Abstract
The mixed-ligand complex formation in the systems Cu2+–Edta4−–L (L = His, Lys, Orn, Arg, Im) has been calorimetrically, pH-potentiometrically, and spectrophotometrically studied in aqueous solution at 298.15 K and the ionic strength of I = 0.5 (KNO3). The thermodynamic parameters of formation of the CuEdtaL, CuEdtaHL, and (CuEdta)2L complexes have been determined. The probable coordination mode for the complexone and the ancillary ligand in the mixed-ligand complexes was discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
EDTA has a widespread clinical application as a so-called chelation therapy agent. Owing to its strong complexing capability, in particular with respect to transition metal ions and radionuclides, it has been used in vivo to remove toxic metal ions or an excess of biometal ions. The formation of relatively stable mixed complexes of the type CuEdtaL at a physiologic pH value should be accounted under chelation therapy and its excretion from the human organism [1]. The account of the interaction of copper (II) complexonate with amino acid and peptide molecules can make the application of chelating agents (complexones) more effective. The sufficiently strong affinity of copper (II) ion to ligands with N donor atoms makes the amino acids containing the so-called additional donor group (His, Lys, Arg, and some others) an interesting object of mixed complexes formation research.
The presence of several donor atoms in amino acids being studied results in various coordination modes. Earlier, the stability of mixed-ligand complexes of Hg(II) ethylenediaminetetraacetate with the above-mentioned amino acids had been studied [2]. It was noted that the chelation number (denticity) of amino acid in mononuclear mixed complexes did not achieve the maximum. The presence of an uncoordinated group of amino acid in HgEdtaL leads to the formation of a binuclear complex in which amino acid has a bridge function. Such effect has been revealed in the systems including histidine, lysine, cysteine, and methionine.
Copper (II)–EDTA–amino acid interaction has been previously studied in [3] by potentiometric titration. The mixed complexes CuEdtaL formation and their possible role in blood plasma models have been investigated. The possible coordination mode of ligands and structure of mixed complexes have not been proposed by the authors. It was interesting to continue the study of systems Cu2+–Edta4−–amino acids in aqueous solution in a wide range of pH in order to reveal the coordination mode of ligands in the mixed complex.
Recently, some metal (II) complexonates (Edta, Edda, Nta) have been investigated by different methods [4, 5]. The mixed complex formation of metal (II) nitrilotriacetates with amino acids has also been studied [6]. Transition metal complexes of complexones (Ida, Nta, Edta) were widely used in biotechnology, particularly in the protein purification technique known as Immobilised Metal-ion Affinity Chromatography (IMAC). It should be noted that calorimetry became a powerful instrument of investigation of metal ion complexation by amino acids, peptides, and other biologically active ligands [7].
Experimental
Materials
Sodium salt of copper (II) ethylenediaminetetraacetate Na2CuEdta·4H2O used in the work was purified by recrystallization from an aqueous-DMFA solution. A solution of the complexonate was prepared from the exact weight of the reagent. Carbonate-free NaOH solution was prepared according to standard procedure. Analytical grade KNO3 used for adjusting the solution ionic strength was doubly recrystallized from distilled water. l-Histidine HHis·HCl (high-purity grade) (Renal), l-Lysine HLys·HCl (Acros), l-Ornithine HOrn HCl (Acros), and l-Arginine HArg·HCl (Renal) were used without further purification. Imidazole was recrystallized from benzene.
pH-metric measurements
The mathematical simulation of the equilibrium compositions of the solutions containing CuEdta2− and amino acid in a wide pH range at various CuEdta2− to L ratios has been carried out by means of the RRSU computer program [8].
