Abstract
Chlorogenic acid and its two structural components, quinic acid and caffeic acid, were pyrolyzed under reaction conditions simulating the typical pyrolysis conditions inside a burning cigarette. Major phenolic products from pyrolysis of the three acids were quantified and compared to evaluate the respective contribution of the quinic and caffeic acid moieties to the overall phenolic yield in chlorogenic acid pyrolysis. The results show that the most prominent phenolic product of chlorogenic acid is catechol, followed in order by phenol, hydroquinone, and alkylcatechols. Among these phenolics, catechol and alkylcatechols are formed mainly from the caffeic acid moiety of chlorogenic acid, while phenol and hydroquinone are produced predominantly from the quinic acid moiety. The quinic acid moiety can thus contribute more than 40 % of the overall phenolic yields in chlorogenic acid pyrolysis (0.54 mol mol−1 chlorogenic acid pyrolyzed at 600 °C). Because considerable amounts of free quinic acid and its derivatives exist in tobacco, the results of this study indicate that quinic acid can be an important source of phenolic compounds, especially hydroquinone and phenol, in tobacco smoke.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds are a main class of chemical constituents of tobacco smoke. Major phenolic compounds identified in tobacco smoke include phenol, catechol, hydroquinone, and their alkyl derivatives [1–4]. Many of these phenolics (e.g., hydroquinone, catechol, and their methyl derivatives) have been shown to be toxic and carcinogenic [4–7]. To minimize these phenolics in tobacco smoke, extensive research has been conducted to identify their source and formation mechanisms during cigarette smoking [3, 8–10]. The results indicate that the predominant phenolic compounds in tobacco smoke are formed from pyrolysis of tobacco constituents at high temperatures in the pyrolysis/distillation region of a burning cigarette [9, 11–13]. Particularly, polyphenolic constituents (e.g., chlorogenic acid and lignin) of tobacco have been considered a major source of the phenolic compounds in tobacco smoke [4, 8–10, 14–16].
Chlorogenic acid is one of the most abundant polyphenolic constituents of tobacco, constituting about 2–3 % of tobacco leaf mass [17]. It is an ester of quinic acid and caffeic acid (see Fig. 1 for the chemical structure of the three acids), and has been identified as an important precursor of phenolic compounds in tobacco smoke [3, 4, 9, 17]. For example, several studies report that chlorogenic acid can contribute at least 13 % of catechol in mainstream smoke [9] and about 11 % of the overall yield of hydroquinone from pyrolysis of tobacco [18]. In addition, the content of chlorogenic acid in tobacco has been shown to correlate linearly with the catechol yield in tobacco smoke [5].
A number of studies have investigated pyrolysis of chlorogenic acid under various reaction conditions [3, 4, 17, 19]. Major phenolic products identified in these studies include phenol, hydroquinone, catechol, and alkylcatechols. For catechol and alkylcatechols, it is easily rationalized that they are formed from the caffeic acid moiety of chlorogenic acid because it has a catecholic structure [17, 19]. However, the source and formation pathways of hydroquinone and phenol are still not well understood.
Several researchers have suggested that phenol is formed mainly from secondary reactions (e.g., dehydroxylation and demethylation) of catechols during chlorogenic acid pyrolysis [17, 19]. In contrast, others have proposed that phenol and hydroquinone are more likely derived from the quinic acid moiety of chlorogenic acid [3, 4]. However, because very few previous studies have investigated pyrolysis of quinic acid and its pyrolysis products, there is still insufficient information in literature for determining the role of quinic acid in the phenolic formation in chlorogenic acid pyrolysis.
