Abstract
Efficiencies of four commercial antioxidants used during the storage of two biodiesel, BS-ethyl and BS-methyl, respectively obtained from soybean oil by ethylic and methylic routes, were evaluated by measuring their oxidative stability using a low pressurized DSC (low P-DSC) method developed by the authors and by Rancimat method, which is specified by the American ASTM D6751 and European EN 14214 standard methods. The operating temperature of the low P-DSC method was the same as that used in the Rancimat procedure (110 °C). The antioxidants, used in 500 mg kg−1 content, have the following active components: phenol, biphenol, phenol + amine mixture, and a hydroquinone + organic acid mixture. Samples of each biodiesel–antioxidant mixture were evaluated simultaneously by the two methods, right after their preparation and 30, 60, 90, and 120 days of storage at 23 ± 1 °C. A non-dimensional oxidative induction time parameter, defined as the ratio between the oxidation induction time (OIT) or induction time (IT) values at a storage time t and at t = 0, was used to evaluate the antioxidant activity. For the same mixture, changes of this non-dimensional parameter calculated from OIT or IT show a similar trend with storage time, indicating that it can be determined either from low P-DSC or Rancimat method data. As the efficiency of the studied antioxidants depends on their composition, their interaction with each biodiesel, and on the storage time, this parameter can be used to indicate the best storage time for each antioxidant–biodiesel mixture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last two decades, many nations have expanded the participation of renewable energy sources in their energy matrix. The main objective was to provide their energy self-sufficiency and to mitigate climate changes in the planet, by using fuels with low CO2 emission, to minimize the effects of greenhouse gases in the atmosphere [1–3].
In this context, there has been an increasing interest in the use of biodiesel as an alternative fuel to petroleum diesel. But, despite its many advantages, like high calorific value, biodegradability, to be nearly free of sulfur and to produce lower CO2 emissions when compared to diesel, biodiesel promotes a slight increase in nitrogen oxide (NOx) emissions and has a high susceptibility to oxidation as well, which may affect its commercialization [4].
Like vegetable oils and fats, biodiesel is subject to oxidation reactions [5, 6], and to improve its oxidative stability, the addition of antioxidants has been studied [7–9]. The oxidation process is a spontaneous phenomenon caused by several factors, such as the presence of oxygen, exposure to light, high temperatures, and contact with metals present in storage tanks or in production units [10–12]. Oxidation is considered the major cause of deterioration of bio-fuels, directly affecting their quality [13].
Thus, oxidation stability is one of the most important parameters to ensure the quality of the biodiesel and its commercialization. The determination of the oxidative stability of biodiesel is based on the oxidation stability index performed according to the standard of the American Oil Chemists’ Society (AOCS). The EN14112 standard method [14] describes the determination using the Rancimat equipment [15–17]. But in recent years, faster and more efficient alternative methods of analysis have been developed for this purpose [18].
As oxidation is an exothermic process, pressurized differential scanning calorimetry (P-DSC) has been widely used in recent years to monitor the stability of vegetable oils, animal fats, and biodiesel [19–21]. P-DSC methods have been used to assess the oxidative stability of biodiesel, with and without added antioxidants [22–25], in a shorter analysis time, when compared to conventional methods [26]. Several studies have also been performed by thermal analysis, and the results are compatible with those obtained by the Rancimat method [27–30].
The commonly used antioxidants that control the oxidation of biodiesel are the synthetic phenolic additives, with substitutions of tert-butyl groups, as well as those that have aromatic amines in their composition [31–34]. In this study, all selected commercial antioxidants have a “No-Harm” certification from the German-based Association for the Quality Management of Bio-Diesel (AGQM) [35].
The objective of this study was to evaluate the efficiency of four commercial antioxidants during the storage of biodiesel/antioxidant mixtures. Two types of biodiesel, BS-ethyl and BS-methyl, were used, respectively obtained from soybean oil by ethylic and methylic routes. For this purpose, a low P-DSC method developed by the authors [28] was used, as well as the Rancimat method, which is specified by the American ASTM D 6751 [36] and European EN 14214 [37] standard methods for the determination of the oxidative stability of biodiesel.
