurana P, Aggarwal S, Narula AK, Choudhary V. Curing and thermal behaviour of epoxy resin in the presence of silicon-containing amide amines. J Appl Polym Sci. 2003;87:1345–53.
Durga G, Kukreja P, Narula AK. Studies on curing kinetics and thermal behaviour of phosphorylated epoxy resin in the presence of aromatic amide–amines. J Appl Polym Sci. 2010;118:3612–8.
Singh D, Malhotra P, Narula AK. Studies on the curing kinetics and thermal stability of diglycidyl ether of bisphenol-A using mixture of novel, environment friendly sulphur containing amino acids and 4,4′-diaminodiphenylsulfone. J Appl Polym Sci. 2009;113:216–25.
Qian L, Ye L, Qui Y, Qu S. Thermal degradation behaviour of the compound containing phosphaphenanthrene and phosphazene groups and its flame retardant mechanism on epoxy resin. Polymer. 2011;52(24):5486–93.
Chen H, Zhang K, Xu J. Synthesis and characterizations of novel phosphorus–nitrogen containing poly(ether sulfone)s. Polym Degrad Stab. 2011;96(2):197–203.
Liu H, Wang X, Wu D. Novel cyclotriphosphazene-based epoxy compound and its application in halogen-free epoxy thermosetting systems: synthesis, curing behaviours, and flame retardancy. Polym Degrad Stab. 2013 (in press).
Acknowledgements
The author (S. Agrawal) wishes to express his gratitude to Guru Gobind Singh Indraprastha University, New Delhi for providing financial support in the form of IPRF.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10973_2013_3391_MOESM1_ESM.tif
Scheme 1 Structures of synthesized phosphorus-containing amines, diamines, cycloaliphatic epoxy and l-tryptophan. (TIFF 1046 kb)
Rights and permissions
About this article
Cite this article
Agrawal, S., Narula, A.K. Curing and thermal behaviour of a flame retardant cycloaliphatic epoxy resin based on phosphorus containing poly(amide–imide)s. J Therm Anal Calorim 115, 1693–1703 (2014). https://doi.org/10.1007/s10973-013-3391-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3391-x