The main objective of this study was therefore to investigate pyrolysis of quinic acid and evaluate its contribution to the phenolic formation in chlorogenic acid pyrolysis. Quinic acid, caffeic acid, and chlorogenic acid were pyrolyzed in a micro semi-batch reactor under reaction conditions simulating those inside a burning cigarette [11]. The major phenolic products from pyrolysis of the three acids were then quantified and compared to evaluate the respective contribution of quinic and caffeic acid moieties to the overall phenolic yield in chlorogenic acid pyrolysis. Thermogravimetric analysis–mass spectrometry (TG–MS) was performed to monitor the evolution profiles of major pyrolysis products of the three acids during TG pyrolysis. Based on the test results, overall reaction pathways for the phenolic formation from chlorogenic acid pyrolysis were then proposed.
Materials and methods
Materials
Chlorogenic acid (95 %), quinic acid (99 %), and caffeic acid (99 %) were purchased from Sigma-Aldrich Inc. The properties of the three compounds are listed in Table 1 [20].
Pyrolysis–gas chromatography–mass spectrometry (Py–GC–MS) analysis
The three acids were pyrolyzed with a Pyroprobe 5200 analytical pyrolyzer (CDS Analytical Inc.) using a heating protocol designed by Baker et al. [11]. Approximately 0.5 mg of the acid sample was inserted into a quartz tube (2 mm i.d., 2.5 cm in length), which was then held at 300 °C for 5 s to simulate the inter-puff smolder period. The temperature was then rapidly increased at a heating rate of 30 °C s−1 to varying final temperatures between 400 and 700 °C. The final temperature was held for another 5 s. This heating protocol simulates the average heating rate, typical temperature range, and pyrolysis time in the pyrolysis/distillation region of a burning cigarette. The volatile products were carried by high-purity helium through a heated tube to a gas chromatograph (GC, Agilent 7890A) that was equipped with a mass spectrometer (5975C MSD) for analysis. The GC inlet was kept at 300 °C, and the inject split ratio was 100:1. The pyrolysis products were separated with an HP-5 MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness, Agilent Technologies, Inc.). The GC oven was programmed to start at 40 °C for 3 min followed by a gradient of 10 °C/min to 200 °C, and then increased at 20 °C min−1 up to a final temperature of 280 °C. The GC–MS interface was kept as 300 °C, and the ionization mode on the MS was “electron impact” at 70 eV. The mass range was from m/z 29 to 400 scanned at 3.84 s−1. All mass spectra were compared to the NIST mass spectrum library to identify the pyrolysis products. Triplicate Py–GC–MS tests were performed for each sample. The yields of major phenolic products were then quantified by comparing the peak area with those of standard solutions (Sigma-Aldrich) that contained target phenolic compounds with known concentrations.
Thermogravimetry–mass spectrometry (TG–MS) analysis
Approximately 10 mg of the acid sample was placed in a thermogravimetric analyzer (TGA/DSC 1 STARe, Mettler Toledo, Switzerland) and pyrolyzed from 50 to 700 °C at a heating rate of 20 °C min−1 under an argon atmosphere. The gaseous effluent from the TG flowed through a heated transporting tube to a downstream mass spectrometer (Thermostar GSD 320, Pfeiffer Vacuum Inc., Nashua, NH) for pyrolysis product analysis. The MS was operated at 70 eV and monitored selected m/z values that were considered to be representatives of target compounds identified in the Py–GC–MS tests. An 38Ar isotope of the carrier argon gas served as an inner standard. The MS intensities were normalized to the intensities of 38Ar isotope to account for changes in ion current caused by a shift in the MS sensitivity [21], and these were then normalized by the mass of the samples.
Results and discussion
Pyrolysis of chlorogenic acid, quinic acid, and caffeic acid
Approximately 30 pyrolysis products were identified in the Py–GC–MS pyrograms for quinic, caffeic, and chlorogenic acids. Table 2 lists the relative abundances of these pyrolysis products based on GC area %. Quinic and caffeic acids produced significantly different pyrolysis products. Major organic products were quinide, phenol, hydroquinone, and benzoic acids for quinic acid, whereas they were catechol, 4-ethylcatechol, 4-vinylcatechol, and 3-(3,4-dihydroxy-phenyl)-2-propenal for caffeic acid. All the major pyrolysis products of both quinic and caffeic acids were observed in the pyrolysis products of chlorogenic acid. In addition, no obvious cross-over products between the quinic and caffeic acid moieties were observed in the pyrolysis products of chlorogenic acid (Table 2), indicating that the two moieties are decomposed almost independently during chlorogenic acid pyrolysis.
