Abstract
Ternary complexes of copper(II) and nickel(II) nitrilotriacetate (MNta) with arginine (Arg), serine (Ser), glycine (Gly), aspartic acid (Asp), and iminodiacetic acid (Ida) have been calorimetrically, potentiometrically, and spectrophotometrically studied in aqueous solution at 298.15 K and ionic strength 0.5 (KNO3). The thermodynamic parameters of mixed-ligand complex formation have been determined. The probable coordination modes of amino acid residues in the heteroligand complexes were discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Study of heteroligand complex formation in the systems including metal complexonates and amino acids is important due to active application of polyaminepolycarboxylic acids in biomedical investigations, particularly in order to remove the toxic excess of metal ions from human organism [1]. The speciation of these metals also becomes more correct if the interaction of metal complexonates with amino acid residues and peptide molecules is taken into account. To separate of peptide molecules during immobilized metal ion affinity chromatography, the immobilization of metal complexonates (Ida, Nta) is used [2]. It also supposes the heteroligand complex formation. Hence, the elucidation of coordination modes of ligands in ternary complexes becomes important problem.
In the first part of this article [3], the ternary complex formation of copper(II) and nickel(II) nitrilotriacetates with basic amino acids (His, Lys, Orn) and dipeptides (GlyGly, AlaAla) [4] has been studied, and probable coordination modes of biomolecules have been revealed on the basis of thermodynamic approach. It was interesting to continue this study in the case of other amino acids with charged side chains (Asp, Arg, Ser).
The ternary systems M-Nta-L (L = Ser, Arg, Asp) have been studied earlier [5, 6]. Unfortunately, only stability constants of heteroligand complexes have been determined by authors [5, 6], but the full thermodynamic parameters of reactions have not been received. Recently, the synthesis and thermal analysis of ternary complexes of Co(II), Ni(II), Cu(II), and Zn(II) nitrilotriacetates with glycine [7] and alanine or phenylalanine [8] have been carried out. The structures of protonated heteroligand complexes were elucidated using molar conductance, magnetic moment, IR, and UV–Vis spectroscopy. However, possible biomedical application of these complexes requires the speciation of ternary systems M(II)–complexone–amino acid in solution. The comprehensive reinvestigation of these systems using reliable computer programs is caused by the absence of critical choice of valid complexation model in these papers. The possible coordination mode of ligands and structure of ternary complexes also have not been proposed in these papers.
Experimental
Materials
The crystalline sodium salts of copper (II) and nickel (II) nitrilotriacetates NaMNta·1.5H2O have been synthesized and identified by means of elemental and thermal analysis. Carbonate-free NaOH solution was prepared according to the standard procedure. l-Arginine HArg·HCl (high purity grade, Reanal), l-Serine HSer (high purity grade, Reanal), and l-Aspartic acid H2Asp (high purity grade, Reanal) were used without further purification. Iminodiacetic acid H2Ida was purified by recrystallization from aqueous solution. Analytical grade KNO3 used for adjusting the solution ionic strength was doubly recrystallized from distilled water.
Potentiometric measurements
Potentiometric measurements have been taken at MNta:L ratios of 1:1 and 2:1. Solutions containing NaMNta, amino acid, and supporting electrolyte KNO3 were pH-metrically titrated by carbonate-free NaOH solution. The emf of the transfer chain including glass and Ag/AgCl electrodes was measured by the compensation method using potentiometer P-37-1 (Russia) with accuracy of ±0.1 mV. The conditions of potentiometric measurements and computer treatment of potentiometric data using the PHMETR program [9] were described in the previous work [3]. The following complexation model has been taken into account:
The potentiometric titration curves fitted significantly better taking into account the formation of ternary complexes [MNtaL]2− and [MNtaHL]−. The protonated mixed complexes formed under reaction:
In the case of serine, the agreement between the theoretical and experimental titration curves was achieved when the complexes of the type [MNtaSer]2− and [MNtaSerH−1]3− formation have been assumed (Fig. 1).
