Abstract
The thermal degradation of a series of three novel ABA block copolymers of different molar mass (M n), were the block A is a poly(2,6-dimethyl-1,4-phenylene oxide) (PPO), while the block B is a random copoly(aryl ether sulfone) P(ESES-co-EES), was studied in both inert (flowing nitrogen) and oxidative (static air) atmospheres, to investigate the effects of M n of the central block on the thermal stability. Copolymers were synthesized with a two step method: in the first stage, a linking molecule is selectively attached as end group to the P(ESES-co-EES) which reacts in the second step with the phenolic hydroxyl group of PPO. Degradations were carried out into a thermobalance, in the scanning mode, at various heating rates, and the characteristic parameters of thermal stability, namely initial decomposition temperature (T i) and the activation energy (E a) of degradation, of the various copolymers were determined. Both T i and degradation E a values increased exponentially as a function of M n of copolymers. The results were discussed and interpreted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phase-segregated polymers exhibit unique chemical and physical properties that can be useful in various applications. This feature is attributable, in part, to the distinct phases that can be optimized to obtain superior multifunction materials having at the same time some desired properties such as mechanical strength and permeation behavior. Sulfonated block copolymers are an example of phase-segregated polymers especially attractive because of their potential use as proton- exchange membranes (PEMs) for hydrogen and methanol fuel cells, which is considered a key technology for future clean energy production and conversion systems for mobile as well as stationary applications. The advantages of fuel cells if compared with conventional energy conversion systems, like, for instance, the combustion of fossil fuels, are their high efficiency and the production of “green energy”.
It is well-known that these systems need proton-exchange membranes (PEM)s, but, substantially, at this moment, the only available membranes for this purpose are based on perfluorosulfonic acid polymers, whose high cost and the dramatic drop in proton conductivity above 80 °C are limiting factors to their use [1]. As a consequence an increasing demand for low cost high performance polymeric materials suitable for the production of (PEM)s is the driving force of research in this field. Sulfonated di-block (AB) and tri-block (ABA) copolymers have been widely studied in the past. These include sulfonated polyether ether ketone [2, 3], sulfonated poly(phenylene oxide) [4], sulfonated polyphosphazene [5], sulfonated polybenzomidazole [6], and a variety of styrene-based block copolymers [7, 8].
Recently, some papers have been reported on the use of block copolymer for toughening thermosets [9, 10]. The use of block copolymers can offer several advantages such as fine tailoring of the morphology leading to nanostructured thermosets [11, 12] or unique properties compared to the simple mix of thermoset with two thermoplastics [13]. In both applications, as production of PEMS or as toughening agent, excellent thermal stability at elevated temperatures is requested and thermogravimetric analysis (TG) offers a simple and effective method for characterizing the degradation behavior of these polymer systems.
In recent years our group has been engaged in the synthesis and characterization of new polymers, which could be used for manufacturing membranes [14, 15] and/or toughening agents for epoxy-resins [15–18]. Regarding this last application our research was focused on the effect of molar masses and end groups of the copolymer [19], of the modifier concentration [20], of the nature of epoxy-resin [21, 22] and of the curing agent [23] on the properties of the epoxy/copolymer blends. On continuing this research we investigated, in this study, some novel ABA block copolymers, were the block A is a poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) while the polymers block B are some random amino ended copoly(aryl ether sulfone)s P(ESES-co-EES)s of different molecular masses (M n). Since in the past, we observed that the absence of phase separation for the system based on diglycidyl ether of bisphenol A (DGEBA) cured by 3,3′-diaminodiphenylsulfone (DDS) for different percentages of copolymer leading to a relatively low toughness [23], we selected ABA block copolymers aiming to tailor the phase separation for the DGEBA/DDS system and to improve both toughness and thermal properties of the blends. The aim of this study was to obtain information about the comprehensive thermal stability of copolymers, to check their suitability for the use as toughening agents for epoxy-resins as well as for manufacturing membranes. To this purpose, the characteristic parameters associated with the thermal stability (initial decomposition temperature and apparent activation energy of degradation values) [24–26] of our ABA block copolymers were determined and compared with each other to verify if and how much thermal stability is affected by the molar mass of P(ESES-co-EES)s. Thermal and thermoxidative degradations were studied by thermogravimetric (TG) and differential thermogravimetric (DTG) analysis.
