Abstract
The enthalpies of dissolution for di(N,N-di(2,4,6,-trinitrophenyl)amino)-ethylenediamine (DTAED) in dimethyl sulfoxide (DMSO) and N-methyl pyrrolidone (NMP) were measured using a RD496-2000 Calvet microcalorimeter at 298.15 K. Empirical formulae for the calculation of the enthalpies of dissolution (Δdiss H) were obtained from the experimental data of the dissolution processes of DTAED in DMSO and NMP. The linear relationships between the rate (k) and the amount of substance (a) were found. The corresponding kinetic equations describing the two dissolution processes were \( {{{\rm{d}}\alpha } \mathord{\left/ {\vphantom {{{\rm{d}}\alpha } {{\rm{d}}t}}} \right. \kern-\nulldelimiterspace} {{\rm{d}}t}} = 10^{ - 2.68} \left( {1 - \alpha } \right)^{0.84} \) for the dissolution of DTAED in DMSO, and \( {{{\rm{d}}\alpha } \mathord{\left/ {\vphantom {{{\rm{d}}\alpha } {{\rm{d}}t}}} \right. \kern-\nulldelimiterspace} {{\rm{d}}t}} = 10^{ - 2.79} \left( {1 - \alpha } \right)^{0.87} \) for the dissolution of DTAED in NMP, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energetic ionic compound is one of the effective ways to develop new kind of high-energy, low-sensitive, and non-toxic materials. Energetic ionic compounds, which are mainly composed of organic cation and inorganic anion or organic anion, include energetic ionic salts and energetic ionic liquids [1, 2]. To meet the requirement of weapons and equipments for multifunctional energetic materials, many new energetic groups are introduced in the chemical structure of cation and anion of energetic ionic compounds by the molecular design [3–6]. This approach makes energetic ionic compounds possess the various excellent functions, such as high density, insensitivity, stability, environmental friendly, and so on [7–9].
Di(N,N-di(2,4,6-trinitrophenyl)amino)-ethylenediamine (DTAED) is a novel type of energetic ionic compound which consists of anion of N,N-di(2,4,6-trinitrophenyl)amine and cation of ethylenediamine, but the solution properties have never been reported so far. Dimethyl sulfoxide (DMSO) and N-methyl pyrrolidone (NMP) as solvents have been used extensively in our production and application, so thermochemical properties of its solution have been studied first by means of a RD496-2000 Calvet microcalorimeter. The aim of this work is to study the dissolution properties of DTAED in DMSO and NMP. At the same time, the kinetic equations of the two dissolution processes are obtained, respectively, which provides valuable information for its applications in the future.
Experimental
Materials
DTAED used as crystalloid was prepared and purified by Beijing Institute of Technology, its purity determined by LC–MS, elemental analysis, and 13C NMR was more than 99.5%. Both DMSO (ρ/g cm−3=1.098–1.102) and NMP (ρ/g cm−3=1.029–1.035) used as solvents were of analysis reagent grade, and their purity was more than 99.5%. The water used in these experiments was deionized with an electrical conductivity of 0.8–1.2 × 10−4 Sm−1, and obtained by two times purification using sub-boiling distillation device.
Equipment and conditions
All measurements were made using a RD496-2000 Calvet microcalorimeter (MianYang CP Thermal Analysis Instrument CO., LTD). The enthalpy of dissolution of KCl (spectrum purity) in distilled water measured by RD496-2000 Calvet microcalorimeter at 298.15 K was 17.234 ± 0.041 kJ mol−1, and the relative error was less than 0.04% compared with the literature value 17.241 ± 0.018 kJ mol−1 [10]. This showed that the device for measuring the enthalpy used in this study was reliable. The enthalpies of dissolution were measured at 298.15 ± 0.005 K.
Results and discussion
Thermochemical behaviors of the dissolution of DTAED in DMSO and NMP
The proper molar sample of DTAED was dissolved in DMSO and NMP at 298.15 K in order to form solution. The enthalpy of the process was detected by the RD496-2000 Calvet microcalorimeter [11–14]. Each process was repeated three times [15, 16]. The dissolution is an exothermic process. The heat flow curves obtained under the same conditions overlap with each other and are shown in Fig. 1, indicating that the reproducibility of test is satisfactory.
The thermochemical data obtained are listed in Tables 1 and 2. In Tables, a is the amount of substance, b is the molality of DTAED, Δdiss H is the molar enthalpy of dissolution, Δdiss H partial is the relative partial molar enthalpy of dissolution, and Δdiss H apparent is the relative apparent molar enthalpy of dissolution.