pH-metric titration curve of a solution (20.09 mL) containing 0.01012 M NaCuNta, 0.01039 M HSer, and 0.48 M KNO3 with a NaOH solution (0.2282 M) (1) and model curves plotted with account of formation of mixed-ligand complex CuNtaSer2− (2) (F min = 8.92), CuNtaSerH 3−-1 (3) (F min = 99.8), and both CuNtaSer2− and CuNtaSerH 3−−1 (4) (F min = 0.73)
Previously [3], it has been shown that the contributions of substitution processes of the type
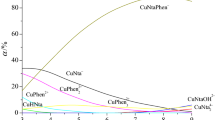
into observed values of pcH and heats of mixing did not exceed the experimental error. The recommended dissociation constants of Nta, Ida, Asp, Arg, and Ser were taken by critical reviews [10–13]. Stability constants of hydroxocomplexonates [MNtaOH]2− have been determined earlier [3]. All determined stability constants of heteroligand complexes are given in the Table 1. The diagrams of the fractional distribution for the CuNta−–L−–H+ (L = Ser, Arg) systems are shown in the Figs. 2 and 3.
Calorimetric measurements
Calorimetric measurements have been taken at 298.15 K using isoperibolic ampoule flow-mixing calorimeter equipped by a 50-mL reaction vessel. The construction of calorimetric cell is original. The calorimeter was verified by the heat of solution of solid KCl in water at 298.15 K. The heats of mixing Δmix H of alkaline solutions of amino acids with solutions containing NaMNta and supporting electrolyte (KNO3) were measured. To account of heat effect of dilution processes, Δdil H blank experiments have been carried out. The experimental data are given in the Tables S1–S8 (in Supporting Information). The heat effect data have been treated using the HEAT computer program [9] based on the minimization of the likelihood function F = Σ(ΔH exp − ΔH calc)2, where ΔH are the experimental and calculated model values of heat effects respectively. The contributions of most important proteolytic and complex formation processes into overall heat effect were calculated according to the following equations:
where n and m denote the processes with known and unknown molar heat effects Δr H i, respectively, B is one of the so-called basic particles (in our case, B is amino acid residue), A is a product of reaction. The molar heats of water ionization and amino acid stepwise protonation were accepted according to the above-mentioned critical reviews. The heat effects of [MNtaOH]2− formation were determined in previous work. Calculated thermodynamic parameters are given in the Table 1. The standard deviations of treated thermodynamic values were calculated as square average deviations taking into account the Student’s coefficients under 95 % confidence level.
ESR and UV–Vis measurements
Visible absorption spectra of solutions containing mixed complexes have been recorded on digital spectrophotometer CFC-3 (Russia) using a cell with 1 cm (Cu) or 5 cm (Ni) optical path length at T = 293 K. The conditions of electronic absorption measurements and computer treatment of spectrophotometric data were similar to [3]. The received absorption spectra in the systems MNta-L (M = Cu, Ni; L = Arg, Ser) are given in the Figs. 4–6. The experimental electronic absorption spectra have been decomposed into individual complex forms using FTMT [9] computer program according to the least-squares fitting procedure. The contribution of each complex ionic form into overall optical density value was treated taking into account molar fractions of all above-mentioned species as calculated from the equilibrium constants by PHMETR program. The distribution-corrected absorption spectra of copper (II) heteroligand complexes are given in the Fig. 7. The standard deviations of calculated molar absorption coefficients do not exceed 0.5 dm3 mol−1 cm−1.
ESR spectra were recorded at the room temperature (291 K) on ELEXSYS II-500 (Bruker) spectrometer. The samples were placed in thin glass tubes with inner diameter 1.1 mm. The background signal of glass tube was eliminated under treatment of experimental ESR spectra. The copper(II) total concentration in solutions was 2 mmol dm−3 (Tables S9 and S10 in Supplementary Material). Simulation of the ESR spectra has been carried out using the original program [15]. The part of experimental and simulated ESR spectra of solutions in Cu-Nta-L ternary system is given in the Fig. S1 (in Supplementary Material). The treated isotropic g0 factors, hyperfine coupling constants A 0, super hyperfine coupling constants A N0 , and rotational correlation times τ R are given in the Table 4.