Experimental
Materials
PPO SA120, which presents a bimodal mass distribution with a polydispersity index of about 7 and M n ≅ 2,000 g mol−1, was kindly supplied by GE Plastics (The Netherlands). The amino ended copoly(aryl ether sulfone)s P(ESES-co-EES) were synthesized according to Ref. [27]. The detailed procedure of synthesis of the studied block copolymer are extensively reported elsewhere [28]. The average numeric molecular masses and the ESES-co-EES/PPO copolymer molar composition were checked by 1 H NMR and were reported in Table 1 together with intrinsic viscosity.
1 H NMR spectroscopy
1 H NMR spectra were recorded by A Varian Unity Inova instrument (1 H 500 MHz), using d6-dimethyl sulfoxide (d6-DMSO) or d2-tetrachloroethane (d2-TCE) as solvent and tetramethylsilane TMS, at a concentration of 30 mg mL−1, as internal standard.
Viscosity measurements
Intrinsic viscosities were measured with an Ubbelohde suspended-level viscosimeter, using solutions of polymers in dimethylformamide (DMF) at 25 ± 0.1 °C.
Thermogravimetric analysis
Thermal degradations were carried out in a Shimadzu DTG-60. The calibrations of temperature and mass were performed following the procedure reported in the instruction manual of equipment [29] using as standard materials: indium (NIST SRM 2232), tin (NIST SRM 2220), and zinc (NIST SRM 2221a) for temperature and a set of exactly weighed samples supplied by Shimadzu for mass. All calibrations of equipment were repeated every two weeks.
Degradations were carried out under flowing nitrogen (0.02 L min−1) and in a static air atmosphere, in dynamic heating conditions, in the temperature range 35–700 °C, at various selected heating rates (Φ = 2, 5, 7.5, 10, 12.5, 15, 17.5, and 20 °C min−1). Samples of about 6.0 × 10−3 g were used for degradations, and their masses as a function of temperature were monitored and recorded by a PC connected with the DTG-60 apparatus. At the end of each run the experimental data were used to plot the percentage of undegraded copolymer (1 − D) % as a function of temperature, where D = (W o − W)/W o, and W o and W were the masses of sample at the starting point and during scanning.
Results and discussion
The 1 H NMR spectra of the 1–3 compounds were first recorded and the molar composition of ESES-co-EES/PPO copolymer as well as the average numeric molar mass of both P(ESES-co-EES)s and PPO were determined and reported in Table 1.
Intrinsic viscosity (η intr) determinations on studied compounds were thus performed. The η intr values obtained increase linearly as a function of copolymer average molar masses, and are reported in Table 1 too.
In order to evaluate a possible dependence of various ABA block copolymers thermal stability on P(ESES-co-EES)s average molar mass, we carried out a comparative study on the thermal and thermo-oxidative degradation of these compounds.
The initial decomposition temperature (T i) is often considered the only parameter to evaluate the thermal stability of polymers, but, in our opinion, it is not strictly correct. The T i value of a polymer must be considered a measure of its resistance to degradation when heated [30–32], but to evaluate its thermal stability it is necessary to take into account also the degradation rate, in particular if we compare compounds with initial decomposition temperatures which are quite close with each other. In this case, the polymer with higher activation energy of degradation might be considered more thermally stable [33].
Two parameters were thus here determined for each compound: the initial temperature of decomposition, obtained by the degradation TG curves as the intersection between the starting mass line and the maximum gradient tangent to the curve, and the apparent activation energy (E a) of degradation, which is correlated with the kinetics of process.
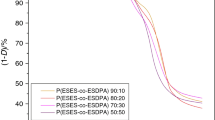
The degradations of the various copolymers were first carried out in the flowing nitrogen, at various heating rates, in the 2–20 °C min−1 range. All studied compounds degraded up to complete mass loss, in temperature intervals ranging, in every case, from 440 to 670 °C about. Since the experimental conditions, and the scanning rate in particular, largely affect both the shape and the position of TG curves, the T i values of each sample at the various scanning rates used were different with each other, though the values of various samples at the same heating rate showed the same trend in every case. For the sake of simplicity only the T i values at a single scanning rate, namely at 10 °C min−1 (selected because it is a medium rate among those we employed for degradation experiments), were considered. The TG curves at 10 °C min−1 of the studied compounds are shown in Fig. 1, while the T i values are listed in Table 2.
Analogous degradation experiments were then carried out in a static air atmosphere. Also in oxidative environment all studied compounds degraded up to complete mass loss, in the same temperature ranges (440–670 °C about) than under nitrogen. The T i values obtained at 10 °C min−1 are reported in Table 2, and the corresponding TG curves are presented in Fig. 2.