The coordination equilibrium was investigated by potentiometric titrations in aqueous solution (I = 0.5 M KNO3 and T = 298.15 ± 0.1 K). The series of pH-potentiometric titrations of solutions containing Na2CuEdta, HL·HCl, and supporting electrolyte KNO3 with a NaOH solution have been performed. The emf of the transfer chain including glass and saturated Ag/AgCl electrodes was measured by the compensation method. The pH glass electrode was calibrated in aqueous solution using standard buffer solutions. The standard potential value was determined using a standard solution of (HCl + KNO3). The constants of complex formation were evaluated from 3 to 4 independent titrations (30–50 data points per titration). The applied ratio of the CuEdta and amino acid was 2:1, 1:1, and 1:2 with the amino acid concentrations varied between 0.01 and 0.02 M. The experimental pH-metric data were processed by means of the PHMETR computer program [8] based on the minimization of the likelihood function F = Σ(p c H exp − p c H calc)2, where p c H = −log[H +] are the experimental values and those calculated by the model, respectively. The possibility of the following reactions has been taken into account:
Computer simulation has shown that the substitution processes of the type CuEdta + nL = CuLn + Edta under a small excess of L have not taken place. The agreement between the theoretic and experimental curves was achieved only when the complexes of the type CuEdtaL3−and CuEdtaHL2− formation were considered (Fig. 1). Unfortunately, the formation constants of binuclear complexes (CuEdta)2L5− have not been potentiometrically determined. The simultaneous treatment of lgK and Δr H of reaction (7) has been calorimetrically performed. The lgK 1 value was received by the extrapolation technique at an ionic strength of I = 0.5 by means of Davis’s equation. The lgK and Δr H values of reactions (2, 3) and (2, 3) were taken from critical reviews [9, 10]. The lgK 4 and Δr H 4 values have earlier been determined by us [11]. The potentiometrically determined lgK values were taken for the reactions (5) and (6). The weighted average values of received equilibrium constants of the addition of L and HL to the CuEdta2− are given in the Table 1.
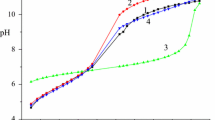
pH-metric titration curve of a solution (20.09 cm−3) containing 0.01021 mol dm−3 Na2CuEdta + 0.01034 mol dm−3 HHis·HCl + 0.48 mol dm−3 KNO3 with a NaOH solution (0.1978 mol dm−3)(2) and model curves plotted with an account for the formation of mixed-ligand complexes CuEdtaHis3− (4) (F min = 7.53), CuEdtaHHis2− (1) (F min = 27.84), and both CuEdtaHis3− and CuEdtaHHis2− (3) (F min = 1.79)
Calorimetric measurements
The heats of the reactions were measured on an isothermal-jacket ampoule flow-mixing calorimeter equipped with a thermistor temperature gage and automated recording of temperature–time curves. The calorimeter was verified against the heat of the solution of KCl in water at 298.15 K. The computer simulation permitted us to choose the optimal reagent concentrations. The heats of mixing Δmix H of a CuEdta solution with alkaline solutions of amino acids containing the supporting electrolyte (KNO3) and the heat of dilution Δdil H of a CuEdta solution in a solution of the supporting electrolyte were measured according to the first method. A weighed sample of a Cu (II) complexonate solution was placed into a glass ampoule. Neutralized amino acid with supporting electrolyte solution was contained in the reaction vessel of the calorimeter. The relative amount of CuEdtaHHis2− did not exceed 50 % and the relative amount of CuEdtaHis3− achieved ~75 % during calorimetric measurements. The series of calorimetric data of mixing of a fully neutralized alkaline solution of L with solutions containing the big excess of Cu (II) complexonate (the second method) demonstrated that the values of Δmix H calculated to the amount of amino acid were significantly more than the heats of the reactions (5) and (6). It may be explained only by binuclear complex formation. The simultaneous treatment of lgK and Δ r H of reaction (7) taking into account the contributions of processes (1)–(6) permitted us to evaluate their full thermodynamic characteristics. The experimental data are given in the Tables 2, 3. The heats of the reactions are computed by means of the HEAT program [8] based on the minimization of the likelihood function F = Σ(ΔH exp − ΔH calc)2, where the ΔH are the experimental and calculated by model values, respectively, of the heat effects. The calculated thermodynamic parameters are listed in the Table 1. The heats of water ionization (Δr H = −56.90 kJ mol−1 [12]), ligand stepwise protonation (Δr H 1 = −45.15 and Δr H 1,2 = −75.65 [13] for His, Δr H 1 = −53.55 and Δr H 1,2 = −100.85 kJ mol−1 [10] for Lys, Δr H 1 = −51.92 and Δr H 1,2 = −99.00 kJ mol−1 [10] for Orn, and Δr H 1 = −57.07 and Δr H 1,2 = −106.53 kJ mol−1 [9] for Arg), and the formation of hydroxocomplexonate CuEdtaOH2− (Δr H = −25.5 kJ mol−1) were accepted according to the published data [11]. The uncertainties of the quantities were calculated as the 95 % confidence intervals according to the Student’s test. The diagrams of the fractional distribution in the Cu2+–Edta4−–His system are shown in the Figs. 2, 3.