Results and discussion
Arginine is classified among basic amino acids with positively charged side chain. It has strongly basic guanidinic and α-amino groups. Being potentially terdentate ligand as rule arginine forms complexes with various metal ions by a glycine-like coordination with the protonated side chain guanidinium group, e.g., MHL and M(HL)2 [11]. The coordination activity of guanidinium fragment is still discussed. Earlier received stability constants of ternary complexes of Cu(II) and Ni(II) nitrilotriacetates with arginine and serine [5] sufficiently well agree with data of our work. But in this paper [5], the formation of mixed complexes of the type [MNtaSerH−1]3− has not been detected. Moreover, complex of the type [MNtaL] (L = Arg) that was considered by authors is essentially respect to [MNtaHArg]− species with zwitterionic form of Arg. At the same time, the ternary complex [MNtaArg]2− with anionic form of arginine has not been studied by authors. For this reason, many of these systems should be comprehensively reinvestigated using computer programs and calorimetric data in order to elucidate the correct complexation model.
The calculated thermodynamic parameters of reactions being studied are given in the Table 1. The addition of amino acid residues to [MNta]− is accompanied by negative enthalpy changes and positive or slightly negative entropy changes. The positive Δr S value is mainly caused by the displacement of water molecules from the inner coordination sphere of coordinatively unsaturated metal(II) nitrilotriacetates [MNta(H2O)2]−. The exothermal character of reaction (15) demonstrates that bonding of amino acid residue to the metal complexonate is enthalpy favorable, and the endothermic effect of dehydration processes of the central ion and the ligands is considerably compensated. It has been shown in previous paper [3] that the Δr S values for Ni(II) complexes are substantially more than for Cu(II) complexes due to tetragonal distortion of Cu2+ ion and displacement of one strongly coordinated water molecule from equatorial position of Cu2+·aq.
The lgK and heat effect values of addition of anionic forms of Ser, Gly, and zwitterionic form of Arg are close (Table 1). Apparently, it demonstrates the glycine-like coordination mode of these amino acids. At the same time, the heat effect of addition of zwitterionic form of Arg is rather more than Δr H 15 values in other cases of copper(II) complexes. It can be explained by participation of positively charged guanidinium group of Arg in the weak interaction with noncoordinated acetate group of Nta by means of hydrogen bonding (II, Fig. 9). Such interaction has been described previously in the case of heteroligand complexes of basic and acidic amino acids [14]. The decrease in denticity of Nta in [CuNtaL] complexes can be suggested according to pentacoordinated character of copper(II) amino acid complexes. Recently [15], it has been shown that only one water molecule was coordinated in the axial position of the inner coordination sphere of the copper(II) amino acid complexes by comparison of NMRD, DFT computations, and structural data.
Addition of aspartic acid residue is also accompanied by the same LgK and Δ r H values. Consequently, chelate number of aspartic acid residue in ternary complex [CuNtaAsp]3− does not exceed two, and formation of additional β-alaninate chelate ring does not take place. In the case of Ida, the exothermic heat effect of reaction (15) is sufficiently low. It seems that competition between donor atoms of monoamine complexones takes place. For nickel(II) complexes, the heat effects of all above-mentioned reactions are in the range −19 ± 1 kJ mol−1, and LgK15 values are practically the same (Table 2). So, in all cases, the {N,O} glycine-like coordination mode of amino acid residues is predominated.
In the system M-Nta-Ser, the acidic dissociation of alcohol group of Ser and its participation in coordination process becomes probable. It leads to various coordination modes of serine residue with dissociated alcohol group: (NH2, COO−), (NH2, O−), (NH2, COO−, O−). The structure and spectral properties of complex are mainly determined by the presence of any donor atoms in equatorial or axial position. Formation of [CuSer2], [CuSer2H−1]−, and [CuSer2H−2]2− complexes and its structure in solution have been elucidated by means of ESR method [16]. It was shown that glycine-like coordination dominating in the complex [CuL2] was replaced by the aminoalcoholate-type equatorial binding of the deprotonated serine molecules in the complex [CuL2H−2]2− in strong alkaline solution.