The degradation E a values of various copolymers, in both flowing nitrogen and static air atmosphere, were thus determined using the data from DTG degradation curves at the various heating rates. The classical Kissinger method [34], widely reported in literature [35–37], was used to this aim, which is based on the following linear equation:
where Φ is the heating rate, T m is the temperature at maximum rate of mass loss (the temperatures of DTG peaks), n is the apparent reaction order, R is the universal gas constant, A is the pre-exponential factor and W m is the mass of sample at the maximum rate of mass loss. The T m values of all studied compounds in both inert and oxidative atmospheres are listed in Tables 3 and 4, respectively. From the least-square treatment of the data in Tables 3 and 4, performed according to the Eq. (1), single linear ln (Φ/T 2m ) versus 1/T m relationships were obtained for all samples in both studied environments. Thus, the E a value could be obtained through the linear dependence of ln (Φ/T 2m ) on 1/T m at various heating rates. The corresponding regression coefficients and the calculated E a values are reported in Tables 5 and 6 for nitrogen and air, respectively.
A preliminary observation on the results obtained: even though all copolymers degraded up to complete mass loss, at all used heating rates, the TG and DTG curves of compounds 1 and 2 in flowing nitrogen were different than those of compound 3. The TG and DTG curves of compounds 1 and 2, showed a first broad stage probably due to the degradation of P(ESES-co-EES)s block [17], followed by another one in the last piece of curve, attributable to the degradation of PPO blocks (Fig. 1). By contrast, the copolymer having higher molar mass (sample 3) completely degraded in a single stage. This behavior can be attributed to higher decomposition temperature of P(ESES-co-EES)s block due to its higher molar mass, thus giving rise to the overlap with the degradations of A and B blocks as evidenced by the shoulder in the DTG curves of sample 3 (Fig. 3).
The degradations TG and DTG curves of studied compounds in oxidative atmosphere exhibited the same behavior observed in inert environment, thus suggesting that the presence of oxygen does not affect the degradation mechanism.
Some considerations are possible on the basis of the data in Table 2, where T i values of the studied copolymers, in both atmospheres, together with the corresponding degradation E a values are reported:
-
the resistance to the thermal degradation of block copolymers here investigated is largely affected by the average molar mass. In particular compound 3, in which the P(ESES-co-EES)s block having the highest M n (16,700 g mol−1) is present, appears the most heat resistant, showing a high increment of initial decomposition temperature values (48 °C under nitrogen and 35 °C in static air atmosphere) in respect to the copolymer having the central block of lower M n (5,000 g mol−1).
-
also the degradation activation energy of copolymer 3, in both studied environments, is higher than those of 1 and 2 samples, thus meaning lower degradation rate and then better thermal stability from the kinetic viewpoint;
-
both initial decomposition temperature (Fig. 4) and apparent activation energy of degradation (Fig. 5) show a very good exponential increase on increasing the average molar mass of P(ESES-co-EES)s block thus indicating a strong dependence of ABA copolymers thermal behavior on the average molar mass of the central block.
Fig. 4
This behavior can be explained with higher percentage of double-bond character due to the increasing number of sulfone groups in copolymer chain, and then with the higher dissociation energy requested for the cleavage of chain links. This result is in agreement with some literature data [38] and with the results of our previous works concerning some polyetherketones and polyethersulfones, in which we found an increase of degradation E a values as a function of glass transition temperature T g [39, 40], owing to it is well-known that glass transition temperature of polymers increases on increasing average molar mass.
Conclusions
The results obtained indicate a strict correlation between the thermal stability of block copolymers here studied and the average numeric molar mass of the central P(ESES-co-EES)s block, not only for the starting point of decomposition but also from the point of view of the degradation kinetics. The better thermal stability of the copolymer 3 in comparison with those of lower average molar mass 2 and 1 copolymers can be attributed to the increase of the number of sulfone groups in copolymer chain which give rise to an increase in percentage of double-bond character of chain bonds. Finally, the high values of initial decomposition temperature and apparent activation energy of degradation found for the ABA block copolymers are encouraging and allow us to plan the use of this compounds as toughening agents of epoxy-resins employed as matrices for advanced composites and as polymeric materials suitable for the production of (PEM)s.
References
Roziere J, Jones DJ. Non-fluorinated polymer materials for proton exchange membrane fuel cells. Annu Rev Mater Sci. 2003;33:503–55.
Manea C, Mulder M. Characterization of polymer blends of polyethersulfone/sulfonated polysulfone and polyethersulfone/sulfonated polyetheretherketone for direct methanol fuel cell applications. J Membr Sci. 2002;206(1–2):443–53.
