Abstract
In the last few years there has been a growing interest in microcalorimetric investigations of microbial processes. Microcalorimetry is a technique based on heat output of bacterial metabolism which can be applied to detect bacterial growth in a few hours. However, there are few studies that attempt to investigate the interactions between two or more bacteria. In this study, we researched the interaction relationship of Enterococcus faecalis and Klebsiella pneumoniae, which are important pathogens responsible for infections in humans. The measures were carried out using a Calvet microcalorimeter, that contains two stainless steel cells of approximately 10 cm3 (reference and experimental). Experiments were carried out at concentration of 103 CFU mL−1 and a constant temperature of 309.65 K. The differences in shape of the heat flow-time curves of E. faecalis and K. pneumoniae and their mixtures were compared. Curves of E. faecalis and K. pneumoniae and their mixtures were analyzed mathematically allowing to calculate thermokinetic parameters such as growth constant (k), generation time (G), detection time (t d) and the amount of heat released (Q). The results revealed that when E. faecalis and K. pneumoniae were put together in the same proportion, the growth character of E. faecalis was not present, due to the inhibitory effects of K. pneumoniae on E. faecalis. Microcalorimetric investigations of microbial interactions is possible and promising.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enterococcus faecalis
The members of the genus Enterococcus are Gram-positive cocci, catalase-negative and facultative anaerobes that occur singly or are arranged in pairs or as short chains. E. faecalis is one of the few pathogenic species to humans in the genus Enterococcus.
The enterococci are predominantly inhabitants of the gastrointestinal tract and are less commonly found in other sites, such as in the genitourinary tract, the oral cavity, and skin, especially in the perineal area. They act as opportunistic agents, causing a variety of infections in humans. They most commonly infect the urinary tract, bloodstream, endocardium, burn and surgical site wounds, catheters, and other implanted medical device.
They are one of the most frequent causes of nosocomial infections. Because, elderly patients with serious underlying diseases and immunocompromised patients who have been hospitalized for prolonged periods, treated with invasive devices, and/or received broad-spectrum antimicrobial therapy are at higher risk to acquire enterococcal infections [1].
Klebsiella pneumoniae
The genus Klebsiella is classified in the family Enterobacteriaceae. They are Gram-negative rods, nonmotile, oxidase-negative and facultative anaerobes. Klebsiella pneumoniae contain a large polysaccharide capsule, which gives rise to large mucoid colonies.
Klebsiella is carried in the nasopharynx and the bowel; however, feces are arguably the most significant source of patient infections.
K. pneumoniae may cause a primary lobar pneumonia community-acquired. Also, this microorganism produces wound infections, soft tissue and urinary tract [1, 2].
Biomedical applications of microcalorimetry
Microcalorimetry has been used as an analytical tool in microbiology for a long time. However, in the last few years microcalorimetric investigations of microbial processes are becoming increasingly popular [3].
This technique is very interesting in microbial research, because of the fast-growing nature of microorganisms and the resultant exuberant amount of heat that is soon produced [4]. Therefore, microcalorimetry can be used to continuously measure of the associated heat production with the metabolism which occurs during the life cycle of a bacterial culture [5]. The results obtained are shown as heat flow versus time curves that are characteristic of each bacterium behaving like a “thermal fingerprint” [6, 7].
In the medical field, it has been employed as an excellent tool for rapid detection of bacterial growth within hours. Recent studies have shown the contribution of microcalorimetry in the early diagnosis of infections or microorganism contamination. It has been employed for detection of the contamination of platelets by different bacteria [8]; bacterial meningitis in cerebrospinal fluid of rats [9]; identification of enterobacteria [6], E. faecalis [10] and Pseudomonas aeruginosa [11] in a culture medium; slow-growing bacteria such as mycobacteria and especially Mycobacterium tuberculosis in culture [12, 13] and urinary tract pathogens in sterile urine [14] or artificial urine [15]. Similarly, it is also possible to determine inhibitory effects and/or the minimal inhibitory concentration for different antimicrobial compounds [16, 17] and antibiotics within hours [18–20]. In addition, calorimetry can allow rapid discrimination of medically important microorganisms, for example the rapid differentiation between methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSA) [21, 22]. Also, the microcalorimetry has been used for study on the antimicrobial properties of silver-containing wound dressings [23].