The coordination of serine residue with acidic dissociation of alcohol group and formation of [MNtaSerH−1]3− complexes is less enthalpy favorable in comparison with the reaction (15). Probably, the exothermic heat effect of coordination of amine nitrogen atom and chelate complex formation does not compensate the endothermic effect of acidic dissociation of alcohol group. The similar situation was observed during dipeptide complexation and dissociation of peptide group [4]. The thermodynamic parameters of [CuNtaSerH−1]3− and [CuSer2H−1]− complex formations are close (Table 2), and participation of dissociated alcohol group in coordination should be proposed. The heats of acidic dissociation of heteroligand complexes [MNtaSer]2− for copper and nickel significantly differ. The heat effect of acidic dissociation of complex [NiNtaSer]2− practically equals to heat of water ionization. It seems that ionization of inner-sphere water molecule takes place in ternary complex [NiNtaSer]2− forming complex of the type [NiNtaOHSer]3−. The complexes [MNtaOHSer]3− and [MNtaSerH−1]3− are potentiometrically undistinguishing. Therefore, the content of inner-sphere should be determined by comparison of thermodynamic and spectral data (Table 3).
Unfortunately, relatively small changes in the spectra of CuNta-Ser system do not provide evidence of acidic dissociation of alcohol group of serine and its coordination activity. First of all, the significant decrease and blueshift of d–d transition band in the spectra of solution containing [CuNta]− and serine at pH range 6–8 demonstrates the N,O glycine-like coordination mode of amino acid residue (Fig. 5). The farther small redshift of absorption band in strong alkaline solutions cannot be unambiguously explained by the aminoalcoholate-type equatorial binding of the deprotonated serine molecule. According to received spectral data, the axial binding of deprotonated alcohol group of serine probably takes place (Fig. 8). Analogically, the coordination activity of guanidinium group cannot be determined reliably only by visible spectrophotometric data. In the spectra of Ni(II) complexes (Fig. 6), the changes in the relative intensities of all transitions 3A2g → 3T1g (P), 3A2g → 3T1g (F), and 3A2g → 3T2g (F) also prove not only heteroligand complex formation but also some structural rearrangement. Increasing and significant blueshift of absorption band with λmax ~ 640 nm (3A2g → 3T1g (F)) is characteristic for coordination of aminocarboxylate ligand via N donor atom. Simultaneously, decrease in absorption band with λmax ~ 400 nm (3A2g → 3T1g (P)) takes place. The similar change in absorption band was observed in the spectra of solution containing NiNtaEn− complex. The further decrease in absorption bands with λ max ~ 400 and 600 nm at pH range 9–11 can be explained by formation of [NiNtaOHSer]3−complex with coordinated hydroxide ion.
Generally, ESR spectra are in agreement with visible absorption data. Decreasing of isotropic g 0 factor and increasing of hyperfine coupling constant A 0 are characteristic of equatorial coordination for Cu(II) complexes. The treated ESR parameters (Table 4) well demonstrate the equatorial coordination of auxiliary ligand: Values of above-mentioned spin-Hamiltonian parameters in the case of heteroligand complexes (2.138 and 57, respectively) significantly differ from those of [CuNta]− complex (2.156 and 43, respectively). At the same time, the close values of g 0 and A 0 parameters provide evidence of glycine-like equatorial coordination of amino acid residue in heteroligand complexes. Taking into account the small changes in ESR parameters under axial coordination, the axial coordination of deprotonated alcohol group of serine can be suggested in the case of [CuNtaSerH−1]3−. Apparently, the molar fraction of isomer of [CuNtaArg]2− complex with coordinated guanidinium group (IV, Fig. 9) is too small.
