Abstract
Chemical looping combustion (CLC) is a promising technology for segregation of carbon dioxide. CLC uses a metal oxide as an oxygen carrier, which transfers oxygen from the air to the fuel avoiding direct contact between them, thus separating the carbon dioxide and nitrogen. Cu-based oxygen carriers are excellent mediums due to high reactivity, environmental friendliness, and favorable thermodynamics. However, there are agglomeration issues due to low melting point of Cu. To solve this issue, a new preparation method as well as a dispersion reagent and a thermal durability-enhanced reagent were applied simultaneously to the oxygen carrier. The carriers were synthesized using both wet and dry impregnation methods. Based on the initial oxygen loading capability tests, the dry impregnation method received additional investigation. The characterizations of the oxygen carriers were evaluated using thermogravimetric analyzer (TG), X-ray diffraction (XRD), scanning electron microscopy (SEM), and surface area analyzer. TG results demonstrate that the enhanced dry impregnation was an effective preparation method, where the mass loss of the oxygen carrier was typically 3.4 %, correlating to almost 17 % loaded CuO. XRD results indicate a new phase, CuAl2O4 spinel, formed after the first few redox cycles, which is responsible for promoting the thermal stability of the oxygen carriers. SEM results show that the addition of the dispersants decreased the agglomeration and the enhanced reagent chemicals greatly improved the strength of the carriers. However, the surface area of the oxygen carriers decreased with the addition of the additives. In addition, with the increasing redox cycles, the surface area also decreased while the pore size increased, indicating that small pores were crushed, but the reactivity of the oxygen carriers did not decrease. In conclusion, the oxygen carriers produced in this manner are suitable for multi-cycle tests, and a major hurdle toward reducing greenhouse gases has been achieved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
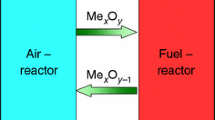
The increasing concentration of carbon dioxide has added to the greenhouse gas effect. Carbon dioxide capture and sequestration (CCS) technology has been pursued to reduce greenhouse gases. Chemical looping combustion (CLC) is one of the most promising CCS technologies with inherent separation of carbon dioxide and nitrogen [1–7]. CLC is carried out by cyclic reduction and oxidation of a metallic oxide, which acts as an oxygen carrier that is exchanged between two interconnected reactors [3]. In the fuel reactor, the oxygen carrier is reduced by the fuel, and in the air reactor, the oxygen carrier is oxidized by the air. With this system, there is no direct contact between fuel and air [1–5]. The exit gas from the fuel reactor contains CO2 and H2O. If the water is condensed, concentrated CO2 streams can be obtained with little energy lost for component separation, thus making CLC systems pivotal in the sequestration of CO2. During the reduction–oxidation cycles, the oxygen carrier plays a significant role, which needs high oxygen-transfer capacity, reactivity, and favorable thermodynamics [1–10]. Many different metal-based oxides have been tested in the literature as potential candidates for CLC systems, for example, NiO, CuO, Fe2O3, and MnO, which are supported on different inert materials, such as Al2O3, SiO2, yttrium stabilized zirconia (YSZ) or TiO2 [1–8]. These supporting materials not only provide high surface area but also act as a binder for increasing mechanical strength and attrition resistance [1, 5]. Different metal oxides, substrates, and preparation methods significantly influence the characterization of the oxygen carriers. Among these metal oxides, copper oxide has been chosen because of high reactivity and favorable thermodynamic behavior [8–10]. In addition, γ-Al2O3 is used as the substrate due to its good fluidization properties and thermal stability [11]. Many preparation methods, such as mechanical mixing [1, 5], precipitation [12], spray drying [13, 14], freeze granulation [2, 4], impregnation [1, 7, 11, 15–17], and sol–gel [18, 19] have been used to prepare the oxygen carriers. Research shows that the impregnation method is one of the best methods for preparing the Cu-based oxygen carriers [1, 15, 16]. In this method, the metal oxide is evenly dispersed on the supporting material, which enabled the oxygen carrier to exhibit high reactivity [1, 15, 16]. However, the copper-based oxygen carrier still faces the problem of a low melting point, which may cause agglomeration and accumulative thermal sintering problems after multi-cycle tests [8–10]. Hence, solving these issues is an important step for widespread usage of Cu-based oxygen carriers. The addition of a dispersant agent and a thermal durability-enhanced reagent is necessary, which will reduce the agglomeration and enhance the thermal durability. In this paper, the dispersant reagent and thermal durability-enhanced reagent were added to the copper-based oxygen carrier during preparation and the characterization of the modified oxygen carrier, such as enhanced as well as used, were evaluated using TG, XRD, SEM, and surface area analyzer for determination of suitable in a CLC system.