Usually high-pressure methods are used in P-DSC oxidative stability time determinations, such as ASTM E1858–08 method for the determination of oxidation induction time (OIT) of hydrocarbons [38, 39], which uses pure oxygen at 3600 kPa. The main differences in the operating conditions of the present low P-DSC method are that air is used as the oxidation ambient and a much lower operating pressure is used (550 kPa), which enable a good resolution in acceptable analysis times.
Experimental
Materials
This article presents a study of the oxidative behavior of two types of biodiesel (BS) made in Brazilian plants, obtained by methylic route (BS-methyl) and ethylic route (BS-ethyl), both produced from the conversion of soybean oil by transesterification reaction [40]. The original biodiesel samples did not contain antioxidants and were stored in plastic bottles in a freezer, before the experiments. Table 1 contains the results of the characterization of biodiesel samples. Except for the oxidation stability, all results were in accordance with the specifications of the Brazilian Government Technical Regulation Agency (ANP 05/2012). This Resolution establishes a minimum of 6 h for the induction time (IT) determined by this method.
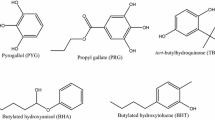
The antioxidants used in this study were selected for their chemical composition and represent the different classes of commercial products mostly used nowadays to improve biodiesel oxidative stability: phenol + amine mixture—PHA (N,N′-di-sec-butyl-p-phenylenediamine + 2,6-di-terc-butylphenol); biphenol—BPH (2,2-methylene-bis-(4-methyl-6-tert butyl-phenol); phenol—PH (2,6-di-tert-butyl, 4-methyl phenol); and organic acid + hydroquinone mixture—HOA(2-tert-butylhydroquinone + citric acid). Their chemical structures are shown in Fig. 1.
Methods
Low P-DSC method
The analysis of biodiesel mixtures with antioxidants was performed in a pressure differential scanning calorimeter, model P-20 Q-DSC from TA Instruments. The equipment calibration was done using standard pure indium metal. About 3.000 mg (±0.2 mg) of sample was used in each analysis in an open platinum pan (110 μL). An empty open pan of the same type was used as reference. Static air at 80 psi (551 kPa) was used, and the samples were initially heated at 10 K min−1, from ambient temperature to 110 °C, followed by an isothermal step at this temperature. The value of the OIT was measured by the onset of the respective DSC exothermic oxidation peak [41, 42] that happens during the isothermal step.
Rancimat method
To determinate the oxidative stability of the biodiesel samples by this traditional method, a Rancimat equipment model 743 from Metrohm was used, according to EN 14112 standard method. It consists of passing an air flow of 10 L h−1 through a sample of 3.000 g ± 0.010 g maintained at 110 °C in a cylindrical vessel [43, 44]. The exiting air current passes by an electrical conductivity cell. The IT is measured when a significant change in the electrical conductivity of the aqueous phase in the cell is registered, due to the action of oxidized products present in the air current.
Sample preparation
Eight initial mixtures (90 g each) were prepared with both biodiesel, having 500 mg kg−1 of each antioxidant. The usual antioxidant content used in commercial biodiesel ranges from 100 to 250 mg kg−1. Thus, the concentration of 500 mg kg−1 was chosen to have an initial IT value for all samples higher than 6 h. This was obtained, except for the BS-methyl/PH antioxidant mixture, the initial IT value of which was 5 h. From each 90 g mixture, 10 g was used for immediate low P-DSC and Rancimat analyses. The remaining mixture was poured into four amber vials of 20 mL capacity, filled completely. The vials, which were kept closed during the storage time, were maintained at a room temperature of 23 °C ± 1 °C. Samples collected every 30 days from respective new bottles were analyzed by low P-DSC and Rancimat methods, for a period of four consecutive months. Both analyses were performed simultaneously.