To further elucidate coordination modes of ligands in mixed complexes, the consideration of thermodynamic parameters of reaction of the type
is useful to some extent. All recommended thermodynamic parameters of binary complex formation were taken according to critical reviews [10–13]. Relatively high LgK20 values (Table 3) demonstrate that heteroligand complexes are well resistant toward degradation to homoligand complexes. Earlier [3], it was shown that these processes were favorable in their entropy. The displacement of inner-sphere water molecules of [ML2] complexes and release of outer-sphere water molecules from hydration shells of [M(Nta)2]4− during heteroligand complex formation result in increase in entropy. Practically in all cases, these processes are slightly endothermic owing to unchanged number of coordinated N donor atoms. Relatively high LgK20 values are caused by increasing of Nta denticity in the complex [MNtaL] compared to that in the complex [M(Nta)2]4−. In the case of M-Nta-Ida systems, the LgK20 values are somewhat more than statistical value. Apparently, the effect of increasing of Nta dentate number is not exposed here. The LgK20 values increase according to a set Ida < Asp < Ser owing to their glycine-like coordination ability.
In the case of arginine, the significant rising of heats of mixing calculated per mol of amino acid residue was observed under increasing of ratio CuNta:Arg. It can be explained by binuclear complex formation due to coordination activity of deprotonated guanidinium group (V, Fig. 9). In mononuclear mixed complexes, the dentate number of amino acids with long aliphatic side chain (Lys, Orn, Arg) does not achieve maximum. For this reason, the noncoordinated side chain donor group can coordinate the second nitrilotriacetate complex. In this case, the predominant ionic form is binuclear complex of the type [(CuNta)2L]3−, where the amino acid residue has a bridge function linking the coordination spheres of two copper(II) nitrilotriacetates. The bridge function of basic amino acids has been shown earlier in the case of Nta [3] and Edta [19–21]. In part I of this paper, the formation of binuclear complexes of the type [(NiNta)2L]3− has not been potentiometrically determined. The calorimetric measurements have been extended in order to simultaneous treatment of equilibrium constant and heat effect of binuclear complexes formation. Both translational term and loss of configurational entropy of amino acid residue with long aliphatic chain can serve the explanation of sufficiently big negative entropy changes in such processes.
Conclusions
To reveal the coordination mode of complexone and amino acids in ternary complexes, the thermodynamic approach was used. Thermodynamic parameters of mixed-ligand complex formation have been treated and compared with those for homoligand complexes. First of all, in the MNtaL mixed complexes, the {N,N} coordination mode was replaced by glycine-like {N,O} mode in order His–Orn–Lys, Arg due to independent character of the additional donor group of amino acid with long aliphatic chain. At the same time, the ability of amino acids to binuclear complexes formation is enhanced in the above-mentioned order. Analysis of thermodynamic parameters of heteroligand complexes formation processes from homoligand complexes reveals that these processes are entropically favored. Sufficiently big Δ r S values are mainly caused by release of outer-sphere water molecules from hydration shells of M(Nta) 4−2 during ternary complex formation. In contrast to this, the binuclear complex formation with the bridging function of amino acid is accompanied by sufficiently big negative entropy change owing to loss both translational and configurational entropy terms of amino acid residues with long aliphatic chain. It should be noted that coordination activity of guanidinium group of Arg and dissociated alcohol group of Ser cannot be determined reliably using only thermodynamic approach.
References
Arena G, Musumeci S, Rizzarelli E, Sammartano S. Copper(II)-EDTA-aminoacid interactions. Stability constants and possible role in blood plasma models. Transit Met Chem. 1985;10:399–401.
Block H, Maertens B, Spriestersbach A, Brinker N, Kubicek J, Fabis R, Labahn J, Schafer F. Immobilized-metal affinity chromatography (IMAC): a review. Methods Enzymol. 2009;463:439–73.
Kiseleva I, Pyreu D, Krivonogikh T, Bazanova M, Hochenkova T, Kozlovskii E. Thermodynamic study of mixed-ligand complex formation of copper(II) and nickel(II) nitrilotriacetates with amino acids in solution. I. Polyhedron. 2013;51:10–7.
Pyreu D, Kozlovskii E, Gruzdev M, Kumeev R. Thermodynamic study of mixed-ligand complex formation of copper(II) and nickel(II) nitrilotriacetates with dipeptides in solution. Inorg Chim Acta. 2014;409:507–11.