Lakshami VV, Choudhary V, Varma IK. Sulphonated poly(ether ether ketone): synthesis and characterisation. Macromol Symp. 2004;210(1):21–9.
Schauer J, Albrecht W, Weigel T. The preparation of microporous membranes from blends of poly(2,6-dimethyl-1,4-phenylene oxide) and sulfonated poly(2,6-dimethyl-1,4-phenylene oxide). J Appl Polym Sci. 1999;73(2):161–7.
Guo Q, Pintauro PN, Tang H, O’Connor SO. Sulfonated and crosslinked polyphosphazene-based proton-exchange membranes. J Membr Sci. 1999;154(2):175–81.
Jones D, Roziere J. Recent advances in the functionalisation of polybenzimidazole and polyetherketone for fuel cell applications. J Membr Sci. 2001;185(1):41–58.
Mani S, Weiss RA, Williams CE, Hahn SF. Microstructure of ionomers based on sulfonated block copolymers of polystyrene and poly(ethylene-alt-propylene). Macromolecules. 1999;32(11):3663–70.
Serpico JM, Ehrenberg SG, Fontanella JJ, Jiao X, Perahia D, McGrady KA, Sanders EH, Kellogg GE, Wnek GE. Transport and structural studies of sulfonated styrene–ethylene copolymer membranes. Macromolecules. 2002;35(15):5916–21.
Ritzenthaler S, Court F, David L, Girard-Reydet E, Leibler L, Pascault JP. ABC triblock copolymers/epoxy–diamine blends. 1. Keys to achieve nanostructured thermosets. Macromolecules. 2002;35(16):6245–54.
Dean JN, Verghese NE, Pham HQ, Bates FS. Nanostructure toughened epoxy resins. Macromolecules. 2003;36(25):9267–70.
Girard-Reydet E, Sautereau H, Pascault JP. Use of block copolymers to control the morphologies and properties of thermoplastic/thermoset blends. Polymer. 1999;40(7):1677–87.
Ritzenthaler S, Court F, Girard-Reydet E, Leibler L, Pascault JP. ABC triblock copolymers/epoxy–diamine blends. 2. Parameters controlling the morphologies and properties. Macromolecules. 2003;36(1):118–26.
Zucchi IA, Galante MJ, Williams RJJ. Comparison of morphologies and mechanical properties of crosslinked epoxies modified by polystyrene and poly(methyl methacrylate) or by the corresponding block copolymer polystyrene-b-poly(methyl methacrylate). Polymer. 2005;46(8):2603–9.
Abate L, Asarisi V, Blanco I, Cicala G, Recca G. The influence of sulfonation degree on the thermal behaviour of sulfonated poly(arylene ethersulfone)s. Polym Degrad Stab. 2010;95:1568–74.
Abate L, Blanco I, Cicala G, Mamo A, Recca G, Scamporrino A. The influence of chain rigidity on the thermal properties of some novel random copolyethersulfones. Polym Degrad Stab. 2010;95:798–802.
Abate L, Blanco I, Cicala G, La Spina R, Restuccia CL. Thermal and rheological behaviour of some random aromatic polyethersulfone/polyetherethersulfone copolymers. Polym Degrad Stab. 2006;91(4):924–30.
Abate L, Blanco I, Cicala G, Recca A, Restuccia CL. Thermal and rheological behaviours of some random aromatic amino-ended polyethersulfone/polyetherethersulfone copolymers. Polym Degrad Stab. 2006;91(12):3230–6.
Blanco I, Cicala G, Lo Faro C, Recca A. Improvement of thermomechanical properties of a DGEBS/DDS system blended with a novel thermoplastic copolymer by realization of a semi-IPN network. J Appl Polym Sci. 2003;88(13):3021–5.
Abate L, Blanco I, Cicala G, Recca G, Scamporrino A. The influence of chain ends on the thermal and rheological properties of some 40/60 PES/PEES copolymers. Polym Eng Sci. 2009;49(8):1477–83.
Blanco I, Cicala G, Lo Faro C, Recca A. Thermomechanical properties and morphology of blends of a novel thermoplastic copolymer and epoxy-resin. J Polym Eng. 2003;23(3):163–76.
Blanco I, Cicala G, Lo Faro C, Recca A. Development of a toughened DGEBS/DDS system toward improved thermal and mechanical properties by the addition of a tetrafunctional epoxy resin and a novel thermoplastic. J Appl Polym Sci. 2003;89(1):268–73.