With the help of the values of b and Δdiss H in Table 1, the empirical formula of enthalpy describing the b versus Δdiss H relation is obtained
where A, B, and C are coefficients for the dissolution equation.
The empirical formulae of relative apparent molar enthalpy (Δdiss H apparent) and relative partial molar enthalpy (Δdiss H partial) calculated by Eq. 1 are
respectively.
According to the values in Table 2, the empirical formula describing the b versus Δdiss H relation, and the empirical formulae of relative apparent molar enthalpy (Δdiss H apparent) and relative partial molar enthalpy (Δdiss H partial) for DTAED in NMP are
respectively.
From Tables 1 and 2, we can see that the values of Δdiss H, the calculated Δdiss H apparent, and Δdiss H partial change with the values of b. We can find that the relationships between Δdiss H and b 1/2 are quadratic equation for DTAED dissolved in DMSO and NMP from Figs. 2 and 3.
The kinetics of dissolution process of DTAED in DMSO and NMP
The kinetic equations describing the dissolution of DTAED in DMSO and NMP are Eqs. 7 and 8 [17–21] which are chosen as the model function describing the dissolution process.
Substituting α = H/H ∞ into the Eq. 9, we get
In these equations, α is conversion degree, f(α) is the kinetic model function, H represents the enthalpy at time of t, i is any time during the process, H ∞ is the enthalpy of the whole process, k is the rate of DTAED dissolved in DMSO and NMP, n is the reaction order, and L is counting number.
The data needed for Eq. 10 are summarized in Tables 3 and 4.
By substituting the original data in Tables 3 and 4, −(dH/dt) i , (H/H ∞ ) i , H ∞ , i = 1,2,…,L, into the kinetic equation (10), the values of n and lnk are obtained and listed in Table 5.
Substituting the values of n and k from Table 5 into Eq. 7, we can get
for dissolution process of DTAED in DMSO, and
for dissolution process of DTAED in NMP.
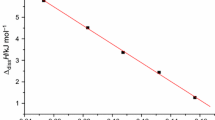
The relationships between k and a are shown in Fig. 4. One can see that the rate (k) for the dissolution processes of DTAED in DMSO and NMP increase with the amount of substance (a) increasing from Fig. 4, the linear relationships exist obviously, and the correlative coefficients (r) are 0.9981 and 0.9978, respectively.
Conclusions
-
(1)
The dissolution process of DTAED in DMSO and NMP were investigated by RD496-2000 Calvet Microcalorimeter at 298.15 K, respectively. The relationship between Δdiss H and b 1/2 of DTAED dissolved in DMSO and NMP are quadratic equations.
-
(2)
The expressions describing values of Δdiss H, Δdiss H apparent, and Δdiss H partial versus the molality (b) of DTAED in DMSO are Δdiss H = −147.76 + 3589.37b 1/2 − 29153.46b, Δdiss H apparent = 3589.37b 1/2 − 29153.46b, and Δdiss H partial = 5384.055b 1/2 − 58306.92b. The expressions describing values of Δdiss H, Δdiss H apparent, and Δdiss H partial versus the molality (b) of DTAED in NMP are Δdiss H = −205.43+4483.26b 1/2 − 33857.7b, Δdiss H apparent = 4483.26b 1/2 − 33857.7b, and Δdiss H partial = 6724.89b 1/2 − 67715.4b, respectively.
-
(3)
The kinetics equations of dissolution processes for DTAED are \( {{{\rm{d}}\alpha } \mathord{\left/ {\vphantom {{{\rm{d}}\alpha } {{\rm{d}}t}}} \right. \kern-\nulldelimiterspace} {{\rm{d}}t}} = 10^{ - 2.68} \left( {1 - \alpha } \right)^{0.84} \) in DMSO, and \( {{{\rm{d}}\alpha } \mathord{\left/ {\vphantom {{{\rm{d}}\alpha } {{\rm{d}}t}}} \right. \kern-\nulldelimiterspace} {{\rm{d}}t}} = 10^{ - 2.79} \left( {1 - \alpha } \right)^{0.87} \) in NMP.
-
(4)
The relationships between k and a are obtained, and the linear relationships exist obviously.
References
Forsyth SA, Pringle JM, Macfarlane DR. Ionic liquids—an over view. Aust Chem. 2004;57:113–9.
Ye TX, Zhang YH, Liu JH, Sun ZC. Study on preparation of ionic liquid N-alkyl imidazolium tetrofluoroborate and its solvent properties. Modern Chem Ind. 2003;23:117–9.