Results and discussion
All low P-DSC and Rancimat measurements were done in duplicate. One of the two sets of all measured low P-DSC results are shown in Figs. 2 and 3, respectively for BS-ethyl and BS-methyl biodiesel, to show typical curves. The results shown in Figs. 4 and 5 represent the mean of duplicate measurements of low P-DSC and Rancimat methods. Maximum deviations from respective mean values were of ±2.5 %.
In Figs. 2 and 3, are shown the overlay of the respective low P-DSC curves obtained for each biodiesel and respective biodiesel–antioxidant mixtures immediately after they were prepared and after their different storage times. From those curves, it can be seen that the immediate OITs for biodiesel–antioxidant mixtures, when compared with biodiesel without antioxidant addition, indicate a retarding effect on oxidation, which decreases with storage time in most of the studied cases.
The highest oxidation rate time is also shown, which corresponds to the respective maximum of low P-DSC peak, which also can be used to evaluate the antioxidant retarding effect and the loss of its activity with storage time. The curves were shifted vertically to allow their better visualization. It can be seen that for both biodiesel, for the whole storage time, the most efficient antioxidant is the BPH. In all cases, right after preparing the mixtures, there is a significant shift of the respective peak maximum (and of the low P-DSC curve) to the right and, as storage time increases, this maximum goes to the left, indicating that there is a loss of antioxidant activity.
Figure 4 shows the evolution of the oxidative stability of the mixtures (BS-ethyl and BS-methyl with antioxidants) as a function of time, during the storage period of 120 days. These results refer to the respective data obtained by low P-DSC (OIT) and Rancimat (IT) methods.
The IT and OIT curves of the BS-ethyl mixture with antioxidant HOA show a gradual decrease of this antioxidant along the storage period (Fig. 4a). The corresponding curves for the BS-methyl, mixed with this antioxidant, exhibit a similar behavior throughout the storage period.
For the BS-ethyl mixtures with PH antioxidant (Fig. 4-B), there is a pronounced decrease in IT and OIT values at the end of the first 30 days. However, there is a tendency for the maintenance of the oxidative stability (measured by the two methods) during the next 60 days. During the last 30 days of storage, IT values present a higher decrease than OIT for this mixture. On a different behavior, the OIT and IT curves for the BS-methyl mixture with PH antioxidant show a continuous decrease during the first 90 days of storage.
The IT and OIT curves of the mixtures of BS-ethyl with BPH and PHA antioxidants (Figs. 4c, d) show that there is an increase of the oxidative stability at the age of 30 days (which is higher for PHA case) and, thereafter, there is a gradual decrease during the following 90 days, which presents a higher total decrease for the PHA case, indicating a better efficiency of the BPH antioxidant. OIT and IT curves for the BS-methyl mixtures with the same antioxidants exhibit a continuous decrease during all the storage periods.
It is important to note that OIT and IT values have both time units, but come from different kinds of measurements that result from oxidation phenomena identified in an analyzed sample, respectively, from exothermic effects or promoted electrical conductivity changes. Thus, theoretically corresponding values obtained by each method cannot be compared directly.
To compare low P-DSC and Rancimat method data, a non-dimensional parameter was defined for each case and analyzed as a function of storage time, as presented in Eq. (1). This parameter (T/T 0 %) actually represents the percentage of each measured OIT or IT values at a particular storage time, with respect to the corresponding OIT and IT values at the beginning of the storage period (i.e., at t = 0).
where T 0 = induction time (OIT or IT) at the beginning of the storage period, T t = induction time (OIT or IT) at t days of storage period (t = 0, 30, 60, 90, and 120 days)
Figure 5 shows a set of comparison among the T/T 0 % values, measured by low P-DSC and Rancimat methods, respectively. The values are shown as a function of storage time. A constant T/T 0 % line corresponding to respective IT = 6 h, calculated by the Eq. (1), where T t = 6 h, is also shown. Results above this line indicate that the stored biodiesel still meets the specification for commercial use. The above non-dimensional parameter used to evaluate the change in oxidative stability, which actually shows how the OIT at a definite storage time changes with respect to the corresponding initial value, allows a better comparison between the data obtained from each measuring method.