Previous studies have suggested that the initial thermal decomposition of chlorogenic acid occurs by breaking the side chain between the quinic and caffeic moieties at various positions, while the central part of the two acid moieties (i.e., alicyclic polyol and catechol nuclear) remains essentially intact [17, 19]. These two central parts then further crack and rearrange to produce secondary products. This may explain that the major pyrolysis products of chlorogenic acid are essentially the same as those produced in pyrolysis of individual quinic acid and caffeic acid (Table 2).
To evaluate the effect of temperature on the yields of phenolic compounds, chlorogenic acid was pyrolyzed at varying temperatures between 400 and 700 °C with a heating rate of 30 °C s−1. Major phenolic compounds were then quantified by comparing the MS peak areas with those of calibration standards. As shown in Fig. 2, the most prominent phenolic product was catechol, followed in order by phenol, hydroquinone, 4-ethylcatechol, 4-methylcatechol, and cresols (including m-, o-, and p-cresols). The yields of all phenolic products increased considerably from 400 to 500 °C. They then only slightly changed with yet further increasing the temperature, indicating that thermal decomposition of chlorogenic acid is almost entirely complete at 500 °C.
The catechol yield did not change much between 500 and 700 °C (approximately 0.21 mol mol−1 chlorogenic acid pyrolyzed), suggesting that it is stable at this testing temperature range. In comparison, the yields of phenol, hydroquinone, and 4-ethylcatechol decreased slightly from 500 to 700 °C, possibly due to secondary reactions at high temperatures [17, 19]. The yield of 4-methylcatechol reached a plateau at 600 °C. This is possibly because 4-methylcatechol is formed via the C=C bond cleavage in the side chain of the caffeic acid moiety [17], which is enhanced at higher temperatures. Cresols (including m-, o-, and p-cresols) increased constantly from 400 to 700 °C (0.005–0.012 mol mol−1), consistent with the previous finding that cresols are formed predominantly at high temperatures possibly from the solid residue of chlorogenic acid [3].
As mentioned previously, cross-over reactions between the quinic and caffeic acid moieties did not play a significant role in chlorogenic acid pyrolysis. This enables us to evaluate the respective contribution of the two moieties to the overall phenolic yields in chlorogenic acid pyrolysis by comparing the pyrolysis products of the three acids. The yields of major phenolic products from pyrolysis of the three acids at 600 °C are compared in Fig. 3. For a better comparison, the phenolic yields are reported as molar yields for the three acids because chlorogenic acid is composed of equimolar quinic and caffeic acids. As shown, chlorogenic and quinic acids produced comparable yields of phenol (0.14 and 0.10 mol mol−1, respectively) and hydroquinone (0.09 and 0.12 mol mol−1, respectively). In contrast, caffeic acid produced only trace amount of phenol and no hydroquinone. On the other hand, chlorogenic and caffeic acids produced considerable amounts of catechol (0.21 and 0.25 mol mol−1, respectively) and alkylcatechols (0.91 and 0.11 mol mol−1, respectively), whereas quinic acid produced only small amounts of catechol and no alkylcatechols. All the three acids produced small amounts of cresols, which are possibly formed from the solid residue of the acids during pyrolysis [3].
The above comparison shows that catechol and alkylcatechols are mainly derived from the caffeic acid moiety during chlorogenic acid pyrolysis, while phenol and hydroquinone are produced predominantly from the quinic acid moiety. In addition, the quinic acid moiety also produces a small fraction of catechol. The quinic acid moiety can thus contribute a significant fraction of the total phenolic yield in chlorogenic acid pyrolysis. For example, the yield of phenol plus hydroquinone (0.23 mol mol−1) accounts for about 43 % of the overall phenolic yield (0.54 mol mol−1) when chlorogenic acid was pyrolyzed at 600 °C (Fig. 2).