Electronic absorption measurements
The absorption spectra of solutions have been recorded after calorimetric measurements on CFC-3 spectrophotometer using a cell with 1 cm optical path length at T = 293 K. The received visible electronic spectra in the systems CuEdta2––L are shown in the Fig. 4.
Absorption spectra of solutions: 1 0.005 mol dm−3 Na2CuEdta; 2 0.005 mol dm−3 Na2CuEdta + 0.01 mol dm−3 HHis; 3 0.005 mol dm−3 Na2CuEdta + 0.0051 mol dm−3 His−; 4 0.005 mol dm−3 Na2CuEdta + 0.02 mol dm−3 Im; 5 0.005 mol dm−3 Na2CuEdta + 0.0070 mol dm−3 Lys−; 6 0.005 mol dm−3 Na2CuEdta + 0.0065 mol dm−3 Orn−; 7 0.005 mol dm−3 Na2CuEdta + 0.0088 mol dm−3 Arg−
Results and discussion
The content and structure of copper (II) histidine complexes have been studied for a long time and various suggestions have been made for the Cu(II)–His bond modes. The presence of three functional groups in the histidine molecule (–NH2, –COOH, and –NIm) makes it a potentially three dentate ligand. Histidine is often coordinated as bidentate ligand resulting in three coordination modes {Nam,NIm}, {Nam,O}, and {NIm,O} due to steric hindrance. Recently, the comprehensive studies of the structure of Cu(II)-His complexes in solution have been performed [14, 15]. The formation of various content complexes CuHnHis2 and their most probable structure has been determined by different types of spectroscopy. Lysine predominantly binds metal ions through the α-amino and carboxylate groups in the glycine mode to form monoprotonated complexes of the type M(HLys)2 in acidic and neutral solutions. Deprotonated complexes of the types MHLys2 and MLys2 form in alkaline solutions. The ε-amino group is not involved in metal binding in solution practically because chelation through the two amino groups would result in an unstable eight-membered ring. So, Lys does not achieve the chelation number 3 in a majority of cases. Thermodynamic and NMR studies of some copper (II)-diaminomonocarboxylate equilibrium systems in aqueous solution were carried out in [16]. It was concluded that Lysine is coordinated to the copper (II) ion in a “glycine-like” manner. The ω-amino groups were coordinated also in the complexes of other diaminomonocarboxylate ligands (dapa, daba, and ornithine) in a fully deprotonated form.
As can be shown from the Table 1, the addition of amino acids to CuEdta2− is accompanied by negative enthalpy changes and slightly positive or negative entropy changes, which are characteristic of mixed-ligand complex formation involving the coordinately saturated copper (II), nickel (II), zinc (II), and cadmium (II) complexonates and occurring with a decrease in the EDTA denticity due to the opening of one or two glycinate chelate rings. The factors that cause such thermodynamic characteristics are comprehensively described in [11].