However, previous reports mainly focused on the study of a single microorganism, few studies have tried to investigate the interactions of two or more microorganisms using the microcalorimetry [24]. In this paper, we study the growth curves of E. faecalis, K. pneumoniae and their mixtures in different proportions using a Calvet microcalorimeter. The differences in shape of the heat flow-time curves and thermokinetic parameters of single microorganisms and their mixtures were compared. The interaction relationship between E. faecalis and K. pneumoniae was analyzed.
Materials and methods
Sample preparation
We have used E. faecalis from the American Type Culture Collection (ATCC 29212) and K. pneumoniae from the Spanish Type Culture Collection (CECT 144).
Samples were inoculated on blood agar plates and incubated at 309.65 K in an incubator for 24 h. The blood agar plates with multiple bacterial colonies were then used to prepare a bacterial suspension with 0.9 % saline solution. This concentration was adjusted to the corresponding of 0.5 on the McFarland scale using a Densichek® optical densitometer and diluting with 0.9 % saline solution to obtain final concentration of 103 CFU mL−1. Below, mixtures of both bacteria were prepared in the following proportions: 50 + 50 % (0.5 mL E. faecalis + 0.5 mL K. pneumoniae), 20 + 80 % (0.2 mL E. faecalis + 0.8 mL K. pneumoniae) and 80 + 20 % (0.8 mL E. faecalis + 0.2 mL K. pneumoniae).
The culture medium used was a liquid enriched with digested soy-casein.
Experimental equipment
The measures were carried out using a Calvet microcalorimeter [25] equipped with a device allowing operation in the absence of vapor phase, and having two Teflon® screw capped stainless steel cells of approximately 10 cm3 (sample and reference). A Philips PM2535 multimeter and a data acquisition system were linked to the microcalorimeter. Calibration was performed electrically using a Setaram EJP30 stabilized current source. The precision in calorimetric signal was ±1 μV. Further details about the experimental method of operation have been published [26].
Experimental setup and data collection
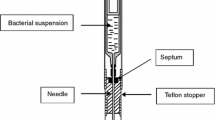
The external environment of the calorimeter was maintained at a constant temperature of 309.65 K. The reference cell was injected with 7 mL of culture medium + 1 mL of saline while the experimental cell was injected with just 7 mL of culture medium (Fig. 1). Both cells were then introduced through two cylindrical holes aligned in parallel, which extended from the upper part of the microcalorimeter to the internal thermopile chamber. The large distance that separates the cells from the entrance permits minimization of heat flow to the exterior. The system is allowed to stabilize for about 2 h, after which 1 mL of the sample is introduced into the experimental cell [6, 10, 11]. The experiment was also carried out with a sample not containing any bacteria (control).
Data were collected using the data collection and processing system, at intervals of 15 s, throughout the duration of the experiment.
Results
By plotting heat voltage difference versus time between the experimental cell and the reference cell, we are able to obtain the exothermic growth curves of E. faecalis, K. pneumoniae and their mixtures at 103 CFU mL−1.
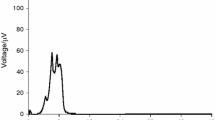
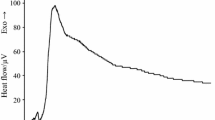
The curve of E. faecalis (Fig. 2) has a unique energetic phase in which stand out three peaks of electromotive force, and the signal is recorded for about 10 h, returning to baseline levels after this time. The curve of K. pneumoniae (Fig. 3) presents a first phase characteristic, which after the trace peak is a plateau prior to a steep drop to very low levels, on the order of 10 μV. After this point, there is an exponential increase of the heat flow, without reaching a peak during the time it takes to complete the experiment.
When we compare the curves of E. faecalis, K. pneumoniae and their mixtures, we can see that graphs of 50 % E. faecalis + 50 % K. pneumoniae (Fig. 4a) and 20 % E. faecalis + 80 % K. pneumoniae (Fig. 4b) have a similar profile to the curve of K. pneumoniae (Fig. 3), with a maximum peak after which there is a small plateau before falling to very low levels, approximately of 10 μV. From this point the heat flow increases through the duration of the experiment. However, in the case of curve 80 % E. faecalis + 20 % K. pneumoniae (Fig. 4c) having a first phase with two peaks with a shape similar to the graph of E. faecalis (Fig. 2) and a second phase similar to the curve K. pneumoniae (Fig. 3), in which after the second peak occurs a decrease of potential up to 10 μV, after which the potential is increased with the duration of the experience.
We have treated the data mathematically, fitting them to exponential equations in ascending and descending phases of each peak of electromotive force and to polynomial-type equations in the areas of higher activity of the curve.