Experimental
Oxygen carrier preparation
The oxygen carriers were prepared from γ-Al2O3 (Puralox NWa-155 Sasol Germany GmbH) and Cu(NO3)2 (Acros, USA). The effective method for preparing oxygen carriers is impregnation, including dry impregnation and wet impregnation [1]. For the wet impregnation method, the substrate γ-Al2O3 was immersed in a saturated solution of Cu(NO3)2 for 12 h, rinsed with DI water, and dried in an oven at 150 °C for 2 h. These procedures were followed by calcination at 500 °C in the oven for 5 h to assure the loaded copper compound was copper oxide (CuO). In the dry impregnation method, a solution of saturated Cu(NO3)2 and a small amount of thermal durability-enhanced reagent (lanthanum nitrate) and dispersant (citric acid) are mixed in a special ratio by mass for 12 h. Following mixing, the solution is applied to the substrate γ-Al2O3 until fully wetted, which took approximately 1 h. Then ethanol was continuously applied to the oxygen carrier for roughly 30 min and dried in the oven at 150 °C for 2 h. After removing from the oven, the oxygen carrier was treated again with the saturated Cu(NO3)2 solution followed by the ethanol treatment and 2 more hours at 150 °C. Subsequently, the oxygen carrier was calcinated in the oven at 500 °C for 5 h. The major difference between “wet” and “dry” methods is removal of moisture occupied inside the pore structure of supporting materials prior to doping procedures. In our process, ethanol was used to facilitate the removal of the moisture.
This study involved three types of oxygen carriers that were prepared according to the wet and dry impregnation methods. Based on the outcome of initial tests, a more complete analysis was performed on the dry impregnation method. The first type oxygen carrier labeled as CW was prepared using the wet method. The second type oxygen carrier labeled as CD was prepared using the dry method, without any additives. The third type oxygen carrier labeled as CD-en is prepared with the dry method, but is also augmented with enhanced reagents and dispersants.
Characterization
The amount of loaded CuO and the thermal stability of the modified oxygen carriers were performed on a TG (TA 2950, US). A 20 mg of sample was placed in a platinum pan and heated to 800 °C in nitrogen flow, and then exposed in a cyclic manner to a reducing gas of 10 % H2 for the reduction period and to air for the oxidation period. The temperature was kept at 800 °C during both reduction and oxidation periods, which were 300 s, respectively. N2 was introduced for 600 s between periods to avoid mixing between H2 and O2. The total flow of the gas into the furnace was 100 mL min−1 and a 10 % portion of the total gas flow, i.e., 10 mL min−1 purge N2 was introduced from the head of TG to keep the balance parts from any corrosive gas. The specific surface area and the pore size distribution of the oxygen carriers were obtained from a Micromeritics ASAP 2020 (Micromeritics Instruments Inc.), where the surface area was evaluated by BET equation in the relative pressure range of 0.05–0.35. The pore size and the pore size distribution were determined by BJH and DFT methods, respectively. The crystalline structures of the oxygen carriers were identified by a SCINTAG X’TRA AA85516 (ThermoARL) X-ray diffractometer equipped with a Peltier-cooled Si solid detector. Monochromatized Cu Kα (0.154 nm) was used as the radiation source. Diffraction patterns were collected at 45 kV–40 mA over 5°–90° (2θ) at a step scan rate of 1.20° min−1. The microstructure of the oxygen carrier was determined by a JEOL JSM 5400-LV scanning electron microscope. The copper distribution in the cross-section was also determined by embedding the particle in resin, polishing to reveal cross-section, and then analysis by SEM.
Results and discussion
TG results
From Figs. 1, 2, and 3, it can be seen that the mass losses of CW, CD, and CD-en were 1.1, 3.8, and 3.4 %, respectively, which correlates to loaded CuO amount of 5.5, 19, and 17 %. Based on the threefold difference in oxygen carrying capacities, the rest of our studies were fully devoted to the dry impregnation method. The CuO content in CD was higher than that of CD-en, suggesting greater oxygen-transfer capacity. However, higher CuO content will lead to agglomeration issues [15]. The typical CuO amount was 17 % for CD-en. After 20 redox cycles of CD and 30 redox cycles of CD-en, both demonstrated good thermal stability, i.e., no further thermal degradation. However, there were small compositional differences between the CD and CD-en oxygen carriers after the multi-cycle tests. The original CD and CD-en oxygen carriers were both composed of CuO and γ-Al2O3; however, after the first few redox cycles, a new phase, CuAl2O4 spinel, formed (Fig. 4), which promoted the thermal stability of the oxygen carrier [1, 15]. In addition, in CD oxygen carrier, another new phase was also formed, Cu2O. This is probably because there were no dispersant reagents, and parts of the Cu agglomerated and emitted large amounts of heat when reacted, which accelerated the decomposition of CuO (Fig. 4). Moreover, the reduction of the oxygen carrier was an exothermic process, as a result, the temperature increased and CuO decomposed to Cu2O. In the reduction process, even after 30 redox cycles, the product was only Cu, indicating that not only CuO but also CuAl2O4 were completely reduced by H2.