The addition of HHis to CuEdta is more an exothermic effect than the addition of anion His−. Previously performed research [2] has revealed that mercury (II) ethylenediaminetetraacetate with histidine and lysine formed the binuclear complexes in which the amino acid acted, most likely, as a bridge linking the coordination spheres of two mercury (II) ethylenediaminetetraacetate complexes. Received data permitted us to assume that the coordination of histidine molecule in complex HgEdtaHis3− was carried out via glycine fragment, but in protonated species HgEdtaHHis2− through imidazole fragment. The similarity of thermodynamic parameters of the secondary ligands HHis± and imidazole addition to HgEdta proved this suggestion. The more basic glycinate fragment was protonated in this case. The formation of CuEdtaIm served as a model process. The addition of Im to CuEdta is accompanied by a well-pronounced exothermic effect. The sufficiently close lgK and Δr H values of the addition of HHis and Im to copper (II) complexonate demonstrate that histidine residue is coordinated via a nitrogen donor atom of imidazole fragment.
At the same time, the reaction (5) for histidine is sufficiently less exothermic in comparison with the reaction (6), but Δ r S 6 value is more negative than Δ r S 5 value. Received thermodynamic data do not exclude the possible existence of the complexes CuEdtaHis3− with ambidentate character of histidine due to (Nam,Nim) or (Nam,O) coordination modes. Apparently, the equilibrium between two forms of mixed complex CuEdtaHis3−, where histidine is coordinated in a glycine-like or histamine-like manner, takes place. The heat effects of reaction (5) in the case of His and Lys are sufficiently close. So, this equilibrium is shifted to the complex with (Nam,O) coordination mode of histidine. In the case of amino acids with a long aliphatic chain (Lys, Orn, Arg), the close values of Δr H 5 and Δr H 6 prove the glycine-like coordination mode of these amino acids in mixed complexes of the types CuEdtaHL and CuEdtaL. Evidently, the ε-amino group is not coordinated by a central ion in mononuclear complexes (Fig. 5).
Earlier [17], it was shown that the noncoordinated amino group in the complexes CuEdtaL (L = (CH2)n(NH2)2, n = 2,6) bound with the second CuEdta residue, resulting in binuclear complex formation with bridging function of diamine. Thermochemical data proved it by means of the second method. Thermodynamic data given in the Table 4 well demonstrate that increasing of the aliphatic chain length of the diamines and diaminocarboxylic acids brings in more negative entropy change value under the binuclear complex formation. The big negative entropy change in reaction (7) is caused not only by translational term, but also by loss of configurational entropy of amino acid residue with the long aliphatic chain. It has been noted in [2] that binuclear complex formation was a result that dentate number of these amino acids did not achieve the maximum value in the mononuclear complexes.
The Fig. 4 shows that the changes in the electronic spectra of copper (II) complexonate in a solution containing amino acid provide evidence of mixed complex formation. The spectrophotometrically received value of the equilibrium constant of reaction (5) for imidazole has a sufficiently good agreement with the same value calorimetrically obtained (Table 1). The visible spectra collected for the systems CuEdta-amino acid indicate a decrease of d–d transition intensity and a small blue shift under the addition of amino acid residue. The same trend has been observed in the systems CuEdta–(CH2)n(NH2)2 (n = 2,6). So, it proves the coordination of amino acid via N donor atom. The close ε values of CuEdtaHHis and CuEdtaIm complexes also provide evidence of coordination of HHis± in mixed complex via imidazole fragment.
Conclusions
So, the basic amino acids can be monodentate in the mononuclear mixed complexes or bidentate with the bridging function in the binuclear complexes. Moreover, the formation of relatively stable mixed complexes of the type CuEdtaL at a physiologic pH value should be accounted under chelation therapy. The account of the interaction of copper (II) complexonates with His and Lys containing peptide molecules can make the application of chelating agents (complexones) more effective.
References
Powell JJ, Burden TJ, Greenfield SM, Taylor PD, Thompson RP. Urinary excretion of essential metals following intravenous calcium disodium edetate: an estimate of free zinc and zinc status in man. J Inorg Biochem. 1999;75:159–65.