From the polynomial equations, by the first derivative we determinate the value of the maximum voltage peak (V max) and the time of it registration (t max) in the thermograms of E. faecalis, K. pneumoniae and their mixtures. Although we cannot establish a relationship of proportionality of the maximum peaks and their appearance time between pure cultures and their mixtures, all experiments show a maximum peak before 10 h (Table 1).
The detection time of the signal (t d) of E. faecalis, K. pneumoniae and their mixtures are shown in Table 1. As can be observed the sample 80 % E. faecalis + 20 % K. pneumoniae has a t d less than 50 % E. faecalis + 50 % K. pneumoniae and 20 % E. faecalis + 80 % K. pneumoniae. Perhaps this could be because E. faecalis has a lower t d K. pneumoniae. In all curves growth can be detected in less than 7 h.
In the logarithmic phase of the growth curve, we adjusted the data to an exponential equation. At this stage of the growth curve, microbial proliferation can be expressed as:
where n 0 is the number of cells at onset (time 0), n t the number of cells at time t, and k the growth constant [6, 10, 11].
If we consider P w as energy released by each cell, then it leads to the following equation:
Taking into account that P 0 is the energy released at onset and P t is the energy released at time t:
The generation time (G) is defined as the time taken by a population to double in size, and is expressed as:
Table 2 shows the parameters k and G of single microorganisms and their mixtures. As can been observed, K. pneumoniae has a k lower and G greater than E. faecalis. Therefore, this would explain that the mixtures 50 % E. faecalis + 50 % K. pneumoniae and 20 % E. faecalis + 80 % K. pneumoniae have k lower and G greater than the mixture 80 % E. faecalis + 20 % K. pneumoniae. All correlation coefficients (R 2) were larger than 0.966, indicating a good linear relationship.
From the curve, we can also calculate the amount of heat released (Q) over the culture time:
where A (μV h) represents the area, calculated by the trapezoidal method, and K is a constant, value, 24.8 J μV−1 h−1, of which was calculated from the electric calibration performed by the Joule effect on the equipment.
In Table 3, we have compared the values of heat released by microorganisms and their mixtures during the first 24 h (Q 24). As was observed, the Q 24 by E. faecalis is approximately half that of K. pneumoniae, because the total life period of E. faecalis is shorter than K. pneumoniae. Therefore, the mixtures 50 % E. faecalis + 50 % K. pneumoniae and 20 % E. faecalis + 80 % K. pneumoniae produce a greater amount of heat than the mixture 80 % E. faecalis + 20 % K. pneumoniae.
Discussion
Microcalorimetry has proven to be an excellent tool for rapid detection of bacterial growth; however, there are few studies that attempt to investigate the interactions between two or more bacteria.
In this paper, we have studied the growth curves of E. faecalis, K. pneumoniae and their mixtures in different proportions, which are important pathogens responsible for infections in humans.
We have prepared three samples mixing of both bacteria in the following proportions: 50 + 50 % (0.5 mL E. faecalis + 0.5 mL K. pneumoniae), 20 + 80 % (0.2 mL E. faecalis + 0.8 mL K. pneumoniae), and 80 + 20 % (0.8 mL E. faecalis + 0.2 mL K. pneumoniae). The measures were carried out using a Calvet microcalorimeter. Then, we have compared and analyzed the differences in shape of the heat flow-time curves of single microorganisms and their mixtures in different proportions.
We have found that the heat flow-time curve of 50 % E. faecalis + 50 % K. pneumoniae (Fig. 4a) is very similar with the K. pneumoniae (Fig. 3), and significantly different from that of E. faecalis (Fig. 2). The possible reason might be that K. pneumoniae has inhibited the growth of E. faecalis, so the characteristic behavior of E. faecalis could not be observed. The curve of 20 % E. faecalis + 80 % K. pneumoniae (Fig. 4b) has a similar profile to the curve of K. pneumoniae (Fig. 3), because of this higher percentage in the sample, the growth character of E. faecalis was not observable. Finally, the curve 80 % E. faecalis + 20 % K. pneumoniae (Fig. 4c) has a first phase shape like the graph of E. faecalis (Fig. 2) and a second phase similar to the curve K. pneumoniae (Fig. 3). That is because there is 80 % of the sample of E. faecalis, predominating its growth profile. Also, its signal was recorded for about the first 10 h, from the moment the signal of K. pneumoniae was recorded until the completion of the experiment.