Blanco I, Oliveri L, Cicala G, Recca A. Effects of novel reactive toughening agent on thermal stability of epoxy resin. J Therm Anal Calorim. 2012;108:685–93.
Blanco I, Cicala G, Motta O, Recca A. Influence of a selected hardener on the phase separation in epoxy/thermoplastic polymer blends. J Appl Polym Sci. 2004;94(1):361–71.
Abate L, Blanco I, Pollicino A, Recca A. Determination of degradation apparent activation energy values of polymers: regression of kinetic parameters derived from TG data. J Therm Anal Calorim. 2002;70:63–73.
Blanco I, Abate L, Bottino FA, Bottino P, Chiacchio MA. Thermal degradation of differently substituted cyclopentyl polyhedral oligomeric silsesquioxane (CP-POSS) nanoparticles. J Therm Anal Calorim. 2012;108:807–15.
Blanco I, Abate L, Antonelli ML. The regression of isothermal thermogravimetric data to evaluate degradation E a values of polymers: a comparison with literature methods and an evaluation of lifetime prediction reliability. Polym Degrad Stab. 2011;96:1947–54.
Puglisi C, Samperi F, Cicala G, Recca A, Restuccia CL. Combined MALDI-TOF MS and NMR characterization of copoly(arylen ether sulphone)s. Polymer. 2006;47(6):1861–74.
Cicala G, Mamo A, Recca G, Restuccia CL. Synthesis and thermal characterization of some novel ABA block copolymers. Macromol Mater Eng. 2007;292:588–97.
Shimadzu Corporation. DTG-60/60H instruction manual. Kyoto: Analytical & Measuring Instruments Division, Shimadzu Corporation; 2000.
Blanco I, Abate L, Bottino FA, Bottino P. Hepta isobutyl polyhedral oligomeric silsesquioxanes (hib-POSS): a thermal degradation study. J Therm Anal Calorim. 2012;108:807–15.
Blanco I, Siracusa V. Kinetic study of the thermal and thermo-oxidative degradations of polylactide-modified films for food packaging. J Therm Anal Calorim. 2012. doi:10.1007/s10973-012-2535-8.
Blanco I, Bottino FA, Bottino P. Influence of symmetry/asymmetry of the nanoparticles structure on the thermal stability of polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym Compos. 2012;33(11):1903–10.
Blanco I, Bottino FA. Effect of the substituents on the thermal stability of hepta cyclopentyl, phenyl substituted-polyhedral oligomeric silsesquioxane (hcp-POSS)/polystyrene (PS) nanocomposites. AIP Conf Proc. 2012;1459:247–9.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
De Angelis Curtis S, Kurdziel K, Materazzi S, Vecchio S. Crystal structure and thermoanalytical study of a manganese(II) complex with 1-allylimidazole. J Therm Anal Calorim. 2008;92:109–14.
Materazzi S, Vecchio S, Wo LW, De Angelis Curtis S. Thermoanalytical studies of imidazole-substituted coordination compounds. Mn(II)-complexes of bis(1-methylimidazol-2-yl)ketone. J Therm Anal Calorim. 2011;103:59–64.
Papadopoulos C, Kantiranis N, Vecchio S, Lalia-Kantouri M. Lanthanide complexes of 3-methoxy-salicylaldehyde: thermal investigation by simultaneous TG/DTG-DTA coupled with MS; Thermal decomposition kinetics. J Therm Anal Calorim. 2010;99:931–8.
Turi EA. Thermal characterization of polymeric materials, vol. 1. San Diego: Academic Press; 1997.
Abate L, Blanco I, Motta O, Pollicino A, Recca A. The isothermal degradation of some polyetherketones: a comparative kinetic study between long-term and short-term experiments. Polym Degrad Stab. 2002;75:465–71.
Abate L, Blanco I, Orestano A, Pollicino A, Recca A. Kinetics of the isothermal degradation of model polymers containing ether, ketone and sulfone groups. Polym Degrad Stab. 2005;87(2):271–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Lorenzo Abate on the occasion of his 70th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blanco, I., Cicala, G., Latteri, A. et al. Thermal and thermo-oxidative degradations of poly(2,6-dimethyl-1,4-phenylene oxide) (PPO)/copoly(aryl ether sulfone) P(ESES-co-EES) block copolymers: a kinetic study. J Therm Anal Calorim 112, 375–381 (2013). https://doi.org/10.1007/s10973-012-2793-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2793-5