Ryzhakov AM, Gruzdev MS, Pyreu DF, Kozlovskii EV, Kumeev RS. Thermodynamics of mixed-ligand complexation of mercury(II) ethylenediaminetetraacetate with histidine and lysine in aqueous solution. Russ J Coord Chem. 2010;36:565–71.
Arena G, Musumeci S, Rizzarelli E, Sammartano S. Copper(II)-EDTA-aminoacid interactions. Stability constants and possible role in blood plasma models. Transition Met Chem. 1985;10:399–401.
Vikram L, Sivasankar BN. Hydrazinium metal(II) and metal(III) ethylenediaminetetraacetate hydrates. Spectral, thermal and XRD studies. J Therm Anal Calorim. 2008;91:963–70.
Rehman S, Arshad M, Masud K, Afzal R, Salma U. Pyrolytical characterization of transition metal complexes of cobalt, nickel, copper and zinc with ethylenediamine-N, N’-diacetate. J Therm Anal Calorim. 2010;102:715–22.
Khalil MMH, Ismail EH, Azim SA, Souaya ER. Synthesis, characterization and thermal analysis of ternary complexes of nitrilotriacetic acid and alanine or phenylalanine with some transition metals. J Therm Anal Calorim. 2010;101:129–35.
Wyrzykowski D, Zarzeczanska D, Jacewicz D, Chmurzynski L. Investigation of copper complexation by glycylglycine using isothermal titration calorimetry. J Therm Anal Calorim. 2011;105:1043–7.
Borodin VA, Vasiliev VP, Kozlovskii EV. Mathematical problems in chemical thermodynamics. Novosibirsk: Nauka; 1985.
Pettit LD. Critical survey of formation constants of complexes of histidine, phenylalanine, tyrosine, l-dopa and tryptophan. Pure Appl Chem. 1984;56:247–92.
Yamauchi O, Odani A. Stability constants of metal complexes of amino acids with charged side chains-Part I: positively charged side chains (Technical Report). Pure Appl Chem. 1996;68:469–96.
Kozlovskii EV, Fridman AYa. Structural and thermodynamic features of addition of mono- and bidentate ligands to the nickel, copper and zinc ethylenediaminetetraacetates in an aqueous solution. Russ J Inorg Chem. 1991;36:1500–2.
Vasiliev VP. Thermodynamic properties of electrolyte solutions. Moscow: Vysschaya Shkola; 1982.
Garavin VA. Canada Science (Chemistry). Dissertation, Ivanovo: Ivanovo Institute of Chemical Technology; 1983.
Mesu JG, Visser T, Soulimani F, Faassen EE, Peinder P, Beale AM, Weckhuysen BM. New insights into the coordination chemistry and molecular structure of copper(II) histidine complexes in aqueous solutions. Inorg Chem. 2006;45:1960–71.
Shtyrlin VG, Zyavkina YI, Gilyazetdinov EM, Bukharov MS, Krutikov AA, Garipov RR, Mukhtarov AS, Zakharov AV. Complex formation, chemical exchange, species structure, and stereoselective effects in the copper(II)- l/dl-histidine systems. Dalton Trans. 2012;41:1216–28.
Gergely A, Farkas E, Nagypál I, Kas E. Thermodynamic and NMR studies of some copper (II)-diaminomonocarboxylate equilibrium systems in aqueous solution. J Inorg Nucl Chem. 1978;40:1709–13.
Pyreu DF, Kozlovskii EV. Thermodynamics of mixed-ligand complex formation of copper(II) ethylenediaminetetraacetate with hexamethylenediamine in an aqueous solution. J Therm Anal Calorim. 2010;100:355–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bazanova, M.A., Pyreu, D.F. & Kozlovskii, E.V. Thermodynamics of mixed-ligand complex formation of copper(II) ethylenediaminetetraacetate with amino acids in solution. J Therm Anal Calorim 112, 1545–1551 (2013). https://doi.org/10.1007/s10973-012-2659-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2659-x