We treated mathematically growth curves and calculated quickly and easily the growth constant (k) and generation time (G) of E. faecalis, K. pneumoniae and their mixtures. In our study, we have seen that the mixtures 50 % E. faecalis + 50 % K. pneumoniae and 20 % E. faecalis + 80 % K. pneumoniae have k lower and G greater than the mixture 80 % E. faecalis + 20 % K. pneumoniae. This is probably because in the samples 50 % E. faecalis + 50 % K. pneumoniae and 20 % E. faecalis + 80 % K. pneumoniae, K. pneumoniae inhibits the growth of E. faecalis, and therefore they present growth rates slower and longer than the sample 80 % E. faecalis + 20 % K. pneumoniae. As can be seen, K. pneumoniae behaves as a dominant bacterium, which is shown in the sample 50 % E. faecalis + 50 % K. pneumoniae, predominating its growth profile. However, in the case of 80 % E. faecalis + 20 % K. pneumoniae, this is due to 80 % of the sample being E. faecalis, resulting in a dominant growth profile.
Also, we quantified the amount of heat released (Q 24) of E. faecalis, K. pneumoniae and their mixtures during the first 24 h. This parameter is important because it allows us to know the heat output of microorganisms and their mixtures in different proportions. In our study, we have observed the Q 24 by E. faecalis is approximately half that of K. pneumoniae. When E. faecalis and K. pneumoniae were mixed, the values of Q 24 for these mixed microorganisms were larger than of E. faecalis and K. pneumoniae (except for the mixture 80 % E. faecalis + 20 % K. pneumoniae that was smaller), which could be due to the interaction of both bacteria. Perhaps this could be explained because K. pneumoniae produces some type of substance that inhibits the growth of E. faecalis, a fact shown in samples containing 50 % and 80 % of K. pneumoniae.
Conclusions
The experiments here presented show that the microcalorimetry is a diagnostic technique that allows studying the behavior of mixed E. faecalis and K. pneumoniae and obtaining in a few hours, the heat flow-time curves a real-time and thermokinetic parameters such as the growth constant (k), generation time (G), detection time (t d), and the amount of heat exchanged (Q). In our study, we have seen that when E. faecalis and K. pneumoniae were put together in the same proportion (50 % E. faecalis + 50 % K. pneumoniae), the growth character of E. faecalis not present, possibly due to the inhibitory effect of K. pneumoniae on E. faecalis. However, it is necessary that more in depth studies be done to know in detail this mechanism.
Microcalorimetric investigations of interaction relationships between bacteria is possible and opens up the possibilities of new studies, for example, to investigate the relationships between different microorganisms (fungi, bacteria, viruses), to study the behavior of two or more bacterial species in the presence of different antimicrobial substances or antibiotics.
References
Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW. Manual of clinical microbiology, vol 1. 10th ed. Washington: ASM Press; 2011.
Murray PR, Rosenthal KS, Pfaller MA. Microbiología médica. 6th ed. España: Elsevier Mosby; 2009.
Braissant O, Wirz D, Göpfert B, Daniels AU. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol Lett. 2010;303:1–8.
Braissant O, Wirz D, Göpfert B, Daniels AU. Biomedical use of isothermal microcalorimeters. Sensors. 2010;10:9369–83.
Sardaro A, Castagnolo M, Trotta M, Italiano F, Cosma P, Agostiano A, et al. Isothermal microcalorimetry of the metabolically versatile bacterium Rhodobacter sphaeroides. J Therm Anal Calorim. 2013. doi:10.1007/s10973-012-2895-0.
Lago N, Legido JL, Arias I, Casás LM. Comparative study of microcalorimetric behavior of Escherichia coli, Proteus mirabili and Klebsiella pneumoniae. Pol J Microbiol. 2012;61(3):199–204.
Tan MR, Ren YS, Yan D, Meng XH, Cheng LH, Qiu LL, et al. Detection of microorganisms in different growth states based on microcalorimetry. J Therm Anal Calorim. 2012;109(2):1069–75.
Trampuz A, Salzmann S, Antheaume J, Daniels AU. Microcalorimetry: a novel method for detection of microbial contamination in platelet products. Transfusion. 2007;47:1643–50.
Trampuz A, Steinhuber A, Wittwer M, Leib SL. Rapid diagnosis of experimental meningitis by bacterial heat production in cerebrospinal fluid. BMC Infect Dis. 2007. doi:10.1186/1471-2334-7-116.
Lago N, Legido JL, Casás LM, Arias I. Microcalorimetric study of the growth of Enterococcus faecalis in an enriched culture medium. J Therm Anal Calorim. 2012;108:665–70.