SEM results
The morphology change and the element distribution of the oxygen carrier were studied by SEM. It can be seen that the particle sizes were <10 μm, and there were many holes on the surface of the oxygen carrier, indicating a higher surface area for the CD and CD-en (Fig. 5a–g). For the CD oxygen carrier, after 20 redox cycles, there was clear agglomeration on the surface of Al2O3, which is exhibited by the dark spots (Fig. 5b). This further explains the compositional difference between CD and CD-en after 20 redox cycles. Based on the agglomeration problem for CD with <20 redox cycles further redox cycles were not attempted. For the CD-en carrier, the agglomeration decreased and the CuO particles were evenly distributed on the surface of the Al2O3 (Fig. 5c, d). After 30 reduction cycles, the copper was evenly distributed on the surface of Al2O3 (Fig. 5e). The images of the cross-section of the original CD-en and CD-en after 20 redox cycles were shown in Fig. 5f and g. It is noted that most of the CuO formed an outer layer on the surface of the Al2O3 and a small amount of CuO migrated into the center of the Al2O3. After 20 redox cycles, the outer layer of the CuO attenuated, moreover, more CuO transferred into the pores of Al2O3. This may be due in part to a chemical reaction or thermal displacement [18]. However, even after 20 redox cycles, the holes can still be observed, indicating that the oxygen carrier was still porous and the reactivity of the oxygen carrier did not decrease even when there was some agglomeration.
Surface area results
The surface area and pore size also influence the reactivity of the oxygen carrier. After redox cycles, not only the surface area but also the pore size changed. Table 1 shows that the surface area of the original γ-Al2O3 was 150 m2 g−1, and original CD and CD-en were 116 and 105 m2 g−1, respectively, demonstrating that with the addition of additives, the surface area decreased. Moreover, with increasing redox cycles, the surface area of CD and CD-en both further decreased from 116 to 42 and 105 to 42 m2 g−1, respectively. But, the average pore size of CD and CD-en increased from 9.0 nm to 17.7 and 18.8 nm, respectively, after 20 redox cycles, implying that some smaller pores were crushed, which may be due to thermal displacement or reactions [18]. As a consequence, pore volume decreased. However, the decreased surface area and increased pore size did not influence the reactivity of the oxygen carrier, which is confirmed by the TG results, Figs. 2, 3 refer. Actually, the pore size has a close relationship with the reactivity of the oxygen carrier. The pore size controls molecular diffusion, which contributes to the reactivity of the carriers. Large pore size promotes the diffusion of molecules to improve the reactivity of the oxygen carrier, but decreases the surface area. Thus, it reduces availability of active sites to reaction and decreases reactivity. So, there is a threshold for the pore size, where the pore size cannot be too large or small. This needs to be further studied. Figure 6a shows that the pore size distribution of both CD and CD-en shifted to the larger pore range. The pore size of CD and CD-en was initially distributed in 2–50 nm. However, after 20 redox cycles, the pore size of both carriers was shifted to 12–50 nm, further confirming that the micropores were crushed. Nevertheless, these pores were still in the mesopore size range, which may be attributed to the oxygen carrier’s long lasting good reactivity. After 30 reduction cycles, the average pore size was 192 nm because of the reduction of copper oxide to Cu. Figure 6b also shows that in the reduction process, the pore size distribution was mainly in 130–500 nm, which is attributed to Cu.
Conclusions
The preparations of Cu-based oxygen carriers were compared using dry and wet impregnation methods. The characterizations of the modified oxygen carriers were evaluated using TG, XRD, SEM, and surface area analyzer. TG results show that enhanced dry impregnation was an effective preparation method, where the loaded CuO amount could reach up to 17 %. Moreover, even after 20 redox multi-cycles, the CD and CD-en demonstrated good thermal stability. XRD results exhibit the formation of a new phase, CuAl2O4 spinel, which promoted the thermal stability of the oxygen carrier. However, in the CD carrier, Cu2O was formed, which may be due in part to a deficiency of dispersant reagents. In the reduction process, the oxygen carriers were completely reduced to Cu. SEM results confirm that the deficiency of dispersant additives led to the agglomeration of the oxygen carriers. BET results point out that with the addition of the enhancers, the surface area of the oxygen carriers decreased. With increasing redox cycles, the surface area of the oxygen carrier decreased but the pore size increased, indicating that the small pores were crushed. The pore size distribution of the CD and CD-en both shifted from 2–50 to 12–50 nm after 20 redox cycles. However, the reactivity of the carriers did not decrease. In conclusion, CD-en is a good oxygen carrier that is suitable for multi-cycle tests.