As observed in BS-ethyl and BS-methyl cases, a gradual decrease in TI and OIT non-dimensional values is noticed as a function of storage time for all tested antioxidants, by both methods, although low P-DSC measurements present a lower percentual decrease than those obtained by Rancimat method.
As can be seen in Fig. 5, the efficiency of each antioxidant depends on the storage time and on the kind of biodiesel. In a general way, up to 30 days of storage, values of the non-dimensional induction time parameter (T t × 100/T o) obtained from both methods are very close to 100 % for BS-ethyl, except for the PH antioxidant.
The same does not occur with BS-methyl data in the same period of time, during which, practically, it presents a decrease, indicating a decrease of the antioxidant activity. For the following storage months, although in some cases, the observed changes obtained from low P-DSC data are lower than those obtained from Rancimat data, in a general way, respective curves show the same trend. This means that the low P-DSC can also be used to monitor the change of the oxidative stability of biodiesel during its storage.
These differences observed between the two methods are due to the fact that, besides each antioxidant having a different interaction with each biodiesel and different parameters being measured by each method as discussed previously, Rancimat IT values are dependent on the formation of volatile compounds during the oxidation steps, while low-P-DSC OIT values are related with the beginning of the exothermic oxidation reactions in liquid phase.
Conclusions
-
Low P-DSC oxidative induction time measurements can be used to monitor commercial antioxidant activity changes during biodiesel storage.
-
Changes of antioxidant activity in mixtures with biodiesel during their storage can also be evaluated by comparing the time needed to attain the maximum of low P-DSC peaks.
-
As OIT and IT values obtained, respectively, from low P-DSC and Rancimat methods have both time units, but come from different kinds of measurements, which also are done at different stages of the biodiesel oxidative process, respective values obtained for the same sample cannot be directly compared.
-
A non-dimensional oxidative induction time parameter defined in this study, which represents the percentage of each measured OIT or IT values at a particular storage time, with respect to the corresponding value at the beginning of the storage period, can be used to evaluate the antioxidant activity and efficiency as a function of storage time.
-
For the same mixture, changes of this non-dimensional parameter, either obtained from OIT or IT values, show a same trend with storage time, indicating that it can be obtained applying low P-DSC or Rancimat methods.
-
As the efficiency of the studied antioxidants depends on their composition, their interaction with each biodiesel, and storage time, this parameter can be used to indicate the best storage time for each antioxidant–biodiesel mixture.
References
Tolmasquim MT, Guerreiro A, Gorini R. Brazilian energetic matrix: a prospective—new studies CEBRAP. 2007;79: 47–69 (In Portuguese), http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-33002007000300003. Accessed Jan 19 2013.
Majer S, Mueller-Langer F, Zeller V, Kaltschmitt M. Implications of biodiesel production and utilization on global climate—a literature review. Eur J Lipid Sci Technol. 2009;111(8):747–62.
Pinzi S, Garcia IL, Lopez-Gimenez FJ, Decastro MDL, Dorado G, Dorado MP. The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications. Energy Fuels. 2009;23(5):2325–41.
Knothe G, Van Gerpen J, Krahl J, editors. The biodiesel handbook. Champaign: AOCS Press; 2005.
Shahidi F, editor. Bailey’s industrial oil and fat products. 6th ed. New York: Wiley; 2005.
Gunstone FD. The chemistry of oils and fats: sources, composition, properties, and uses. Boca Raton: CRC Press; Oxford: Blackwell Pub; 2004.
McCormick RL, Graboski MS, Alleman TL, Herring AM, Tyson KS. Impact of biodiesel source material and chemical structure on emissions of criteria pollutants from a heavy-duty engine. Environ Sci Technol. 2001;35(9):1742–7.
Hess MA, Haas MJ, Foglia TA, Marmer W. The effect of antioxidant addition on NOx emissions from biodiesel. 228th ACS Natl Meet Prepr Pap Am Chem Soc Div Fuel Chem. 2004;49(2): 852–53.
