Abstract
Biodiesel can contain unsaturated fatty acids, which are susceptible to oxidation, being able to change into polymerized compounds. Oxidative stability is very important in the quality control of oils and biodiesel. In this study, biodiesel samples were produced through the methyl route, using a homogeneous catalyst. The determination of methyl esters was performed by gas chromatography in order to confirm the conversion of the carboxylic acids present in the raw material for the methyl esters. Also proved the presence of methyl linoleate and methyl oleate to the major constituent of biodiesel. The thermal and oxidative stability of sunflower and cotton oils and their biodiesel, using TG and P-DSC techniques were investigated. The use of P-DSC to measure the oxidative induction time was very important. These measurements were used to evaluate the cotton and sunflower oils, and their respective biodiesel. It was found that the thermal-oxidative stability of vegetable oils and their biodiesel were similar, due to the fact that both presented chemical composition and percentages of fatty acids similar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to replace the petroleum-based fuels, biodiesel became a significant alternative between the biomass products as consequence of its economic, social and environmental positive impacts [1–5]. It is currently the most widely accepted as alternative fuel for diesel engines because of its technical, environmental, and strategic advantages. It has enhanced biodegradability, reduced toxicity and improved lubricity in comparison with conventional diesel fuels. Being derived from biomass resources, biodiesel is regarded as a renewable and biodegradable fuel [6]. Biodiesel is defined as the monoalkyl esters of vegetable oils or animal fats.
The vegetable biodiesel can be obtained from different sources, as soy, cotton, sunflower, palm bean or simply oil of domestic fry. This is made through a chemical process called transesterification where by the glycerin is separated from the fat or vegetable oil. The process generates two products: methyl or ethyl esters (the chemical name for biodiesel) [7]. Although the renewable origin and biodegradability of the biodiesel are widely presented as advantage, its decomposition profile confers to itself lower oxidative stability in comparison to mineral diesel [2, 8]. Biodiesel, an alternative fuel prepared by transesterification of vegetable oils or animal fats, is susceptible to autoxidation. They are oxidized by metal traces, oxygen and temperature, and their stability is also influenced by the unsaturated fatty acid composition [9–12]. Generally, the rate of oxidation of fatty compounds depends on the number of double bonds and their position. The oxidation chain reaction is usually initiated at the positions allylic to double bounds. Thus, fatty acids (FA) with methylene-interrupted double bonds, for example, linoleic acid [(9Z, 12Z)-octadecadienoic acid], are more susceptible to oxidation because they contain methylene groups that are allylic to two-double bonds. Fatty acids with conjugated double bonds, for example, linolenic acid [(9Z, 12Z, 15Z)-octadecatrienoic acid], are even more susceptible to oxidation [13]. In order to evaluate the susceptibility to oxidation, the biodiesel is submitted to accelerated oxidation under controlled conditions and may be evaluated by thermal analysis techniques by using pressure differential scanning calorimetry (P-DSC) [14, 15].
The purpose of this study was to evaluate the thermal and oxidative stability of the cotton and sunflower biodiesels obtained by the methanol route, using thermal analysis by non-isothermal TGA and P-DSC.
Experimental
Sunflower and cotton oil were converted to biodiesel using alkaline transesterification. The sample was prepared in 1:6 molar ratio of oil / methanol. In this reaction 1% wt catalyst (potassium hydroxide) in relation to oil was dissolved in methanol, and then added to the oil being stirred at a constant temperature for 2 h. After the decantation process, the glycerine was removed and the biodiesel were purified with the addition of water, then the biodiesel were dried and characterized. The analyses of the oil and pure biodiesel (B100) were performed as indicated by the Resolution Nº 7 of ANP, the Brazilian National Agency of Petroleum, Natural Gas and Biofuels [16].
The obtained sunflower and cotton biodiesel were analyzed by gas chromatography (GC) with a flame ionization detector, in order to determine the conversion of triacylglyceride to methyl esters of the corresponding fatty acids upon the transesterification reaction. A Thermo Trace GC-FID gas chromatograph was used. The FID detector temperature was 250 °C. The oven program to carry out the analysis of ester content had initial temperature of 150 °C for 6 min, next, the first heating rate was 10 °C min−1 up to 210 °C for 5 min, finally a second heating rate was 5 °C min−1 up to 240 °C for 2 min.
The thermogravimetric (TG) experiments were carried out using a Thermobalance Mettler-STGA 851 model, in the temperature range of 30–600 °C, under nitrogen atmosphere flowing in a rate of 25 mL min−1, using alumina crucible of 75 μL and heating rates 10 °C min−1.