Lago N, Legido JL, Paz-Andrade MI, Arias I, Casás LM. Microcalorimetric study of the growth and metabolism of Pseudomonas aeruginosa. J Therm Anal Calorim. 2011;105:651–5.
Rodriguez D, Daniels AU, Urrusti JL, Wirz D, Braissant O. Evaluation of a low-cost calorimetric approach for rapid detection of tuberculosis and other mycobacteria in culture. J Appl Microbiol. 2011;111(4):1016–24.
Braissant O, Wirz D, Göpfert B, Daniels AU. The heat is on: rapid microcalorimetric detection of mycobacteria in culture. Tuberculosis. 2010;90(1):57–9.
Bonkat G, Braissant O, Widmer AF, Frei R, Rieken M, et al. Rapid detection of urinary tract pathogens using microcalorimetry:principle, technique and first results. BJU Int. 2012;110(6):892–7.
Bonkat G, Braissant O, Rieken M, Solokhina A, Widmer AF, Frei R, et al. Standarization of isothermal microcalorimetry in urinary tract infection detection by using artificial urine. World J Urol. 2012. doi:10.1007/s00345-012-0913-2.
Kong W, Li Z, Xiao X, Zhao Y, Zhang P. Activity of berberine on Shigella dysenteriae investigated by microcalorimetry and multivariate analysis. J Therm Anal Calorim. 2010;102:331–6.
Xu XJ, Chen CQ, Wang ZY, Zhang Y, Hou AX, Li CH, et al. Study of antibacterial activity of ZnZnbisporphyrin complexes and its free components on Staphylococcus aureus by microcalorimetry. Biol Trace Elem Res. 2009;127:269–77.
von Ah U, Wirz D, Daniels AU. Isothermal micro calorimetry: a new method for MIC determinations: results for 12 antibiotics and reference strains of E. coli and S. aureus. BMC Microbiol. 2009. doi:10.1186/1471-2180-9-106.
Howell M, Wirz D, Daniels AU, Braissant O. Application of a microcalorimetric method for determining drug susceptibility in Mycobacterium species. J Clin Microbiol. 2012;50(1):16–20.
Yang LN, Sun LX, Xu F, Zhang J, Zhao JN, Zhao ZB, et al. Inhibitory study of two cephalosporins on E. coli by microcalorimetry. 2010;100:589–92.
von Ah U, Wirz D, Daniels AU. Rapid differentiation of methicillin-susceptible Staphylococcus aureus from methicillin-reistant S. aureus and MIC determinations by isothermal microcalorimetry. J Clin Microbiol. 2008;46(6):2083–7.
Baldoni D, Hermann H, Frei R, Trampuz A, Steinhuber A. Perfomance of microcalorimetry for early detection of methicillin resistance in clinical isolates of Staphylococcus aureus. J Clin Microbiol. 2009;47:774–6.
O′Neill MAA, Vine GJ, Beezer AE, Bishop AH, Hadgraft J, Labetoulle C, et al. Antimicrobial properties of silver-containing wound dressings: a microcalorimetric study. Int J Pharm. 2003;263(1–2):61–8.
Kong WJ, Xing ZY, Xiao XH, Zhao YL, Wei JH, Wang JB. Effect of berberine on Escherichia coli, Bacillus subtilis, and their mixtures as determined by isothermal microcalorimetry. Appl Microbiol Biotechnol. 2012;96:503–10.
Calvet E, Prat H. Microcalorimétrie: applications physico-chimiques et biologiques. Paris: Masson el Cie Editeurs; 1956.
Paz Andrade MI, Les Dévelopements Récents de la Microcalorimétrie et de la Thermogenese, 1st edn. Paris: CRNS; 1967.
Acknowledgements
We thank María Perfecta Salgado Gonzalez and Sofia Baz Rodríguez for their collaboration with the technical measures. We are also thankful for the financial support provided by the projects EM 2012/141, C269 131H 64502, CN 2012/285, and “Agrupación Estratégica de Biomedicina (INBIOMED)” by “Xunta de Galicia” and the project FIS 2011-23322 funded by Ministry of Science and Innovation of Spain. All these projects are co-financed with FEDER funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vázquez, C., Lago, N., Legido, J.L. et al. Microcalorimetric study of the growth of Enterococcus faecalis, Klebsiella pneumoniae and their mixtures in an enriched culture medium. J Therm Anal Calorim 113, 1415–1420 (2013). https://doi.org/10.1007/s10973-013-3287-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3287-9