Gan S, NG HK. Effects of antioxidant additives on pollutant formation from the combustion of palm oil methyl ester blends with diesel in a non-pressurised burner. Energy Convers Manag. 2010;51(7):1536–46.
Knothe G. Review: some aspects of biodiesel oxidative stability. Fuel Process Technol. 2007;88:669–77.
Knothe G, Dunn RO. Dependence of oil stability index of fatty compounds on their structure and concentration and presence of metals. JAOCS. 2003;80(10):1021–6.
McCormick RL, Westbrook SR. Storage stability of biodiesel and biodiesel blends. Energy Fuels. 2010;24(1):690–8.
Monyem A, Van Gerpen JH. The effect of biodiesel oxidation on engine performance and emissions. Biomass Bioenergy. 2001;20:317–25.
EN 14112. Fat and oil derivatives. Fatty acid methyl esters (FAME). Determination of oxidative stability (accelerated oxidation test), EN 14112:2003. Brussels, Belgium: European Committee for Standardization (CEN); 2003.
Waynick JA. Characterization of biodiesel oxidation and oxidation products—SwRI® Project No. 08-10721, 2005-Southwest Research Institute—National Renewable Energy Laboratory, U.S. Department of Energy, Golden, Colorado, USA.
Erhan SZ, Dunn RO, Knothe G, Moser BR. Fuel properties and performance of biodiesel. In: Hou CT, Shaw JF, editors. Biocatalysis and bioenergy. Hoboken: Wiley. doi:10.1002/9780470385869.ch1, ISBN: 978-0-470-13404 608; 2008.
Prankl H. High biodiesel quality required by European standards. Eur J Lipid Sci Technol. 2002;104(6):371–5.
Jain S, Sharma MP. Review of different test methods for the evaluation of stability of biodiesel. Renew Sustain Energy Rev. 2010;14(7):1937–47.
Tan CP, Che Man YB. Recent developments in differential scanning calorimetry for assessing oxidative deterioration of vegetable oils. Trends Food Sci Technol. 2002;13(9–10):312–8.
Ramalho EFSM, Albuquerque AR, Souza AL, Barro AK, Maia AS, Santos IMG, Souza AG. Use of different techniques in the evaluation of the oxidative stability of poultry fat biodiesel. J Therm Anal Calorim. 2011;106:787–91.
Zhao H, Cao Y, Orndorff W, Cheng Y-H, Pan Wp. Thermal behaviors of soy biodiesel. J Therm Anal Calorim. 2012;109(3):1145–50. doi:10.1007/s10973-012-2551-8.
Galvão LPFC, Santos AGD, Gondim AD, Barbosa MN, Araujo AS, Souza L, Fernandes Junior VJ. Comparative study of oxidative stability of sunflower and cotton biodiesel through P-DSC. J Therm Anal Calorim. 2011;106:625–9.
Dunn RO. Review: antioxidants for improving storage stability of biodiesel, antioxidants for improving. Ltda Biofuels Bioprod Bioref. 2008;2:304–18.
De Guzman R, Tang H, Salley S, NG KYS. Synergistic effects of antioxidants on the oxidative stability of soybean oil and poultry fat-based biodiesel. J Am Oil Chem Soc. 2009;86:459–67.
Candeia RA, Sinfrônio FSM, Bicudo TC, Queiroz N, Barros Filho AKD, Soledade LEB, Santos IMG, Souza AL, Souza AG. Influence of the storage on the thermo-oxidative stability of methyl and ethyl esters by PDSC. J Therm Anal Calorim. 2011;106(2):581–6.
Kowalski B, Gruczynska E, Maciaszek K. Kinetics of rapeseed oil oxidation by pressure differential scanning calorimetry measurements. Eur J Lipid Sci Technol. 2000;102(5):337–41.
Dantas MB, Albuquerque AR, Soledade LEB, Queiroz N, Maia AS, Santos IMG, Souza AL, Cavalcanti EHS, Barro AK, Souza AG. Biodiesel from soybean oil, castor oil and their blends oxidative stability by PDSC and Rancimat. J Therm Anal Calorim. 2011;106:607–11.