Furthermore, the results suggest that although quinic acid does not have a phenolic chemical structure, it can be an important source of phenolic compounds, especially phenol and hydroquinone in tobacco smoke. This is consistent with the recent finding of McGrath et al. [3], who reported that adding 3 % quinic acid to tobacco leaves significantly increased the yields of phenol, hydroquinone, and catechol in tobacco smoke, whereas adding caffeic acid increased only catechol.
The formation of catechol and alkylcatechols from the caffeic acid moiety is easily rationalized considering the polyphenolic structure of caffeic acid [3, 17, 19]. In contrast, the formation of phenol, hydroquinone, and catechol from the quinic acid moiety have not been well understood. To get more insight into the reaction pathways for phenolic formation in pyrolysis of the three acids, we performed TG–MS tests for chlorogenic, quinic, and caffeic acids.
TG–MS of chlorogenic acid, quinic acid, and caffeic acid
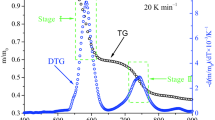
All the three acids were thermally decomposed at temperatures below their respective boiling points (416.8–665 °C, see Table 1) during TG heating (Fig. 4a). The majority of mass loss occurred in the temperature range of 200–400 °C for all the three acids. The minimal mass loss at temperatures above 400 °C are attributed to the secondary decomposition of char [3, 17, 22]. During TG heating, volatiles released from the three acids were monitored by a downstream online MS. An array of MS responses (i.e., m/z values) was monitored and assigned to the species of concern, e.g., m/z = 18, m/z = 44, m/z = 94 for H2O, CO2, and phenol, respectively [23]. The MS response of m/z = 110 can be ascribed to the ion fragments of both catechol and hydroquinone. Based on the Py–GC–MS testing results, we perceived that m/z = 110 is attributed mostly to hydroquinone for quinic acid and to catechol for caffeic acid, whereas it can be attributed to both catechol and hydroquinone for chlorogenic acid. Alkylcatechols were not detected because they have high boiling temperatures and thus were easily condensed in the interface and transporting line between the TG and MS [24–26].
As the TG and DTG curves show (Fig. 4a), the mass loss of quinic acid occurred mainly within 180–400 °C, and it produced almost no solid residue at the end of TG pyrolysis. The initial mass loss at approximately 180–220 °C is mainly attributed to the loss of H2O molecule from quinic acid, which can be discerned by the MS response of H2O (m/z = 18). This dehydration reaction produces quinide (i.e., γ-lactone), an internal ester of quinic acid as the primary product [27, 28]. As temperature increased yet further, quinide was further decomposed to produce secondary products. As the MS curve of H2O (m/z = 18) shown, H2O continuously evolved with broad overlapped peaks within the temperature range of approximately 180–500 °C, indicating that the hydroxyl groups of quinide are successively detached to produce H2O. CO2 (m/z = 44) was formed from decarboxylation reactions of the internal ester of quinide at temperatures above approximately 280 °C. Concurrently, hydroquinone (m/z = 110) and phenol (m/z = 94) began to evolve at about 280 °C. The coincidence of MS peaks of hydroquinone, phenol, CO2, and H2O all occurring at approximately 280–500 °C suggests that hydroquinone and phenol are formed via decarboxylation and dehydration reactions of quinide.