The curves of differential scanning calorimeter under pressure (P-DSC) were obtained from a calorimeter mark TA Instruments MDSC model 2920 coupled with cell DSC Cell Pressure, pressure 1400 kPa, in an atmosphere of oxygen. Analyses with P-DSC were performed by two methods: dynamic and isothermal. The curves obtained by the dynamic method were performed using a heating ramp of 10 °C min−1, from room temperature to 600 °C. In the isothermal method the test is started at 50 °C and then subjected to a heating rate of 10 °C min−1 to 110 °C, where it is kept at constant temperature until the complete oxidation of the sample.
Results and discussion
As the data shown in Table 1, the oils analyzed showed physico-chemical properties suitable for use as raw material in the synthesis of biodiesel. The Biodiesel from those presented in accordance with the specifications of Resolution No 7 of the ANP.
Observed that the viscosities of biodiesel are lower than of the raw materials that gave rise to them. This decrease is a positive factor, since high values can lead to engine problems. This significant decrease is partly because the chain of the esters to be lower than that of triglycerides. This property serves as indication that the conversion of carboxylic acids into methyl esters was effective, since the change in values is significant.
The determination of methyl esters was performed by gas chromatography in order to confirm the conversion of the carboxylic acids present in the raw material for the methyl esters. As presented in the Table 2, it is observed the conversion of triglycerides to methyl esters of 98.01 % for cotton biodiesel and 97.35 % for sunflower to biodiesel. The composition of the biodiesel showed methyl linoleate and methyl oleate as the major constituents.
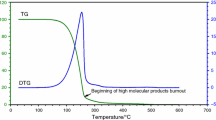
The Fig. 1 illustrates the thermal behavior of cotton oil in inert atmosphere presented one mass loss step between 304 and 490 °C attributed to the triacylglycerides volatilization and combustion processes, which are responsible for approximately 97.5% of mass loss. The TG curve of the cotton biodiesel, methanol route, presented one mass loss step, between 135 and 344 °C with mass loss of 98.6%. The step is ascribed to the volatilization and/or combustion of the methyl esters, with the prevalence of methyl linoleate. It was also demonstrated in Fig. 1 that oil sunflower presented only one mass loss step of 98.7%, between 300 and 495 °C. The sunflower biodiesel, whose major component is methyl linoleate, also presented one loss step, 115–315 °C, of 98.1%. It was observed that the thermogravimetric profiles of the samples are similar. This suggests a similarity between the chemical compositions of vegetable oils in the study.
In Table 3 was calculated the residual masses, in which the sunflower oil (1.3%) showed better results in residual masses compared with cotton oil (2.5%). Because of its chemical composition have more unsaturated compounds become less stable than cotton oil.
Regarding mass loss may be a correlation with gas chromatography. The thermogravimetry analysis may serve as a qualitative analysis of the conversion of fatty acid methyl esters. Whereas within the experimental errors, the values are exactly similar and that thermogravimetry can replace the gas chromatography (GC) with time savings, cost and without quality loss in the results unless there is a need to identify the esters.
The oxidative stability can be used for evaluating the quality of oils, fats and biodiesels. Such property depends on chemical composition, the quality of the raw material, the conditions of refining processes (oils and fats), transesterification route (biodiesel), and the storage conditions [17–19]. The onset is the temperature at which it initiates the oxidation process. Figures 2 and 3 show the dynamic P-DSC curves and Table 4 the values of onset. It may be noted that raw materials have a higher oxidative stability than their respective biodiesel. This fact expected, since the methyl esters are more susceptible to suffer the oxidation process [20]. The values close to biodiesel can be attributed to similarity in their composition.
Oxidative induction time (OIT) indicated that the as extracted oils have higher stability than the biodiesel comes from them (Table 5). This occurs because of the ester functional group present in biodiesel, which that are more sensitive to oxidation than carboxylic acids present in vegetable oil. The sunflower oil showed induction time of 193 min versus 203 min for cotton oil. These close induction times, were attributed to the similarity in chemical composition of vegetable oils studied.
The Fig. 4 shows the isothermal P-DSC curves of biodiesel (cotton and sunflower). The induction time of cotton biodiesel was 140 min and of the sunflower biodiesel was 120 min, showing similar oxidative stability profiles. However, there were differences in the values of OIT around 15% which can be attributed to a slightly higher percentage of unsaturated methyl esters present in sunflower biodiesel. This can be correlated by the analysis of gas chromatography discussed.
Conclusions
The use of P-DSC to measure the oxidative induction time showed to be useful and efficient. For both the dynamic and isothermic methods, it was found that the thermal-oxidative stabilities of these vegetable oils and their respective biodiesel were similar, due to their chemical compositions present fatty acids in equivalent percentages.