Li N, Chai CP, Gan YZ, Luo YJ. Review on molecular design and performance of energetic ionic compounds. Chin J Energ Mater. 2010;18:467–74.
Huang HF, Meng ZH, Zhou ZM, Gao HX, Zhang J, Wu YK. Energetic salts and energetic ionic liquids. Prog Chem. 2009;21:152–64.
Ye CF, Shreeve JM. Rapid and accurate estimation of densities of room-temperature ionic Liquids and salts. J Phys Chem A. 2007;111:1456–61.
Wang SH, Wang XR. Ionic liquids and their applications. Text Auxiliaries. 2007;24:1–4.
Hiskey M, Chavez D. Progress in high-nitrogen chemistry in explosives, propellants and pyrotechnics. In: Proceedings of 27th International Pyrotechnics Seminar, Colorado, USA; 2000. p. 3–14.
Xu SL, Yang SQ. A novel method of preparing bis-(triaminoguanidinium)-5, 5′-zaotetrazolate from 5-aminotetrazole. Chin J Synth Chem. 2005;13:486–8.
Hiskey M, Goldman N, Stine J. High-nitrogen energetic materials derived from azotetrazolate. J Energ Mater. 1998;16:119–27.
Marthada VK. The enthalpy of solution of SRM 1655 (KCl) in H2O. J Res Nat Bur Stand. 1980;85:467.
Xing XL, Xue L, Zhao FQ, Gao HX, Hu RZ. Dissolution properties of 1,1-diamino-2,2-didinitrorthylene (FOX-7) in dimethyl sulfoxide (DMSO). Thermochim Acta. 2009;32:53–7.
Xing XL, Xue L, Zhao FQ, Gao HX, Pei Q, Hu RZ. Evaluating the thermal hazard of double-base propellant SQ-2 by using microcalorimetry method. Chin J Chem. 2010;28:1369–72.
Xing XL, Xue L, Zhao FQ, Yi JH, Gao HX, Xu SY, Pei Q, Hao HX, Hu RZ. Dissolution properties of the CL-20 in ethyl acetate and acetone. J Therm Anal Calorim. 2010;99:703–7.
Zhao FQ, Xue L, Xing XL, Hu RZ, Zhou ZM, Gao HX, Yi JH, Xu SY, Pei Q. Thermochemical properties and thermokinetic behavior of energetic triazole ionic salts. Scientia Sin Chim. 2010;40:1430–43.
Xue L, Zhao FQ, Xing XL, Gao HX, Xu SY, Hu RZ. Dissolution properties of 1,3,3-trinitroazetidine (TNAZ) in ethyl acetate and N,N-dimethylformamide. Acta Phys Chim Sin. 2009;25:2413–6.
Xue L, Zhao FQ, Xing XL, Gao HX, Xu SY, Hu RZ. Dissolution properties of 3,4-dinitrofurazanfuroxan (DNTF) in N-methyl-2-pyrrolidone and dimethyl sulfoxide. Chin J Explos Propel. 2009;32:53–7.
Gao HX, Zhao FQ, Hu RZ. Differential and integral isoconversional non-linear methods and their application to energetic materials. Chin J Chem. 2008;26:1973–8.
Xue L, Zhao FQ, Xing XL, Zhou ZM, Wang K, Xu SY, Gao HX, Yi JH, Hu RZ. Thermal behavior of 3,4,5-triamino-1,2,4-triazole dinitramide. J Therm Anal Calorim. 2010;102:145–7.
Zhao FQ, Heng SY, Hu RZ, Gao HX, Han F. A study of kinetic behaviours of the effective centralite/stabilizer consumption reaction of propellants using a multi-temperature artificial accelerated aging test. J Hazard Mater. 2007;145:45–50.
Yi JH, Zhao FQ, Xu SY, Zhang LY, Ren XN, Gao HX, Hu RZ. Effect of pressures on decomposition reaction kinetics of double-base propellant catalyzed with cerium citrate. J Therm Anal Calorim. 2009;92:318–21.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008.
Acknowledgment
This study was financially supported by the Science and Technology Foundation of Science and Technology on Combustion and Explosion Laboratory of China (Grant No. 9140C350303110C3504).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, L.B., Xing, X.L., Fan, X.Z. et al. Thermochemical properties of di(N,N-di(2,4,6-trinitrophenyl)amino)-ethylenediamine in dimethyl sulfoxide and N-methyl pyrrolidone. J Therm Anal Calorim 110, 1431–1436 (2012). https://doi.org/10.1007/s10973-011-2036-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2036-1