Caffeic acid exhibited two stages of mass loss at temperature range of approximately 200–400 °C, and produced considerable solid residue (about 18 mass %) at the end of TG heating (700 °C) (Fig. 4b). The first sharp peak (200–250 °C) in the DTG curve is mainly ascribed to the decarboxylation reaction of caffeic acid, which produced CO2 and alkylcatechols as the major pyrolysis products [17]. Correspondingly, a sharp peak was observed at approximately 240 °C in the MS curve of CO2 (m/z = 44). The second peak (250–400 °C) in the DTG curve can be mainly attributed to evaporation of catechols, whose boiling points are generally higher than 245 °C (e.g., catechol 245.5 °C, 4-methylcatechol of 251 °C, and 4-ethylcatechol of 273.3 °C). The MS response of m/z = 110 shows that catechol was primarily formed at temperatures above approximately 300 °C when the bond between the alkyl side chain and benzene ring of alkylcatechols was cleaved. However, no obvious MS peak of phenol (m/z = 94) was observed during TG pyrolysis of caffeic acid, consistent with our previous Py–GC–MS result that caffeic acid did not produce much phenol during pyrolysis. The result indicates that secondary reactions of catechol to phenol are negligible under the testing conditions, agreeing with the finding of Lomnicki et al. [29], who reported that catechol is stable within the temperature range of 550–850 °C under inert atmosphere and produces a maximum phenol yield of only about 0.2 % at 700 °C.
Compared with quinic acid and caffeic acid, chlorogenic acid released mass more gradually and produced the highest solid residue (approximately 29 mass %) during TG pyrolysis (Fig. 4c). The TG curve shows that the decomposition of chlorogenic acid occurred mainly below 400 °C, similar to the result of Sharma et al. [17]. A broad DTG peak was observed within the temperature range of ~200–420 °C, indicating that the thermal decomposition of chlorogenic acid involves a series of simultaneous and consecutive reaction such as dehydration, decarbonylation, and decarboxylation [17]. The DTG shoulder at about 220 °C can be attributed to the dehydration of quinic acid moiety, which produces chlorogenic acid lactone [27, 30]. CO2 (m/z = 44) is produced from decarboxylation reactions of the ester functional group between the quinic and caffeic acid moieties and the internal ester of quinide. As the temperature increases, the side chain between the quinic and caffeic acid moieties breaks at various positions [17]. Phenol, hydroquinone, catechol, and alkylcatechols are then formed via the similar reaction pathways described above for pyrolysis of quinic and caffeic acids.
Overall reaction pathways of phenolic formation in chlorogenic acid pyrolysis
Based on the above information, the overall reaction pathways of phenolic formation in pyrolysis of chlorogenic acid is proposed in Fig. 5. This is a modification to the previous schemes proposed by Sharma et al. [17] and Nowakowski et al. [19], showing the contribution of quinic acid to the phenolic formation in chlorogenic acid pyrolysis. In addition, by taking into account the Py–GC–MS results (Table 2), it can be concluded that benzene, which has been previously assumed to be produced from the successive reduction of catechol [17], and benzoic acids are more likely derived from the quinic acid moiety.
The Py–GC–MS test results indicate that chlorogenic acid is a potent precursor of phenolic compounds in tobacco smoke. High yields of phenolic compounds can be formed from the pyrolysis of chlorogenic acid. The most prominent phenolic products include catechol, phenol, hydroquinone, and 4-ethylcatechol (~0.21, 0.14, 0.09, and 0.07 mol mol−1 at 600 °C, respectively). Among these phenolic products, catechol and alkylcatechols are derived predominantly from the caffeic acid moiety of chlorogenic acid, whereas phenol and hydroquinone are derived mainly from the quinic acid moiety. The quinic acid moiety can thus contribute more than 40 % of the overall phenolic yields (in molar yields) in chlorogenic acid pyrolysis (see Fig. 3).