References
de Diego LF, Garcia-babiano F, Adanez J, Gayan P, Abad A, Corbella BM, Palacios JM. Development of Cu-based oxygen carriers for chemical-looping combustion. Fuel. 2004;83:1749–57.
Zafar Q, Abad A, Mattisson T, Gevert B. Reaction kinetics of freeze-granulated NiO/MgAl2O4 oxygen carrier particles for chemical-looping combustion. Energy Fuels. 2007;21:610–8.
Moghtaderi B, Song H. Reduction properties of physically mixed metallic oxide oxygen carriers in chemical looping combustion. Energy Fuels. 2010;24:5359–68.
Cho P, Mattisson T, Lyngfelt A. Comparison of iron-, nickel-, copper- and manganese-based oxygen carriers for chemical-looping combustion. Fuel. 2004;3:1215–25.
Adanez J, de Diego LF, Garcia-labiano F, Gayan P, Abad A. Selection of oxygen carriers for chemical-looping combustion. Energy Fuels. 2004;18:371–7.
Gu H, Shen LH, Xiao J, Zhang SW, Song T. Chemical looping combustion of biomass/coal with natural iron ore as oxygen carrier in a continuous reactor. Energy Fuels. 2011;25:446–55.
Mattisson T, Jardnas A, Lyngfelt A. Reactivity of some metal oxides supported on alumina with alternating methane and oxygen-application of chemical-looping combustion. Energy Fuels. 2003;17:643–51.
Cao Y, Pan WP. Investigation of chemical looping combustion by solid fuels. 1. Process analysis. Energy Fuels. 2006;20:1836–44.
Cao Y, Casenas B, Pan WP. Investigation of chemical looping combustion by solid fuels. 2. Redox reaction kinetics and product characterization with coal, biomass, and solid waste as solid fuels and CuO as an oxygen carrier. Energy Fuels. 2006;20:1845–54.
Cao Y, Li B, Zhao HY, Lin CW, Sit SP, Pan WP. Investigation of asphalt (bitumen)-fuelled chemical looping combustion using durable copper-based oxygen carrier. Energy Procedia. 2011;4:457–64.
Dueso C, Aba A, Garci-labiano F, Diego LF, Gayan P, Adanez J, Lyngfelt A. Reactivity of a NiO/Al2O3 oxygen carrier prepared by impregnation for chemical-looping combustion. Fuel. 2010;89:3399–409.
Gayan P, Dueso C, Aba A, Adanez J, Diego L, Garcia-labiano F. NiO/Al2O3 oxygen carriers for chemical-looping combustion prepared by impregnation and deposition–precipitation methods. Fuel. 2009;88:1016–23.
Moldenhauer P, Ryden M, Mattisson T, Lyngfelt A. Chemical-looping combustion and chemical-looping with oxygen uncoupling of kerosene with Mn- and Cu-based oxygen carriers in a circulating fluidized-bed 300 W laboratory reactor. Fuel Process Technol. 2012;104:378–89.
Jernadl E, Mattisson T, Thijs I, Snijkers F, Lyngfelt A. NiO particles with Ca and Mg based additives produced by spray-drying as oxygen carriers for chemical-looping combustion. Energy Procedia. 2009;1:479–86.
Diego LF, Gayan P, Garacia-Labiano F, Celaya J, Abad A, Adanez J. Impregnated CuO/Al2O3 oxygen carriers for chemical-looping combustion: avoiding fluidizing be agglomeration. Energy Fuels. 2005;19:1850–6.
Dennis JS, Muller CR, Scott SA. In situ gasification and CO2 separation using chemical looping with a Cu-based oxygen carrier: performance with bituminous coals. Fuel. 2010;89:2353–64.
Zhao HY, Cao Y, Kang ZZ, Wang YB, Pan WP. Thermal characteristics of Cu-based oxygen carriers. J Therm Anal Calorim. 2012;109:1105–9.
Zhao HB, Liu LM, Xu D, Zheng CG, Liu GJ, Jiang LL. NiO/NiAl2O4 oxygen carriers prepared by sol–gel for chemical-looping combustion fueled by gas. J Fuel Chem Technol. 2008;36:261–6.
Wang BW, Zhao HB, Zheng Y, Liu ZH, Yan R, Zheng CG. Chemical looping combustion of a Chinese anthracite with Fe2O3-based and CuO-based oxygen carriers. Fuel Process Technol. 2012;96:104–15.
Acknowledgements
We gratefully acknowledge financial supports through projects by Cenovus, Canada and the United State Department of Energy (DE-FC26-FE0001808).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Hy., Cao, Y., Orndorff, W. et al. Study on modification of Cu-based oxygen carrier for chemical looping combustion. J Therm Anal Calorim 113, 1123–1128 (2013). https://doi.org/10.1007/s10973-012-2889-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2889-y