References
Dantas MB, Conceição MM, Fernandes VJ Jr, Santos NA, Rosenhaim R, Marques ALB, Santos IMG, Souza AG. Thermal and kinetic study of corn biodiesel obtained by the methanol and ethanol routes. J Therm Anal Calorim. 2007;. doi:10.1007/s10973-006-7780-2.
Meher LC, Sagar DV. Technical aspects of biodiesel production by transesterification—A review. Renew Sustain Energ Rev. 2006;. doi:10.1016/j.rser.2004.09.002.
Van Gerpen J. Biodiesel processing and production. Fuel Process Tech. 2005;. doi:10.1016/j.fuproc.2004.11.005.
Candeia RA, Freitas JCO, Sousa MAF, Conceição MMI, Santos MG, Soledade LEB, Soledade LEB, Souza AG. Thermal and rheological behavior of diesel and methanol biodiesel blends. J Therm Anal Calorim. 2007;. doi:10.1007/s10973-006-7861-2.
Santos NA, Tavares MLA, Rosenhaim R, Silva FC, Fernandes VJ Jr. Thermogravimetric and calorimetric evaluation of babassu biodiesel obtained by the methanol route. J Therm Anal Calorim. 2007;. doi:10.1007/s10973-006-7765-1.
Benjumea P, Agudelo J, Agudelo A. Basic properties of palm oil biodiesel–diesel blends. Fuel. 2008;. doi:10.1016/j.fuel.2007.11.004.
Barnwal BK, Sharma MP. Prospects of biodiesel production from vegetable oils in India. Renew Sustain Energ Rev. 2005;. doi:10.1016/j.rser.2004.05.007.
Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Tech. 2005;. doi:10.1016/j.fuproc.2004.11.002.
Ramalho VC, Jorge N. Antioxidantes utilizados em óleos, gorduras e alimentos gordurosos. Quím Nova. 2006;29(4):755–60.
Suarez PAZ, Meneghetti SMP, Meneghetti MR, Wolf CR. 70º aniversário do biodiesel em 2007: evolução histórica e situação atual no Brasil. Quím Nova. 2007;. doi:10.1590/S0100-40422007000800046.
Knothe G. Analysis of oxidized biodiesel by 1H-NMR and effect of contact area with air. Eur J Lipid Sci Tech. 2006;. doi:10.1002/ejlt.200500345.
Knothe G, Dunn RO. Oxidative stability of biodiesel in blends with jet fuel by analysis of oil stability index. J Am Oil Chem Soc. 2003;80:1047–8.
Bouaid A, Martinez M, Jaracil J. Long storage stability of biodiesel from vegetable and used frying oils. Fuel. 2007;. doi:10.1016/j.fuel.2007.02.014.
Santos NA, Santos JRJ, Sinfrônio FMS, Bicudo TC, Santos IMG, Antoniosi Filho NR, Fernandes VJ Jr, Souza AG. Thermo-oxidative stability and cold flow properties of babassu biodiesel by pdsc and tmdsc techniques. J Therm Anal Calorim. 2009;97:611–4. doi:10.1007/s10973-008-9719-2.
Rodrigues FMG, Souza AG, Santos IMG, Bicudo TG, Silva MCD, Sinfrônio FSM, Vasconselos AFF. Antioxidative properties of hydrogenated cardanol for cotton biodiesel by PDSC and UV/VIS. J Therm Anal Calorim. 2009;97:605–9. doi:10.1007/s10973-008-9600-3.
Resolution ANP No. 7, of 13.3.2008. Available at http://www.anp.gov.br.
Garcia-Mesa JA, Luque de Castro MD, Valcarcel M. Factors affecting the gravimetric determination of the oxidative stability of oils. J Am Oil Chem Soc. 1993;70:245–7.
Gutierrez F. Determination of the oxidative stability of virgin olive oils: comparison of the active oxygen and the Rancimat methods. Grasas y Aceites. 1989;40:1–5.
Hill SE, Perkins EG. Determination of oxidation stability of soybean oil with the oxidative stability instrument: operation parameter effects. J Am Oil Chem Soc. 1995;72:741–3.
Conceição MM, Fernandes VJ, Bezerra AF, Silva MCD, Santos IMG, Silva FC, Souza AG. Dynamic kinetic calculation of castor oil biodiesel. J Therm Anal Calorim. 2007;. doi:10.1007/s10973-006-8194-x.
Acknowledgements
The authors acknowledge the ANP (Agência Nacional do Petróleo, Gás Natural e Biocombustíveis) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galvão, L.P.F.C., Santos, A.G.D., Gondim, A.D. et al. Comparative study of oxidative stability of sunflower and cotton biodiesel through P-DSC. J Therm Anal Calorim 106, 625–629 (2011). https://doi.org/10.1007/s10973-011-1411-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1411-2