In addition to being structural component of chlorogenic acid, quinic acid exists in appreciable amounts as free acid and structural components of many compounds in tobacco [1–3, 31, 32]. The content of free quinic acid in green tobacco leaves varies widely from trace amounts to 3.5 % (by dry mass) [31], and a concentration of 0.23 % (by dry mass) has been reported for flue-cured tobacco leaves [32]. Moreover, considerable amounts of quinic acid derivatives such as feruloylquinic acid, syringoylquinic acid, and p-coumarylquinic acid have been identified in tobacco [2, 3]. Despite the significant existence of quinic acid in tobacco, the importance of quinic acid as a precursor of phenolic compounds in tobacco smoke may have been somewhat underestimated before. This is possibly because quinic acid often exists in associated forms (e.g., chlorogenic acid and p-coumarylquinic acid) with phenolic constituents of tobacco (e.g., caffeic acid and p-coumaryl alcohol), which have conventionally been considered the major sources of phenolic compounds in tobacco smoke [10]. However, given the high yields of phenol and hydroquinone in pyrolysis of quinic acid (Fig. 3), our results indicate that quinic acid can be an important source of phenolic compounds, especially, hydroquinone and phenol in tobacco smoke.
Conclusions
Pyrolysis of chlorogenic acid produces high yields of phenolic compounds, including catechol, phenol, hydroquinone, and alkylcatechols. Among these phenolics, phenol and hydroquinone are predominantly produced from the quinic acid moiety, while catechol and alkylcatechols are formed primarily from the caffeic acid moiety of chlorogenic acid. The results of this study also indicate that quinic acid can be an important source of phenolic compounds in tobacco smoke.
References
Johnstone RAW, Plimmer JR. The chemical constituents of tobacco and tobacco smoke. Chem Rev. 1959;59(5):885–936.
Stedman RL. Chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68(2):153–207.
McGrath TE, Brown AP, Meruva NK, Chan WG. Phenolic compound formation from the low temperature pyrolysis of tobacco. J Anal Appl Pyrolysis. 2009;84(2):170–8.
Czégény Z, Blazsó M, Várhegyi G, Jakab E, Liu C, Nappi L. Formation of selected toxicants from tobacco under different pyrolysis conditions. J Anal Appl Pyrolysis. 2009;85(1–2):47–53.
Schlotzhauer WS, Snook ME, Chortyk OT, Wilson RL. Pyrolytic evaluation of low chlorogenic acid tobaccos in the formation of the tobacco smoke co-carcinogen catechol. J Anal Appl Pyrolysis. 1992;22(3):231–8.
Van Duursen MBM, Sanderson JT, De Jong PC, Kraaij M, Van den Berg M. Phytochemicals inhibit catechol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for catechol estrogen-induced DNA damage. Toxicol Sci. 2004;81(2):316–24.
Kaur N, Lacasse M, Fürtös A, Waldron KC, Morin A. Sequential fractionation with concurrent chemical and toxicological characterization of the combustion products of chlorogenic acid. J Chromatogr A. 2009;1216(23):4703–12.
Schlotzhauer WS, Martin RM, Snook ME, Williamson RE. Pyrolytic studies on the contribution of tobacco leaf constituents to the formation of smoke catechols. J Agr Food Chem. 1982;30(2):372–4.
Carmella SG, Hecht SS, Tso TC, Hoffmann D. Roles of tobacco cellulose, sugars, and chlorogenic acid as precursors to catechol in cigarette smoke. J Agr Food Chem. 1984;32(2):267–73.
Torikaiu K, Uwano Y, Nakamori T, Tarora W, Takahashi H. Study on tobacco components involved in the pyrolytic generation of selected smoke constituents. Food Chem Toxicol. 2005;43(4):559–68.
Baker RR, Bishop LJ. The pyrolysis of non-volatile tobacco ingredients using a system that simulates cigarette combustion conditions. J Anal Appl Pyrolysis. 2005;74(1–2):145–70.
Maskos Z, Khachatryan L, Dellinger B. Precursors of radicals in tobacco smoke and the role of particulate matter in forming and stabilizing radicals. Energy Fuels. 2005;19(6):2466–73.
Zhou S, Wang CH, Xu YB, Hu Y. The pyrolysis of cigarette paper under the conditions that simulate cigarette smouldering and puffing. J Therm Anal Calorim. 2011;104(3):1097–106.