Leonardo RS, Murta Valle ML, Dweck J. An alternative method by pressurized DSC to evaluate biodiesel antioxidants efficiency. J Therm Anal Calorim. 2012;108:751–9.
Tavares MLA, Queiroz N, Santos IMG, Souza AL, Cavalcanti EHS, Barros AKD, Rosenhaim R, Soledade LEB, Souza AG. Sunflower biodiesel: use of P-DSC in the evaluation of antioxidant efficiency. J Therm Anal Calorim. 2011;106:575–9.
Polavka J, Paligová J, Cvengroš J, Šimon P. Oxidation stability of methyl esters studied by differential thermal analysis and Rancimat. J Am Oil Chem Soc. 2005;82(7):519–24.
Mittelbach M, Schober S. The impact of antioxidants on biodiesel oxidation stability. Eur J Lipid Sci Technol. 2004;106:382–9.
Karavalakis G, Stournas S. Impact of antioxidant additives on the oxidation stability of diesel/biodiesel blends. Energy Fuels. 2010;24:3682–6. doi:10.1021/ef1004623.
Chen Y-H, Luo Y-M. Oxidation stability of biodiesel derived from free fatty acids associated with kinetics of antioxidants. Fuel Process Technol. 2011;92:1387–93.
Tang H, Guzman RCD, Ng KYS, Salley SO. Effect of antioxidants on the storage stability of soybean-oil-based biodiesel. Energy Fuels. 2010;24(3):2028–33. doi:10.1021/ef9012032.
German-based Association for the Quality Management of Bio-Diesel; http://www.agqm-biodiesel.de/en/services/no-harm-list/. Accessed in Jan 22, 2013.
ASTM D6751. Standard specification for biodiesel fuel blend stock (B100) for middle distillate FUELS, ASTM D6751-10 In: ASTM annual book of standards. West Conshohocken: ASTM International; 2010.
EN 14214. Automotive fuels—fatty acid methyl esters (FAME) for diesel engines—requirement methods, EN 14214:2008. Brussels: European Committee for Standardization (CEN); 2009.
ASTM E1858. Standard Test method for determining oxidation induction time of hydrocarbons by differential scanning calorimetry, ASTM E1858-08. In: West Conshohocken: ASTM International; 2008.
Dunn RO. Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process Technol. 2005;86:1071–85.
Van Gerpen J. Biodiesel processing and production. Fuel Process Technol. 2005;86(10):1097–107.
Blaine RL, Harris MB. A proposed reference material for oxidative induction time by differential scanning calorimetry. In: Riga AT, Patterson GH, editors. Oxidative behavior of materials by thermal analytical techniques, ASTM STP 1326. American Society for Testing and Materials; 1997.
Blaine RL, Lundgren CJ, Harris MB. Oxidative induction time: a review of DSC experimental effects. In: Riga AT, Patterson GH, editors. Oxidative behavior of materials by thermal analytical techniques. Philadelphia: ASTM Publication; 1997. pp. 3–15.
Hadorn H, Zurcher K. Zur bestimmung der oxydationsstabilitat von olen und fetten. Deutsche Lebensmittel Rundschau. 1974;70(2):57–65.
Farhoosh R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J Am Oil Chem Soc. 2007;84(3):205–9.
Acknowledgments
To the Laboratory of Biodiesel Stability Evaluation of the UFRJ Chemistry School and to COPPE/UFRJ Laboratory of Structures and Materials, both at Federal University of Rio de Janeiro, Brazil, where respectively, Rancimat and P-DSC experiments were performed by the authors and to the Brazilian National Council of Research (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dweck, J., Leonardo, R.S. & Valle, M.L.M. Evaluating antioxidants efficiency during storage of ethylic and methylic biodiesel by low pressurized DSC and Rancimat methods. J Therm Anal Calorim 113, 1317–1325 (2013). https://doi.org/10.1007/s10973-013-3028-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3028-0