Israeli J, Cecchetti M. Complexes mixtes de la serine et de l’arginine. Can J Chem. 1968;46:3821–4.
Israeli J, Cecchetti M. Complexes mixtes des nitrilotriacetates metalliques avec l’acide glutamique ou avec l’acide aspartique. Talanta. 1968;15:1031–4.
Souaya ER, Ismail EH, Mohamed AA, Milad NE. Preparation, characterization and thermal studies of some transition metal ternary complexes. J Therm Anal Calorim. 2009;95:253–8.
Khalil MMH, Ismail EH, Azim SA, Souaya ER. Synthesis, characterization and thermal analysis of ternary complexes of nitrilotriacetic acid and alanine or phenylalanine with some transition metals. J Therm Anal Calorim. 2010;101:129–35.
Borodin VA, Vasiliev VP, Kozlovskii EV. Mathematical problems in chemical thermodynamics. Novosibirsk: Nauka; 1985.
Anderegg G. Critical survey of stability constants of NTA complexes. Pure Appl Chem. 1982;54:2693–758.
Yamauchi O, Odani A. Stability constants of metal complexes of amino acids with charged side chains-Part I: positively charged side chains. Pure Appl Chem. 1996;68:469–96.
Berthon G. The stability constants of metal complexes of amino acids with polar side chains. Pure Appl Chem. 1995;67:1117–240.
Anderegg G, Arnaud-Neu F, Delgado R, et al. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications. Pure Appl Chem. 2005;77:1445–95.
Sakurai T, Yamauchi O, Nakahara A. Mixed ligand copper(II) complexes of α-amino acids with ligand-ligand interactions. Bull Chem Soc Japan. 1976;49:169–73.
Bukharov MS, Shtyrlin VG, Mukhtarov ASH, et al. Study of structural and dynamic characteristics of copper(II) amino acid complexes in solutions by combined EPR and NMR relaxation methods. Phys Chem Chem Phys. 2014;16:9411–21.
Szabo-Planka T, Rockenbauer A, Korecz L. An ESR study of coordination modes in copper(II) complexes of l-serine in aqueous solution at ligand excess above pH 7. Polyhedron. 1999;18:1969–74.
Kiss T, Simon C, Vachter Z. J Coord Chem. 1987;16:225.
Kiss T, Sovago I, Gergely A. Critical survey of stability constants of complexes of glycine. Pure Appl Chem. 1991;63:597–638.
Pyreu DF, Kozlovskii EV. Thermodynamics of mixed-ligand complex formation of copper(II) ethylenediaminetetraacetate with hexamethylenediamine in an aqueous solution. J Therm Anal Calorim. 2010;100:355–60.
Bazanova MA, Pyreu DF, Kozlovskii EV. Thermodynamics of mixed-ligand complex formation of copper(II) ethylenediaminetetraacetate with amino acids in solution. J Therm Anal Calorim. 2013;112:1545–51.
Pyreu DF, Kozlovskii EV, Gruzdev MS, Kumeev RS. Thermodynamic and NMR studies of mixed-ligand complex formation of cadmium ethylenediaminetetraacetate with diamines in an aqueous solution. J Therm Anal Calorim. 2011;103:1073–7.
Acknowledgements
We thank A.V. Kulikov from the Institute of Problems of Chemical Physics of the Russian Academy of Science (Chernogolovka) for ESR spectra recording. Appreciation is expressed to V.G. Shtyrlin and M.S. Bukharov from Kazan Federal University for ESR spectra treatment. This work was supported by Russian Foundation for Basic Research (Project No. 14-03-00360-a) and Ministry of Science and Education of Russian Federation (Project No. 526).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pyreu, D., Molkova, T., Ryzkova, S. et al. Thermodynamic study of heteroligand complex formation of copper(II) and nickel(II) nitrilotriacetates with amino acids in solution. J Therm Anal Calorim 124, 1003–1011 (2016). https://doi.org/10.1007/s10973-015-5212-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5212-x