Nakanishi M, Ogi T, Fukuda Y. Thermogravimetric analysis with gas chromatograph mass spectrometry of Japanese fir wood (Abies sachalinensis) in helium with or without steam-oxygen. J Therm Anal Calorim. 2013;111(1):929–37.
Li XY, Su L, Wang YJ, Yu YQ, Wang CW, Li XL, et al. Catalytic fast pyrolysis of kraft lignin with HZSM-5 zeolite for producing aromatic hydrocarbons. Front Environ Sci Eng. 2012;6(3):295–303.
Yu YQ, Li XY, Su L, Zhang Y, Wang YJ, Zhang HZ. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl Catal A Gen. 2012;447:115–23.
Sharma RK, Fisher TS, Hajaligol MR. Effect of reaction conditions on pyrolysis of chlorogenic acid. J Anal Appl Pyrolysis. 2002;62(2):281–96.
Halliwell BB, Poulsen HE. Cigarette smoke and oxidative stress. 1st ed. New York: Springer; 2006.
Nowakowski DJ, Jones JM. Uncatalysed and potassium-catalysed pyrolysis of the cell-wall constituents of biomass and their model compounds. J Anal Appl Pyrolysis. 2008;83(1):12–25.
Xie J. The constituents of tobacco and tobacco smoke. 1st ed. Beijing: Chemical Industry; 2010.
Wang YJ, Cannon FS, Salama M, Fonseca DA, Giese S. Characterization of pyrolysis products from a biodiesel phenolic urethane binder. Environ Sci Technol. 2009;43(5):1559–64.
Sharma RK, Hajaligol MR, Smith PAM, Wooten JB, Baliga V. Characterization of char from pyrolysis of chlorogenic acid. Energy Fuels. 2000;14(5):1083–93.
Ahamad T, Alshehri SM. Thermal degradation and evolved gas analysis of epoxy (DGEBA)/novolac resin blends (ENB) during pyrolysis and combustion. J Therm Anal Calorim. 2013;111(1):445–51.
Meszaros E, Jakab E, Varhegyi G, Szepesvary P, Marosvolgyi B. Comparative study of the thermal behavior of wood and bark of young shoots obtained from an energy plantation. J Anal Appl Pyrolysis. 2004;72(2):317–28.
Wang YJ, Zhang Y, Su L, Li XY, Duan L, Wang CW, et al. Hazardous air pollutant formation from pyrolysis of typical Chinese casting materials. Environ Sci Technol. 2011;45(15):6539–44.
Quan C, Li AM, Gao NB. Research on pyrolysis of PCB waste with TG-FTIR and Py-GC/MS. J Therm Anal Calorim. 2012;110(3):1463–70.
Farah A, De Paulis T, Trugo LC, Martin PR. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J Agr Food Chem. 2005;53(5):1505–13.
Scholz BM, Maier HG. Isomers of quinic acid and quinide in roasted coffee. Z Lebensm Unters Forsch. 1990;190(2):132–4.
Lomnicki S, Truong H, Dellinger B. Mechanisms of product formation from the pyrolytic thermal degradation of catechol. Chemosphere. 2008;73(4):629–33.
Jiang DS, Peterson DG. Role of hydroxycinnamic acids in food flavor: a brief overview. Phytochem Rev. 2010;9(1):187–93.
Palmer JK. Occurrence of l-quinic acid in tobacco leaves. Science. 1957;126(3272):504–5.
Nagasawa M. A microcolorimetric method for the determination of quinic acid and its content in flue-cured tobacco. B Agr Chem Soc Japan. 1958;22(4):205–7.
Acknowledgments
This research is supported by the Funding of Shanghai Tobacco (Group) Corporation (2011-1-010), a grant from the NSFC project (51008175), and the National Special Program of Water Pollution Control and Management (No. 2012ZX07301-005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Li, X., Zhen, S. et al. The important role of quinic acid in the formation of phenolic compounds from pyrolysis of chlorogenic acid. J Therm Anal Calorim 114, 1231–1238 (2013). https://doi.org/10.1007/s10973-013-3142-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3142-z